Ageing is associated with a net loss of muscle mass, also in master athletes( Reference Faulkner, Larkin and Claflin 1 – Reference Klitgaard, Ausoni and Damiani 3 ). Since muscle mass is a significant determining factor for muscle strength and function, preservation of muscle mass during ageing is essential for preserving individuals’ ability to live independent lives. Muscle mass and strength are lower in women than in men( Reference Ford, Detterline and Ho 4 – Reference Lee, Janssen and Heymsfield 6 ). Furthermore, women experience accelerated reduction in muscle mass, strength and function when they enter menopause( Reference Kurina, Gulati and Everson-Rose 7 – Reference Samson, Meeuwsen and Crowe 9 ). The age-dependent accumulation of non-contractile tissue (fat, connective tissue) within skeletal muscle tissue increases after menopause( Reference Jubrias, Odderson and Esselman 10 , Reference Taaffe, Sipila and Cheng 11 ) and this reduces muscle quality (strength relative to muscle cross-sectional area)( Reference Phillips, Rook and Siddle 8 , Reference McGregor, Cameron-Smith and Poppitt 12 , Reference Lynch, Metter and Lindle 13 ). Therefore, both the muscle quality and skeletal muscle mass in women are negatively affected by the transition to the post-menopausal state. Since life expectancy is also higher in women than in men, women are particularly vulnerable to age-related frailty and morbidity. Therefore, it is important to evaluate the effectiveness of preventive strategies to postpone loss of muscle function in women. This will be all the more important in coming years due to the increasing numbers of post-menopausal women in developed countries who will challenge healthcare systems if preventive strategies to reduce physical disabilities are not implemented.
Loss of muscle mass takes place when the synthesis rate of structural contractile muscle proteins is lower than the breakdown rate. Furthermore, the turnover of structural muscle, tendon and ligament proteins may influence the composition and function of the tissue( Reference Hansen, Kongsgaard and Holm 14 ). The aim of the present paper is to review the literature regarding the effect of (lack of) oestrogen/female hormones on muscle and tendon collagen protein turnover and mass. The present review will refer primarily to data from human trials. The direct effect of oestrogen on muscle strength and power, independent of the effect of muscle mass, is beyond the scope of the present review, but has been reviewed by others (for review, see( Reference Greising, Baltgalvis and Lowe 15 , Reference Lowe, Baltgalvis and Greising 16 )).
Female hormone levels across the lifespan
Oestrogens are steroid hormones, which mediate their action by binding to a number of tissue receptors, including specific nuclear oestrogen receptors (ER; α and β) and plasma membrane-associated ER( Reference Marino, Galluzzo and Ascenzi 17 ). ER α and β function as transcription factors once bound to their ligand. ER α and β are expressed and localised within skeletal muscle tissue and in tendons and ligaments( Reference Wiik, Ekman and Morgan 18 – Reference Sciore, Frank and Hart 23 ), suggesting a direct effect of oestrogen.
The level of circulating oestrogen (17-β estradiol), the primary female sex hormone, increases during puberty and is about four times higher in women than men during adulthood until menopause. Oestrogen and the other female hormones (progesterone, luteinising hormone, follicle-stimulating hormone) fluctuate during each menstrual cycle. The concentration of circulating oestrogen is relatively low during the early follicular phase (menses phase), increasing progressively in the late part of the follicular phase until ovulation. After this, the level decreases, but it is still maintained at a high level during the luteal phase until menses. The concentration of progesterone is low during the follicular phase, but markedly increased during the luteal phase. During pregnancy, both oestrogen and progesterone are markedly enhanced( Reference Charlton, Coslett-Charlton and Ciccotti 24 ). The level of oestrogen at the end of pregnancy is about 100 times or more higher than the level experienced during the late follicular phase. After delivery, the hormone levels drop again within a few days. Commonly, women in their mid-40s experience the menopausal transition where the level of female hormones and menstrual pattern become irregular. Menopause is defined by the permanent cessation of menstrual period and is experienced by women in their late 40s or early 50s. After menopause, oestrogen is reduced to a negligible level in most women. The implication of the latter is that women spend more than one-third of their life with low levels of oestrogen and progesterone.
Sex differences in muscle mass and muscle protein turnover across the lifespan
The sex difference in muscle mass becomes marked during the teenage years when boys experience accelerated muscle growth( Reference Kyle, Genton and Hans 25 , Reference Wells 26 ). From young adulthood until the menopausal transition, skeletal muscle mass can at an individual level be changed positively or negatively by training and immobilisation/disuse, but otherwise the sex difference in muscle mass is relatively stable until the age of about 50 years.
The observation of no major sex-related changes in relative muscle mass in young subjects is supported by the majority of published studies, which in young subjects report no sex difference in muscle protein synthesis in the post-absorptive state( Reference Fujita, Rasmussen and Bell 27 – Reference West, Burd and Churchward-Venne 31 ) in response to feeding( Reference Smith, Atherton and Reeds 30 , Reference West, Burd and Churchward-Venne 31 ) and strenuous exercise( Reference West, Burd and Churchward-Venne 31 , Reference Miller, Hansen and Olesen 32 ). Similarly, the limited available data for measurements of muscle protein breakdown have shown no difference between young men and women( Reference Fujita, Rasmussen and Bell 27 , Reference West, Burd and Churchward-Venne 31 ) or muscle loss during disuse( Reference Yasuda, Glover and Phillips 33 ). These observations may seem counterintuitive based on the anabolic effect of the male steroid testosterone( Reference Kraemer and Ratamess 34 ) and women have a testosterone level that is 10–15-fold lower than in men( Reference West, Burd and Churchward-Venne 31 ) and do not experience an increase in circulating testosterone post-resistance exercise as has been seen in men( Reference West, Burd and Churchward-Venne 31 , Reference Kraemer, Fleck and Dziados 35 ). Therefore, it can be hypothesised that other stimulating factors (e.g. oestrogen) in young women may compensate for the lower testosterone level since testosterone evidently supports muscle growth in response to resistance training( Reference Kraemer and Ratamess 34 ). Nevertheless, not all data from training studies suggest the response to regular strength training in muscle mass is equal between young men and women( Reference Ivey, Tracy and Lemmer 36 ). A greater increase in muscle volume was observed in eleven young men than in eleven young women after 9 weeks of knee extension exercises, three times weekly (also after adjustment for baseline muscle volume)( Reference Ivey, Tracy and Lemmer 36 ). Nevertheless, the latter findings are in conflict with several other strength training studies, which have reported no sex difference in muscle growth and strength gain( Reference O'Hagan, Sale and MacDougall 37 – Reference Staron, Karapondo and Kraemer 39 ). Still, the number of studies, which have directly compared women and men in relation to the effect of strength training on muscle growth, is limited. Furthermore, the majority of studies include less than ten participants within each training group, which enhance the risk of statistical type II error.
During the menopausal transition, muscle loss is accelerated in women( Reference Aloia, McGowan and Vaswani 40 ), after which the rate of muscle loss slows down( Reference Kyle, Genton and Hans 25 , Reference Forbes and Reina 41 ). The accelerated loss of muscle mass during the post-menopausal transition occurs even though a higher post-absorptive muscle protein synthesis rate is observed in post-menopausal women than in premenopausal women and age-matched men( Reference Smith, Reeds and Hall 42 , Reference Smith, Yoshino and Reeds 43 ). These results suggest that the net negative muscle protein balance in post-menopausal women is caused by an up-regulation of skeletal muscle protein breakdown rate, although this hypothesis has not directly been tested in human subjects. However, catabolic genes (e.g. MuRF1 and FOXO3 mRNA expression) are up-regulated in ageing muscle in women( Reference Smith, Yoshino and Reeds 43 , Reference Raue, Slivka and Jemiolo 44 ), which may help to explain the counterintuitive observation of loss of muscle mass even though the post-absorptive muscle protein synthesis rate is enhanced in post-menopausal women.
A reduced responsiveness to anabolic stimuli such as exercise and feeding when female hormone levels decline at menopause may be an alternative explanation for the net loss of muscle mass in elderly women. In line with this notion, post-menopausal women experience a diminished anabolic response to feeding( Reference Smith, Atherton and Villareal 45 , Reference Smith, Villareal and Sinacore 46 ) and resistance exercise( Reference Smith, Villareal and Sinacore 46 , Reference Hansen, Skovgaard and Reitelseder 47 ) compared with young subjects and age-matched men. Also, the accumulated response to resistance exercise training (three times per week in 26 weeks) has been reported to be lower in elderly women than in elderly men( Reference Bamman, Hill and Adams 48 ). In agreement, after a 10-week unilateral knee-extension strength-training programme, including fifty-four men and eighty-two post-menopausal women (50–85 years) a significant greater training-induced increase in muscle volume was observed in men than women, which corresponded to 9·1 % in men and 7·5 % in women( Reference Walts, Hanson and Delmonico 49 ). In contrast, training studies including women without regard to use of hormone replacement therapy (HRT) and with less than ten subjects in each group have not been able to observe significant differences between old women and men in response to resistance training( Reference Roth, Ivey and Martel 50 ), which may be related to lack of statistical power.
Influence of female hormones on skeletal muscle
In comparative studies, including women and men, it is not possible to test the isolated effect of the individual sex hormones on skeletal muscle. Furthermore, when testing young women, several hormones fluctuate during the menstrual cycle and inter-individual levels of female hormones exhibit great variation. This may be due to genetic factors as well as nutritional status( Reference Mountjoy, Sundgot-Borgen and Burke 51 ). In a cross-sectional trial, we did not observe any differences in the myofibrillar protein synthesis rate in eight young females tested 2–3 d after the onset of menses (the follicular phase), and seven females tested in the luteal phase 4 d after a positive ovulation test( Reference Miller, Hansen and Olesen 32 ). In the luteal phase compared with the early follicular phase, circulating oestrogen was on average twice as high and the progesterone markedly higher, but there was great variation and overlap in oestrogen between the phases (Fig. 1). Therefore, to elucidate a clear effect of oestrogen on muscle protein synthesis rate independent of progesterone, it would have been more appropriate to measure the synthesis rate in the early follicular phase v. late the late follicular phase in a cross-over trial. Another approach could be to administer estradiol and progesterone separately to post-menopausal women who have an existing low circulation level of oestrogen and progesterone. Accordingly, Smith et al.( Reference Smith, Yoshino and Reeds 43 ) in a parallel-randomised controlled trial enhanced circulating estradiol to a level that corresponded to the mid-to-late-follicular phase by administering transdermal oestrogen replacement therapy (ERT) or administrated progesterone in a dose that enhanced circulating progesterone to a level corresponding to the mid-luteal in young girls. The administration of progesterone was associated with a 50 % increase in muscle protein synthesis rate, whereas ERT did not affect muscle protein synthesis rate in the post-absorptive state. This suggests that oestrogen may not have any marked effect on the post-absorptive muscle protein synthesis rate( Reference Smith, Yoshino and Reeds 43 ). Nonetheless, oestrogen may reduce muscle protein breakdown and/or enhance sensitivity to anabolic stimuli. In support of the former, HRT has been reported to reduce muscle loss or increase muscle mass and strength in post-menopausal women in several, but not all( Reference Ribom, Piehl-Aulin and Ljunghall 52 – Reference Kenny, Dawson and Kleppinger 54 ), randomised controlled trials( Reference Taaffe, Sipila and Cheng 11 , Reference Taaffe, Newman and Haggerty 55 – Reference Sipila, Taaffe and Cheng 57 ). Furthermore, positive associations have been observed between serum estradiol and muscle mass and strength in post-menopausal women( Reference Taaffe, Newman and Haggerty 55 ). In line with this, a twin study, including thirteen pairs of monozygotic post-menopausal twin pairs showed that use of HRT was associated with greater muscle power and higher walking speed than no use of HRT after a 1-year intervention( Reference Ronkainen, Kovanen and Alen 58 ). In addition, in a randomised controlled trial lean tissue cross-sectional area was increased significantly (6·3 %) after 12-month administration of HRT compared with the control group (0·7 %), which underline that HRT influence muscle protein balance positively( Reference Sipila, Taaffe and Cheng 57 ). In further support of an oestrogen mediated reduction in skeletal muscle breakdown, HRT has been reported to counteract post-menopausal-related enhancement of protein degradation( Reference Pollanen, Ronkainen and Suominen 59 ). In a randomised double-blinded trial, reduction in lean body mass along with transcriptional changes in the ubiquitine–proteosome system was observed in women in the early post-menopausal years after a 1-year intervention( Reference Pollanen, Ronkainen and Suominen 59 ). In contrast, amongst the women receiving HRT during the intervention period, lean body mass was increased and no transcriptional changes in the ubiquitine–proteosome system were observed( Reference Pollanen, Ronkainen and Suominen 59 ). Therefore, there are several findings, which suggest that ERT/HRT may indirectly influence skeletal muscle protein turnover and counteract the age-related loss of muscle mass and strength. In addition, it should be noted that oestrogen is an antioxidant and sarcolemma membrane stabiliser, which may positively influence the contractile properties of skeletal muscle and protect against muscular damage. Moreover, oestrogen lowers the age-related increase of pro-inflammatory cytokines( Reference Girasole, Giuliani and Modena 60 ) that otherwise may contribute to muscle loss by increasing muscle breakdown( Reference Tsujinaka, Ebisui and Fujita 61 ). Accumulation of fat in the skeletal muscle may also be counteracted by ERT/HRT( Reference Taaffe, Sipila and Cheng 11 ), thus reducing the impairment of muscle quality observed in the elderly( Reference McGregor, Cameron-Smith and Poppitt 12 ).
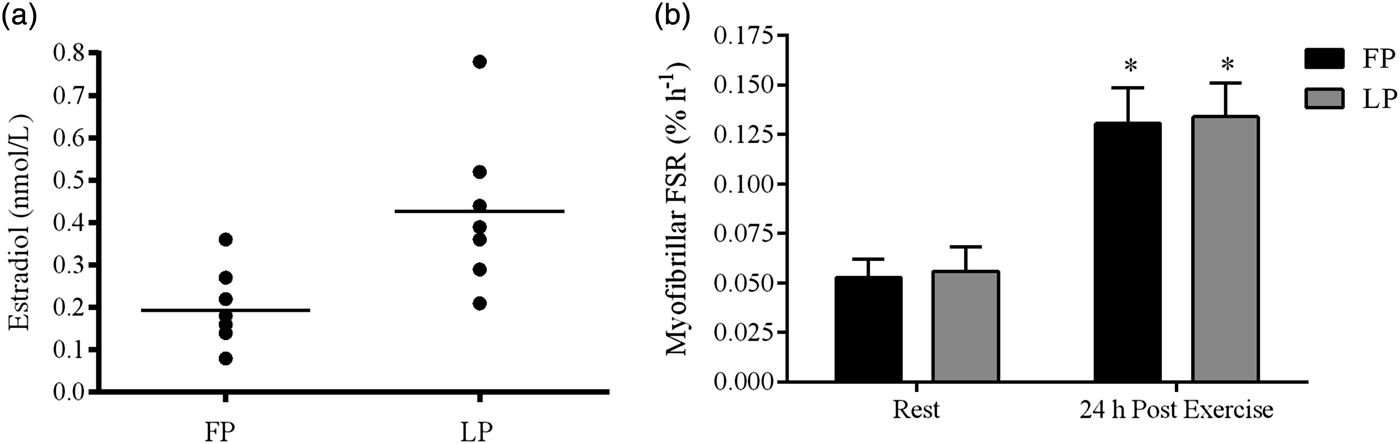
Fig. 1. Plasma estradiol (a) and resting and post-exercise myofibrillar fractional synthesis rates (FSR) (b) in the follicular phase (FP) and luteal phase (LP) of the menstrual cycle. Straight line in (a) represents the average estradiol level. *P < 0·05. Significantly different from contralateral resting leg within each menstrual phase. Copyright© 2006 The American Physiological Society. Used with permission( Reference Miller, Hansen and Olesen 32 ).
A reduced sensitivity to anabolic stimuli in post-menopausal women may provide an alternative explanation for the accelerated loss of muscle mass in the early menopause. Several observations support that oestrogen has an important positive role in regards to increasing sensitivity to training, reducing exercise-induced muscle damage and improving recovery (discussed later). Part of this positive effect may be explained by the idea that oestrogen seems to be important for satellite cell expansion, differentiation, and self-renewal and thereby muscle function( Reference Kitajima and Ono 62 , Reference Enns and Tiidus 63 ) (for review see( Reference La Colla, Pronsato and Milanesi 64 )). Therefore, the reduction in oestrogen at menopause may compromise satellite cell function and have negative impact on the training response and increase the risk of sarcopenia( Reference La Colla, Pronsato and Milanesi 64 ). In addition, in a cross-sectional study that included oral ERT users we observed an increase in myofibrillar protein synthesis rate in response to resistance exercise( Reference Hansen, Skovgaard and Reitelseder 47 ). In contrast, age-matched post-menopausal controls with estradiol concentration under the analytic threshold showed no change in myofibrillar protein synthesis rate when measured 24 h post-exercise, even though the women had performed a very strenuous bout of unilateral knee extensor exercise (ten sets of ten repetitions, corresponding to ten to twelve repetitions maximum (10–12 RM))( Reference Hansen, Skovgaard and Reitelseder 47 ). Moreover, Taaffe et al.( Reference Taaffe, Sipila and Cheng 11 ) observed a synergistic effect when combining training with HRT (oestrogen and synthetic progesterone) on leg muscle cross-sectional area compared with no HRT or training alone. Furthermore, transcriptional data from analysis of muscle samples from post-menopausal women support positive synergetic effects of training and use of HRT on skeletal muscle mass( Reference Pollanen, Fey and Tormakangas 65 , Reference Dieli-Conwright, Spektor and Rice 66 ), and animal data show that oestrogen is important for regaining muscle mass in ovariectomised rats after muscle loss( Reference Brown, Foley and Ferreria 67 – Reference Sitnick, Foley and Brown 69 ).
Oestrogen may also influence the response to training in young girls, but probably not when combined with a high circulating level of progesterone, as in the luteal phase. No difference in the myofibrillar protein synthesis rate in response to an acute bout of strenuous exercise is observed between girls in the early follicular phase where circulating oestrogen and progesterone are low compared with the mid-luteal phase where both hormones are elevated( Reference Miller, Hansen and Olesen 32 ) (Fig. 1). Also, animal data indicate that the individual effect of oestrogen and progesterone on net muscle protein balance may counteract each other when present simultaneously (as in the luteal phase) (see review( Reference Oosthuyse and Bosch 70 )). In the follicular phase, especially in the late part of the follicular phase, only oestrogen is enhanced. This may hypothetically induce an enhanced possibility for muscle growth if resistance training is performed in this phase of the menstrual cycle. In support, Wikström-Frisén et al.( Reference Wikström-Frisén, Boraxbekk and Henriksson-Larsen 71 ) observed greater improvements in muscle strength and muscle mass in response to 4 months resistance training in girls who had undertaken intensified resistance exercise training (five times per week) during the follicular phase compared with girls who undertook intensified resistance exercise training in the luteal phase. This observation is supported by others( Reference Reis, Frick and Schmidtbleicher 72 , Reference Sung, Han and Hinrichs 73 ), but not all( Reference Sakamaki-Sunaga, Min and Kamemoto 74 ). In general, the number of studies within the area is still limited. In addition, in the most well-controlled study by Wikström-Frisén et al.( Reference Wikström-Frisén, Boraxbekk and Henriksson-Larsen 71 ) with fifty-nine participants who completed the training protocol, the groups consisted of a mix of non-users and users of oral contraceptives (OC), which makes it difficult to separate between the effects of endogenous and synthetic female hormones. Nevertheless, it should be noted that the OC users who experienced an increase in muscle growth and strength when training in the first 2 weeks of the pill-circle primarily used triphasic OC with a low content of synthetic progesterone in the first part of the pill period.
In young women, a lower myofibrillar protein synthesis rate was observed in women using OC containing a constant amount of ethinyl estradiol and gestogen (third generation OC) compared with non-users of OC( Reference Hansen, Langberg and Holm 75 ). In contrast, myofibrillar protein synthesis rate in the non-users of OC and users of second generation OC containing ethinyl estradiol and norgestimate was comparable( Reference Hansen, Langberg and Holm 75 ). These observations indicate that the synthetic type of progesterone (gestagen) have differential (anti-) androgen effects on myofibrillar protein synthesis rate when combined with ethinyl estradiol. However, mostly the type of OC is not reported in the literature or type of OC has not been taken into account in the data analysis. The present data underline the importance of clarifying the specific effect of the different types of OC on skeletal muscle in future studies.
In summary, oestrogen may be important for muscle maintenance and muscle growth in response to training in young and post-menopausal women regardless of no potential direct effect of oestrogen on muscle protein synthesis at rest in the post-absorptive state( Reference Smith, Yoshino and Reeds 43 ). However, future human trials need to clarify the individual female hormones’ effect on net muscle protein balance alone and in combination under differential circumstances (e.g. in the post-absorptive state, in response to protein feeding and/or in response to exercise/training).
Influence of female hormones on tendon and ligaments
Any influence of female hormones on the biomechanical properties of tendon and ligaments will have impact on locomotion( Reference Kjaer, Langberg and Heinemeier 76 ). Therefore, it is interesting to note that sex differences are observed in connective tissue. However, the effect of individual female hormones alone and combined on skeletal muscle connective tissue is a puzzle, which may be related to differential direct and indirect effects of endogenous and exogenous female hormones( Reference Hansen and Kjaer 77 , Reference Hansen and Kjaer 78 ).
Tendon structural quality seems to be lower in women than in men( Reference Magnusson, Hansen and Langberg 79 – Reference Carroll, Dickinson and Haus 82 ). Isolated female tendon fascicles rupture at a lower load compared with fascicles from men( Reference Magnusson, Hansen and Langberg 79 ). Also, a lower tendon dry mass per mg tendon wet weight( Reference Lemoine, Lee and Trappe 80 ) and a higher expression of type III collagen mRNA( Reference Sullivan, Carroll and Jemiolo 81 ) in women compared with men has been reported. In line with these observations, tendon stiffness during maximal loading is lower in women, indicating less resistance to deformation during loading( Reference Carroll, Dickinson and Haus 82 ). Reduced tendon and ligament stiffness may explain why muscle damage after eccentric non-weight-bearing muscle contractions is lower in women than in men because of reduced tensile loading of the myofilaments during muscular contractions( Reference Sewright, Hubal and Kearns 83 , Reference Minahan, Joyce and Bulmer 84 ). Conversely, reduced stiffness may also help to account for the observed 2–8 times higher risk of sustaining an anterior cruciate ligament (ACL) rupture in active women than in comparably active men( Reference Hewett, Myer and Ford 85 , Reference Huston, Greenfield and Wojtys 86 ). The idea that oestrogen may negatively impact tendon and ligament resistance against rupture during loading is further supported by the findings that load to failure is significantly lower in ACL from rabbits treated with a high dose of oestrogen than in controls( Reference Slauterbeck, Clevenger and Lundberg 87 ). Nevertheless, in regard to tendinopathy, ERT/HRT may be beneficial for post-menopausal women in preventing tendinopathy, especially in active elderly women( Reference Cook, Bass and Black 88 ).
Sex may also influence the ability of tendons and ligaments to respond to training. Collagen is the most abundant structural protein in tendons and ligaments, and the tendon collagen synthesis rate is markedly lower in women than in men both at rest and in response to acute exercise( Reference Miller, Hansen and Olesen 89 ). Furthermore, in a cross-sectional trial, we included young untrained controls and experienced female and male runners who had been running at least 40 km/week for the previous 5 years (men average 58 km/week and women average 54 km/week)( Reference Westh, Kongsgaard and Bojsen-Moller 90 ). The results showed that the weight-normalised cross-sectional area of the patellar tendon and Achilles tendon in trained and untrained women were comparable( Reference Westh, Kongsgaard and Bojsen-Moller 90 ). In contrast, the cross-sectional area of the tendons in trained men were greater than untrained and trained women, but also greater than untrained men( Reference Magnusson, Hansen and Langberg 79 , Reference Westh, Kongsgaard and Bojsen-Moller 90 ). These data indicate that the hypertrophic effect of regular exercise on the patellar and Achilles tendons is lower in young trained women than in similarly trained men( Reference Magnusson, Hansen and Langberg 79 , Reference Westh, Kongsgaard and Bojsen-Moller 90 ) (Fig. 2). Based on these findings, we hypothesised that oestrogen has an inhibiting effect on tendon and ligament collagen synthesis, which was supported by some( Reference Yu, Liu and Hatch 91 ), but not all animal findings( Reference Lee, Liu and Smith 92 ) dependent on species. Nevertheless, we found that elderly women using ERT had a higher tendon collagen synthesis than age-matched post-menopausal women, and that the estradiol level correlated positively with the tendon collagen synthesis rate (Fig. 3)( Reference Hansen, Kongsgaard and Holm 14 ). Therefore, the higher tendon collagen synthesis in men compared to women may be caused by a dominating effect of another factor (e.g. testosterone). But on comparing women to women the higher tendon collagen synthesis rate in ERT users than in non-users was associated with a relatively lower tendon stiffness( Reference Hansen, Kongsgaard and Holm 14 ). Similarly, we observed in a group of female handball players a negative correlation between serum estradiol and tendon stiffness (adapted from( Reference Hansen, Couppe and Hansen 93 )). Furthermore, a significantly higher knee joint laxity was observed in women in their third trimester (week 30) compared with 5–7 weeks postpartum. Knee joint laxity was reduced in thirty-eight of forty women postpartum( Reference Charlton, Coslett-Charlton and Ciccotti 24 ). The latter observation underlines the fact that biomechanical properties can change over a relatively short time. In a well-controlled trial, Lee et al.( Reference Lee, Petrofsky and Daher 94 ) collected blood samples seven times during a menstrual cycle and measured anterior tibia displacement simultaneously. In the late follicular phase, when the level of serum estradiol peaks and progesterone is low, they observed significantly greater knee laxity compared with other time points during the menstrual cycle. This observation is confirmed by others( Reference Shultz, Sander and Kirk 95 – Reference Deie, Sakamaki and Sumen 97 ) and is connected with a greater risk of sustaining an ACL rupture in the late follicular phase of the menstrual cycle( Reference Hewett, Zazulak and Myer 98 , Reference Adachi, Nawata and Maeta 99 ). It seems surprising that tissue structure is able to change within days. However, results from engineered ligaments have shown that short-term exposure to oestrogen (48 h) can inhibit the activity of the crosslinking enzyme lysyl oxidase( Reference Lee, Lee-Barthel and Marquino 100 ). As a result, the tissue structure was destabilised, the relative stiffness was lowered and the ultimate stress before rupture was reduced( Reference Lee, Lee-Barthel and Marquino 100 ). Notably, this finding comes from a study that involved engineered ligaments, so further research is needed to confirm whether similar inhibition of the cross-linking enzyme in tendon and ligaments takes place in human subjects in vivo.
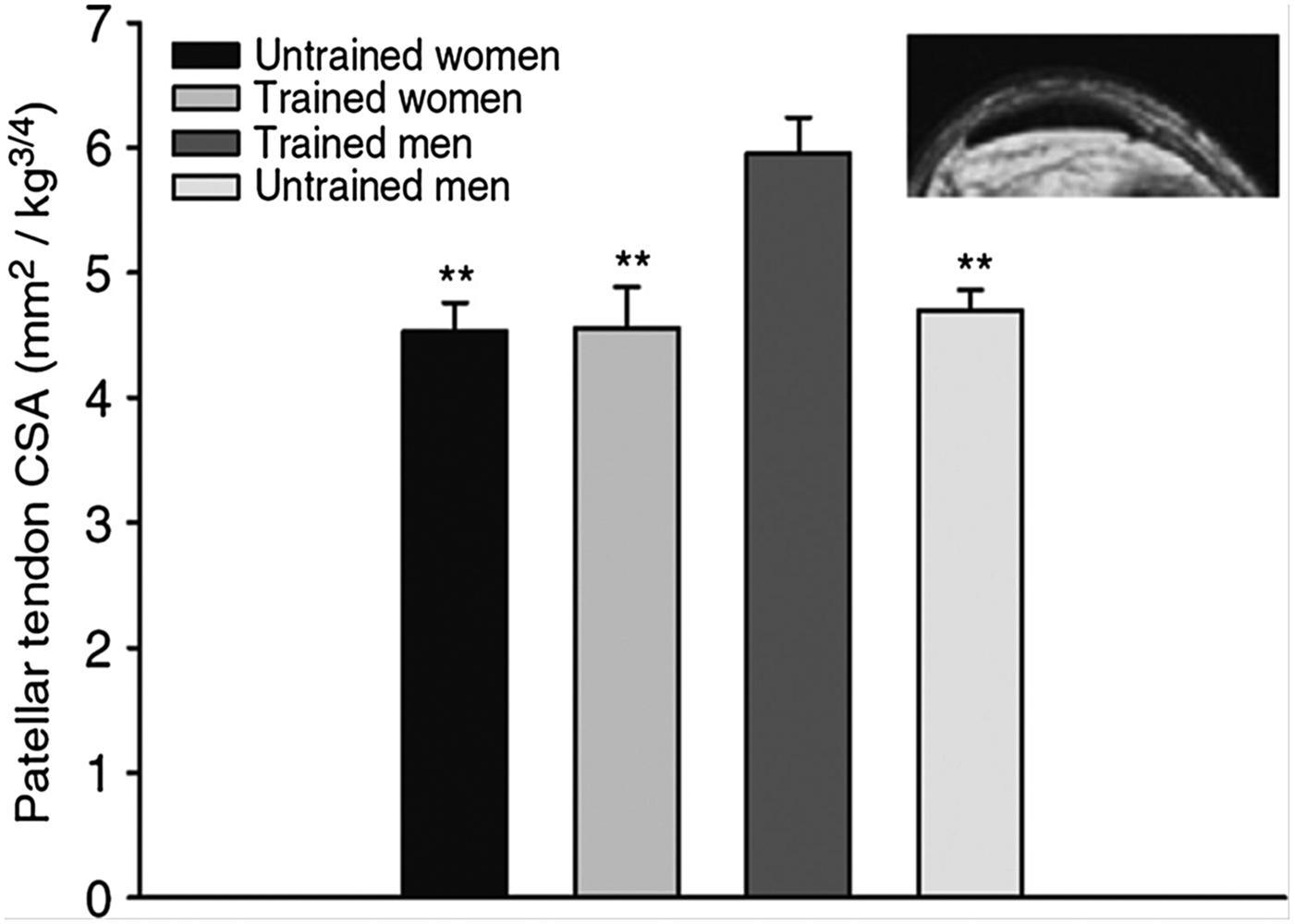
Fig. 2. The MRI determined patellar tendon cross-sectional area (CSA) for trained and untrained men and women normalised to body mass. Trained men had a greater CSA than untrained men (P<0·01); however, note that trained women had a similar CSA compared with untrained women( Reference Magnusson, Hansen and Langberg 79 , Reference Westh, Kongsgaard and Bojsen-Moller 90 ). Copyright 2007 John Wiley and Sons. Used with permission.
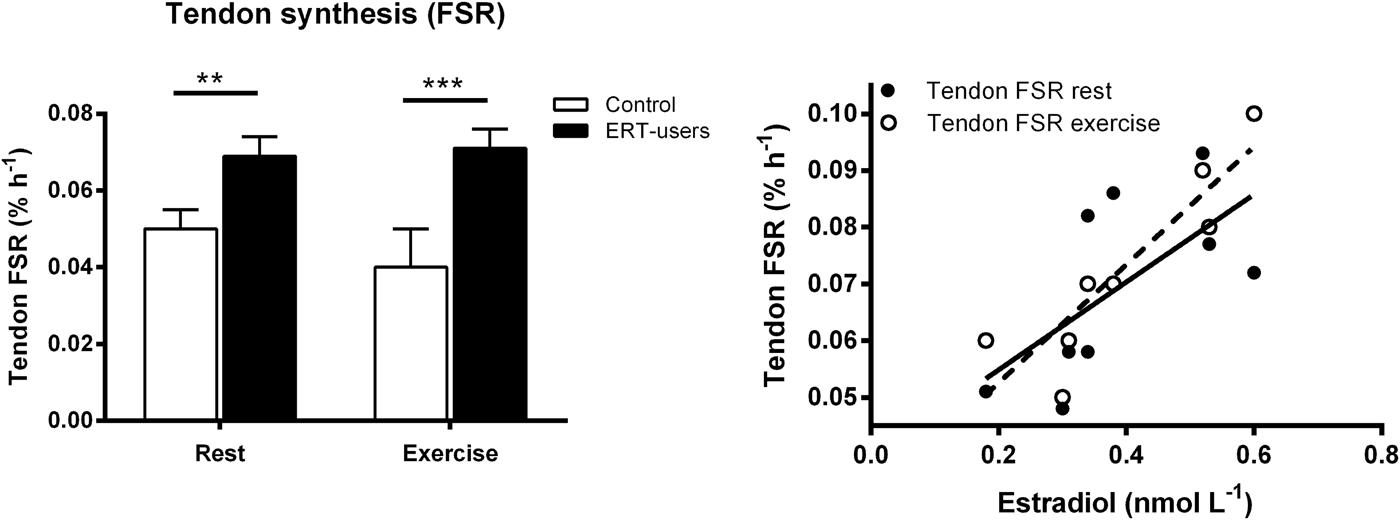
Fig. 3. Left: Patellar tendon collagen fractional synthesis rates (FSR) at rest and 25 h after exercise in post-menopausal women who used oestrogen replacement therapy (ERT) and post-menopausal women who did not use ERT (control) **P < 0·01 and **P < 0·001, unpaired t test, control v. ERT users. Right: Relationship between tendon FSR and serum (s)- estradiol in ERT users at rest (r 2 0·41, P = 0·06) and post-exercise (r 2 0·80, P < 0·001)( Reference Hansen, Kongsgaard and Holm 14 ). Copyright© 2009 The American Physiological Society. Used with permission.
Administration of OC to young women seems to have opposite effect of tendon and ligaments than HRT to postmenopausal women( Reference Hansen and Kjaer 78 ). In OC users, tendon collagen synthesis rate is lower than in age-matched controls( Reference Hansen, Miller and Holm 101 ). This is in contrast to the higher tendon collagen synthesis rate observed in elderly women using ERT compared with age-matched controls( Reference Hansen, Kongsgaard and Holm 14 ). Furthermore, use of OC is associated with lower ACL elasticity in several studies( Reference Lee, Petrofsky and Daher 102 , Reference Martineau, Al-Jassir and Lenczner 103 ), but not all( Reference Pokorny, Smith and Calus 104 ). This is also contrary to the lower relative tendon stiffness in elderly ERT users( Reference Hansen, Kongsgaard and Holm 14 ). The influence of OC on tendon and ligaments seem to influence injury risk. A case–control study including 4497 operatively treated patients after ACL rupture and 8858 age-matched controls with no ACL injury concluded that the relative risk for sustaining an ACL injury was lower in OC-users( Reference Rahr-Wagner, Thillemann and Mehnert 105 ). The latter may be explained by OC users having a lower endogenous level of estradiol and they do not experience a peak in estradiol during the pill-period as do non-users of OC. Still, it has not been clarified whether synthetic estradiol (ethinyl estradiol) or the synthetic gestagens in OC influence tendon and ligament collagen turnover directly or indirectly. It is noteworthy that, use of OC is associated with markedly lower insulin-like growth factor-I (IGF-I) levels in young female OC users( Reference Hansen, Miller and Holm 101 ). IGF-I enhances tendon collagen synthesis( Reference Hansen, Boesen and Holm 106 ). Therefore, the lower IGF-I level in OC-user may be a major explanatory factor in regards to the lower tendon collagen synthesis rate( Reference Hansen, Miller and Holm 101 ). The IGF-I level is already relative low in elderly women( Reference Vestergaard, Hansen and Frystyk 107 ). Therefore, the further small reduction in IGF-I induced by oral ERT in elderly women may have negligible influence on tendon collagen synthesis rate, whereas the stimulating influence of an enhanced estradiol level on tendon collagen synthesis may overrule the consequence of a lowered IGF-I level in elderly ERT-users( Reference Hansen and Kjaer 78 ).
It is noteworthy that the presented findings have focused on the effect of female hormones on ACL, Achilles or patellar tendon. It is too simple to assume that female hormones influence the structure and biomechanical properties equally in all tendons and ligaments, independent of anatomical position and function (e.g. stabilisation or elastic properties). Differential distribution of ER in different tissues may, for example, induce differential effects on collagen protein turnover( Reference Khalid and Krum 108 ). Furthermore, there are many types of OC and HRT with either estradiol or ethinyl-estradiol, and different types of synthetic progesterone with differential androgenic properties. Nevertheless, the latter has not be elucidated in regards to the effects on tendon and ligament collagen synthesis rate, and only sparely in regards to the effect on myofibrillar protein synthesis rate( Reference Hansen, Langberg and Holm 75 ).
In conclusion, sex differences in muscle protein turnover in young subjects seem to be negligible. Still, oestrogen may play an important role for obtaining a positive anabolic effect of training. However, oestrogen also reduces tendon and ligament stiffness, which for the young female athletes probably enhance the risk for ACL rupture. In postmenopausal, administration of ERT/HRT has beneficial effects on skeletal muscle protein maintenance and may improve sensitivity to anabolic stimuli and thereby enhance muscle mass and strength. Furthermore, ERT/HRT may be beneficial for post-menopausal women in preventing tendinopathy and reduce tendon stiffness. Nevertheless, individual's risk profile should be considered before initiating HRT/ERT and it must be underlined that no evidence-based optimal dose, type of ERT/HRT, duration or timing of initiation of treatment is currently outlined. Therefore, currently, post-menopausal women should be recommended to follow evidence-based guidelines for diet and regular resistance training (with or without use of HRT/ERT), since these are well-documented strategies for counteracting age-related loss of muscle mass and function, but also other age-related degenerative changes in men and women( Reference Chase, Phillips and Brown 109 – Reference Witard, McGlory and Hamilton 111 ). Furthermore, physically active post-menopausal women report fewer symptoms related to menopause than sedentary women( Reference Moilanen, Aalto and Hemminki 112 ).
Acknowledgements
I thank the Nutrition Society for the invitation to give a presentation at the Spring Conference 2017 in Stirling, Scotland, and the opportunity to prepare this paper.
Financial Support
None.
Conflict of Interest
None.
Authorship
M. H. conducted the literature search and drafted the paper.






