Protection from obesity is one of several possible health-beneficial actions of the soya isoflavone genistein( Reference Behloul and Wu 1 ). We hypothesised that this effect is due to promoting a beige, rather than white, adipocyte phenotype. We thus determined how genistein affected the expression profile of a panel of genes characteristic of white or beige adipocytes in the mouse NIH3T3-L1 adipocyte cell line model. Since protection from obesity is an action also of the dietary polyphenol resveratrol( Reference de Ligt, Timmers and Schrauwen 2 ), which can increase Sirt1 expression( Reference de Ligt, Timmers and Schrauwen 2 ), we hypothesised also that some of these actions of genistein are mediated through affecting Sirt1 expression and/or activity.
Genistein (10–100 μM) was added as a component of the medium used to achieve differentiation of NIH3T3-L1 cells into adipocytes. RNA was prepared after 3–12 d and expression of a panel of test genes was measured by RT-qPCR. Mitochondrial oxygen consumption was measured 12 d after inducing differentiation. A low concentration of genistein (10 μM) and/or shorter exposure (3 d) promoted differentiation to white adipocytes, indicated by large fat droplets and expression of adipocyte marker genes( Reference Wu, Boström and Sparks 3 ) at higher levels. However, at higher concentration of genistein (100 μM) and after longer exposure (12 d) cells had smaller fat droplets and lower expression of these genes, coupled with expression of genes characteristic of beige adipocytes( Reference Wu, Boström and Sparks 3 ) at higher levels. Table 1 shows data for expression of white adipocyte marker genes induced by exposure to 10 μM genistein over 8 d. Table 2 shows data for expression of beige adipocyte marker genes induced by exposure to 100 μM genistein over 12 d.
Table 1. Values are for n = 3 normalised to control using TOP1 and NONO as the reference gene in RT-qPCR; **P < 0·01; **P < 0·001 by one-way ANOVA followed by Dunnett's test
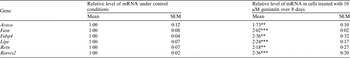
Table 2. Values are for n = 6 normalised to control using TOP1 and NONO as the reference gene in RT-qPCR; **P < 0·01; **P < 0·001 by one-way ANOVA followed by Dunnett's test

Genistein at 100 μM also increased Sirt1 expression after 12 d (1·68 ± 0·10 vs 1·00 ± 0·03; P < 0·001) and the Sirt1 inhibitor ST527 (10 μM added at 48 h post-confluence for 12 d) attenuated the effects of 100 μM genistein to increase UCP1 mRNA (6·62 ± 2·92 vs 19·10 ± 5·41; P < 0·05). In addition, basal and proportion of uncoupled mitochondrial oxygen consumption were higher in cells treated with genistein than in control cells (305 ± 7 vs 253 ± 11 pmol/min/mg; 0·87 ± 0·06 vs 0·54 ± 0·04, respectively (P < 0·001 by Student's t-test) consistent with a switch from white to beige metabolic phenotype.
Dietary genistein may thus protect against obesity by promoting the development of beige, rather than white, adipose tissue, through a mechanism that may involve increased action of Sirt1.




