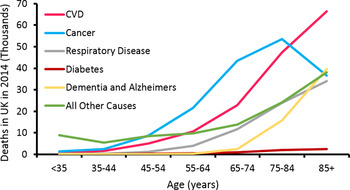
Life expectancy has increased dramatically (Fig. 1). In the UK, males and females born in 1982, had a life expectancy of 71·1 and 77·0 years, respectively, while the projected values for 2082 are 89·7 and 92·6 years( 1 ). Thus, we are witnessing a staggering demographic shift in favour of older people (Fig. 2). For instance, it has been estimated the percentage of individuals in the UK over 60 years of age will double to 22 % by 2050, when compared to 2000( 2 ). Remaining disease free is a significant challenge faced by older people, as the prevalence of many conditions increases with age (Fig. 3). Of the diseases associated with advancing age, CVD is the leading cause of morbidity in individuals over 60 years of age( Reference Prince, Wu and Guo 3 ). The dysregulation of cholesterol metabolism is intimately associated with the pathogenesis of CVD( Reference Gould, Davies and Alemao 4 ), and age-related alterations in the metabolism of cholesterol are implicated in the disturbance of this system( Reference Morgan, Mooney and Wilkinson 5 ). These include, a decrease in LDL-cholesterol clearance; a potential increase in cholesterol absorption; a decrease in bile acid synthesis; a decrease in bacterial bile acid modification( Reference Millar, Lichtenstein and Cuchel 6 – Reference Hopkins and Macfarlane 8 ). It is likely that these alterations play a role in the accumulation of LDL-cholesterol and disease pathogenesis. The accumulation of plasma cholesterol can also be moderated by diet, while pharmaceutical and pre- and probiotic administration have largely been associated with reduced LDL-cholesterol levels and CVD risk( Reference Guo, Liu and Zhang 9 – Reference Beserra, Fernandes and do Rosario 13 ).

Fig. 1. UK life expectancy by year of birth. Data from Clio-Infra( 132 ).

Fig. 2. (Colour online) UK population by age and sex in 1982 and 2012. Data from United Nations Statistics Division( 133 ).

Fig. 3. (Colour online) UK causes of death by age. Data from British Heart Foundation( Reference Townsend, Bhatnagar and Wilkins 134 ).
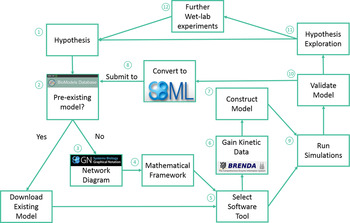
Traditionally, when nutritionists have investigated complex metabolic pathways, such as cholesterol metabolism, they have utilised conventional wet laboratory techniques. However, studying cholesterol metabolism and its interaction with both diet and ageing using conventional approaches is challenging, due to the integrated nature of this system, and the time scales involved in studying the effects of the ageing process. Traditional in vivo or in vitro techniques can also be limited when testing a hypothesis, as such approaches can be resource-intensive, expensive, impractical and potentially unethical( Reference Mc Auley, Proctor and Corfe 14 ). Thus, utilisation of the systems biology approach is becoming an increasingly important tool in nutrition-based research, as systems biology overcomes a number of the challenges outlined earlier, but more importantly, facilitates the integration of data generated from a diverse range of sources( Reference Mc Auley, Proctor and Corfe 14 ), leading to an improved understanding of how cellular dynamics influence the behaviour of tissues and ultimately the health of whole-organ systems( Reference Auffray and Hood 15 ). Thus, the systems biology approach seeks to understand complex biological systems by studying them in a more holistic manner, in contrast to the reductionist approach regularly adopted in human nutrition. At the core of the systems biology approach is computational modelling. Computational modelling is an abstract process that is used to represent the dynamics of a biological system in a precise manner using mathematics. The steps involved in building a computational model are outlined in Fig. 4. Computational models are now used to model a diverse range of complex nutrient centred pathways including cholesterol metabolism for a number of reasons. Firstly, computational models are capable of providing quantitative data on the interaction of molecular components( Reference Kitano 16 ). Secondly, nutrient-based interactions are inherently complex and often non-linear in nature( Reference Patti and Kahn 17 – Reference Gianchandani, Brautigan and Papin 19 ), and can involve complex feedback and feedforward loops( Reference Lamb 20 – Reference Pappu, Steiner and Connor 22 ). Thus, it is challenging and even unfeasible to reason about these by human intuition alone. Computational modelling offers an alternative means of handling this complexity; thus, utilisation of computational modelling alongside the experimental work provides a means of representing the dynamics of complex biological systems. Models can be used to simulate intrinsic perturbations, such as those associated with ageing and extrinsic perturbations, such as diet. Output from the model provides an overview of how these changes impact the dynamics of the system, and the implications this has for healthspan. In this review, we present an overview of cholesterol metabolism and discuss how ageing impacts its regulatory mechanisms. We also discuss how diet influences cholesterol metabolism, and how the dysregulation of this system influences heath. Next, we discuss therapeutic strategies for the treatment of hypercholesterolaemia, namely dietary, pharmacological and probiotic intervention. Finally, we describe how we are using the systems biology framework to investigate cholesterol metabolism and the impact ageing has on it. Specifically, there is a focus on how we have used computational modelling and how we are exploring this approach with simulated digestive tracks.

Fig. 4. (Colour online) Modelling overview. (1) Identify the system to model and hypothesis formation. (2) Identify pre-existing models; using the BioModels Database, a repository for peer reviewed models. (3) If no model of the system of interest exists: produce a network diagram. If a model does exist: download model and move to step 5, then step 7. (4) Establish mathematical framework. (5) Identify a suitable modelling tool; there are several available including: COPASI, which we utilised in our updated model of cholesterol metabolism( Reference Morgan, Mooney and Wilkinson 123 ), CellDesigner, Mathematica and MATLAB. (6) Obtain initial concentrations of species, rate laws and kinetic data to construct the model. The online resources BRENDA and SABIO-RK provide a substantial volume of kinetic data. (7) Run simulations. (8) Validate the model. (9) Explore the hypotheses, and determine if the model accurately represents the biological system, and can be used to make predictions, or if the model needs refining. (10) Conduct further wet laboratory experiments based upon model output. (11) Code the model in the exchange format, Systems Biology Markup Language and deposit in the BioModels Database. Adapted from Mc Auley and Mooney( Reference Mc Auley and Mooney 135 ).
Overview of cholesterol metabolism
Cholesterol plays a vital role in the body as a component of cell membranes, and precursor to steroid hormones and bile acids. Whole-body cholesterol metabolism is encapsulated by cholesterol ingestion, absorption, excretion and synthesis. These factors interact in a coordinated fashion to regulate whole-body cholesterol balance, with subtle changes to individual components influencing the behaviour of the others, so that cholesterol balance is maintained. In the next sections, we will outline in detail the complexities of cholesterol metabolism and how ageing interacts with it, thus emphasising the need for a systems biology approach when investigating it.
Cholesterol ingestion and absorption
In the UK, the average daily intake of cholesterol is 304 and 213 mg for males and females, respectively( Reference Henderson, Gregory and Irving 23 ), 10–15 % of which is in the esterified form( Reference Iqbal and Hussain 24 ). In the small intestine, esterified cholesterol is hydrolysed to form free cholesterol, which is more readily incorporated into bile acid micelles, which facilitate the absorption of cholesterol via Niemann–Pick C1-like 1 protein (NPC1L1)( Reference Betters and Yu 25 , Reference Ikeda, Matsuoka and Hamada 26 ). Additionally, phytosterols can also be absorbed via NPC1L1( Reference Davis, Zhu and Hoos 27 ). Intestinal absorption of cholesterol and phytosterols can be limited by heterodimer ATP-binding cassette (ABC) G5/G8, which effluxes these sterols back to the intestinal lumen( Reference Yu, Qian and Jiang 28 ). Acyl CoA: cholesterol acyltransferase 2 esterifies internalised cholesterol, which is then incorporated into a nascent chylomicron via microsomal TAG transfer protein( Reference Chang, Li and Chang 29 , Reference Atzel and Wetterau 30 ). The nascent chylomicron then exits the enterocyte by exocytosis into the lymphatic system before entering the blood stream( Reference Van Dyck, Braem and Chen 31 ). The nascent chylomicron is converted to a mature chylomicron upon acquisition of apo C-II and E from HDL. Apo C-II activates lipoprotein lipase on the capillary endothelium of adipose or muscle tissue, which in turn catalyses the hydrolysis of TAG( Reference Kersten 32 , Reference Olivecrona and Beisiegel 33 ). Apo C-II is then returned to HDL, and hepatic LDL receptors (LDLr) and LDLr-related protein recognise apo B-48 and E, initiating the absorption of the chylomicron remnants( Reference Cooper 34 ).
Cholesterol synthesis
Cholesterol is synthesised endogenously in all nucleated cells in the body from acetyl CoA( Reference Bloch 35 ). Renfurm et al.( Reference Renfurm, Bandsma and Verkade 36 ) observed endogenous cholesterol was synthesised at a rate of 9·8 (sd 6·2) mg/kg per d in healthy adults with a mean age of 32 years and mean weight of 64 kg. This equates to 627·2 mg/d synthesised cholesterol, a similar value to the 710 mg/d observed by Jones and Schoeller( Reference Jones and Schoeller 37 ). Interestingly, cholesterol consumption can influence the synthesis of endogenous cholesterol; an increase from 173 to 781 mg/d dietary cholesterol has been observed to decrease the rate of sterol synthesis by 34 %( Reference Parker, McNamara and Brown 38 ).
Cholesterol synthesis commences when acetoacetyl CoA thiolase catalyses the interconversion of acetyl CoA and acetoacetyl CoA. One molecule acetyl CoA and one molecule acetoacetyl CoA undergo a condensation reaction by 3-hydroxy-3-methylglutaryl (HMG) CoA synthase to form a molecule of HMG CoA. HMG CoA reductase, with the addition of two NADPH molecules, and then catalyse the conversion of HMG CoA to mevalonate. As the rate-limiting enzyme of cholesterol biosynthesis, HMG CoA reductase is the therapeutic target of statins, for the treatment of hypercholesterolaemia, and the prevention of atherosclerosis( Reference Sirtori 39 ). Phosphorylation of mevalonate by the enzyme mevalonate kinase forms mevalonate-5P, which undergoes further phosphorylation to form mevalonate-5PP via the enzyme phosphomevalonate kinase. Decarboxylation and dehydration by mevalonate-5PP decarboxylase create isopentenyl-PP and thus its isoform dimethylallyl-PP via isopentenyl diphosphate delta isomerase. Farnesyl diphosphate synthase initiates the condensation of dimethylallyl-PP with one molecule of isopentenyl-PP and NADPH to create geranyl-PP. Further condensation and the addition of another molecule of isopentenyl-PP and NADPH create farnesyl-PP. Condensation of two farnesyl-PP molecules by squalene synthase and NADPH forms squalene, which is then converted to squalene epoxide by squalene epoxidase, NADPH, and O2, before undergoing cyclisation by oxidosqualene cyclase to form lanosterol( Reference Hoshino, Chiba and Abe 40 ). A series of reactions, including the branching of 7-dehydrodesmosterol to either desmosterol or 7-dehydrocholesterol, both of which can then be converted to the end product cholesterol via the enzymes 24-dehydrocholesterol reductase and 7-dehydrocholesterol reductase concludes the de novo synthesis of cholesterol( Reference Luu, Hart-Smith and Sharpe 41 , Reference Risley 42 ).
Lipoprotein dynamics and reverse cholesterol transport
VLDL-cholesterol is formed from the hepatic pool of cholesterol to transport endogenously synthesised TAG to the tissues( Reference Havel 43 ). Partial hydrolysis of VLDL by lipoprotein lipase forms intermediate density lipoprotein, with subsequent hydrolysis of intermediate-density lipoprotein by hepatic lipase forming LDL, which acts to deliver cholesterol to the peripheral tissue( Reference Havel 43 ). VLDL-cholesterol, intermediate-density lipoprotein-cholesterol and LDL-cholesterol can be removed from the circulation by hepatic LDLr, while LDL-cholesterol can also be absorbed independently( Reference Spady, Turley and Dietschy 44 , Reference Veniant, Zlot and Walzem 45 ). Reverse cholesterol transport transfers cholesterol from the tissues to the liver via HDL, reducing the risk of cholesterol accumulation and atherosclerosis( Reference Shen, Peng and Peng 46 ). Cholesterol can be effluxed from the tissues by the receptors ABC-A1, and scavenger receptor class B member 1, or via receptor independent passive diffusion to nascent HDL( Reference He, Zhang and Li 47 – Reference Gillotte, Davidson and Lund-Katz 49 ). The incorporated cholesterol is then esterified by lecithin-cholesterol acyltransferase( Reference Sorci-Thomas, Babiak and Rudel 50 ). Cholesterol enters the liver either directly, via the receptor scavenger receptor class B member 1, or via the enzyme cholesteryl ester transfer protein (CETP). CETP mediates the 1:1 exchange of cholesterol from HDL with TAG from VLDL and LDL( Reference Zhang, Charles and Tong 51 ). Once in the liver, cholesterol can be removed from the body.
Cholesterol excretion
Cholesterol can be removed from the body by two mechanisms: directly via the hepatic ABCG5/G8 receptor and effluxed to the gall bladder or alternatively, cholesterol can be converted to bile acids for faecal excretion( Reference Brown and Goldstein 52 , Reference Repa, Berge and Pomajzl 53 ). Approximately 98 % of bile acids are conjugated to either taurine or glycine, as conjugation increases polarity, which reduces passive transport from the intestinal lumen into enterocytes, and allows the movement of bile acids to be tightly regulated, and under receptor control; in addition to improving solubility( Reference Aldini, Montagnani and Roda 54 ). Removal of the amino acid from conjugated bile acids, by bacterial bile salt hydrolase (BSH), decreases reabsorption efficiency, thus unconjugated bile acids make up 98 % of the 5 % of bile acids that are excreted daily( Reference Batta, Salen and Rapole 55 , Reference Gérard 56 ). This modification is of significant interest, as the production of more readily excreted bile acids, may lead to the increased conversion of cholesterol to bile acids to replace those lost, in turn lowering serum cholesterol( Reference Oner, Aslim and Aydas 57 ).
Cholesterol and healthspan
Intrinsic ageing
The ageing process has been associated with an increase in both total cholesterol (TC) and LDL-cholesterol. For instance, Ericsson et al. reported an increase in TC from 4·8 mm/l in the young (aged 20–39 years), to 5·14 mm/l in the middle aged (aged 40–59 years), and to 5·44 mm/l in old aged (aged 60–80 years) healthy Scandinavian volunteers( Reference Ericsson, Eriksson and Vitols 58 ). Furthermore, LDL-cholesterol increased with age, from 3·37 in the young, to 3·76 in the middle aged and to 4·05 mm/l in the old aged. Additionally, VLDL-cholesterol has been observed to either remain steady or increase with age, while HDL-cholesterol appears to be unaffected by the ageing process( Reference Ericsson, Eriksson and Vitols 58 , Reference Abbott, Garrison and Wilson 59 ). Abbott et al. also found sex influences the lipoprotein profile. For example, females exhibited higher levels of LDL-cholesterol, especially in those using oestrogen hormones, and increased HDL-cholesterol, whereas VLDL-cholesterol was greater in males( Reference Abbott, Garrison and Wilson 59 ). The age-associated dysregulation of cholesterol metabolism, and accumulation of LDL-cholesterol, has been associated with alterations to several key mechanisms, including cholesterol absorption, LDL-cholesterol clearance, bile acid synthesis and subsequent intestinal bacterial modification (Fig. 5)( Reference Morgan, Mooney and Wilkinson 5 ).

Fig. 5. (Colour online) Overview of age-related changes to cholesterol metabolism.
Intestinal cholesterol absorption varies greatly among individuals, with estimates ranging from 20·0 to 80·1 %( Reference Abbott, Garrison and Wilson 59 , Reference Bosner, Lange and Stenson 60 ). Evidence from murine models suggests cholesterol absorption increases significantly with age( Reference Wang 7 ). Rodent studies have demonstrated this is mediated by a significant increase in NPC1L1 in both the duodenum and jejunum, while ABCG5/G8 expression is suppressed( Reference Duan, Wang and Ohashi 61 ). It has been estimated this increase in cholesterol absorption and concurrent reduction in efflux from enterocytes, confers a 19–40 % increase in cholesterol absorption with age( Reference Duan, Wang and Ohashi 61 ). Moreover, it was observed that high levels of oestrogen up-regulated NPC1L1 and ABCG5/G8 mRNA expression. Oestrogen and ageing have been reported to enhance cholesterol absorption via the estrogen receptor-α pathway( Reference Duan, Wang and Ohashi 61 ). It is important to note that these findings have not yet been observed in human individuals( Reference Bosner, Lange and Stenson 60 ).
Bile acid metabolism is also affected by the ageing process, most significantly, there is a reduction in bile acid synthesis. Wang( Reference Wang 7 ) found ageing resulted in a significantly reduced biliary bile acid output, from 192 to 211 to 124–157 µm/h per kg in mice. Additionally, Wang( Reference Wang 7 ) demonstrated intrinsic ageing resulted in a reduction in bile acid synthesis, with a 33·3–57·1 and 41·7–56·3 % decrease in cholesterol 7α-hydroxylase (CYP7A1) activity in male and female mice, respectively, dependent on dietary and genetic factors. Similarly in human subjects, an inverse correlation between age and CYP7A1 expression has been described( Reference Bertolotti, Gabbi and Anzivino 62 ). For instance, cholesterol 7α-hydroxylation rates were reduced by 50 % for individuals over 65 years, compared with individuals below 65 years of age in one Italian cohort( Reference Bertolotti, Abate and Bertolotti 63 ). Bertolotti et al.( Reference Bertolotti, Abate and Bertolotti 63 ) estimated by linear regression analysis a 60 mg/d (about 150 µm/d) decline every 10 years, in cholesterol undergoing cholesterol 7α-hydroxylation, while Einarsson et al.( Reference Einarsson, Nilsell and Leijd 64 ) estimated an 80 mg/d (200 µm/d) reduction over the same time period. Bertolotti et al.( Reference Bertolotti, Gabbi and Anzivino 62 ) propose the age-related reduction in CYP7A1 expression could be related to the concomitant decline in hepatic nuclear factor four and co-activator CYP7A1 promoter-binding factor/liver receptor homologue-1, mediated by the decline in growth hormone and insulin-like growth factor.
Once bile acids reach the small intestine, modification by digestive microflora occurs influencing enterohepatic circulation. Many digestive bacteria produce the enzyme BSH, which deconjugates bile acids, decreasing reabsorption efficiency, and enhancing excretion. For example, Tanaka et al.( Reference Tanaka, Doesburg and Iwasaki 65 ) reported 59 and 98 % of Lactobacillus and Bifidobacteria strains, isolated from faeces are BSH positive. With age there are several changes to the gut microflora, including a decline in the number and species diversity of Lactobacillus and Bifidobacterium ( Reference Hopkins and Macfarlane 8 , Reference Woodmansey, McMurdo and Macfarlane 66 ). Therefore, it is possible that the age-related decline of these bacterial species reduces bile acid deconjugation, and in turn reduces the conversion of cholesterol to bile acid. This may play a role in the accumulation of cholesterol with age( Reference Morgan, Mooney and Wilkinson 5 ).
It has also been reported, the clearance rate of LDL-cholesterol is affected by the ageing process. The apo B-100 containing lipoproteins, LDL-cholesterol and VLDL-cholesterol are removed from the blood via hepatic LDLr, for elimination, either by direct efflux or conversion to bile acids. Millar et al.( Reference Millar, Lichtenstein and Cuchel 6 ) determined the mean LDL apo B-100 residence time was 2·42 d for younger male adults (mean age 31 (sd 6) years) and 3·46 d for older male adults (mean age 61 (sd 10) years). With age, a decline in LDLr activity and/or numbers is thought to be responsible for the reduction in LDL clearance rate and increase in residence time. The reduced conversion of cholesterol by CYP7A1 may play a role in reduction of LDLr. Additionally, proprotein convertase subtilisin/kexin type 9, a proprotein convertase responsible for the degradation of LDLr has been correlated with age( Reference Lagace, Curtis and Garuti 67 ). Interestingly, proprotein convertase subtilisin/kexin type 9 has also been correlated with BMI, TC, LDL-cholesterol and TAG( Reference Cui, Ju and Yang 68 ). Furthermore, Millar et al.( Reference Millar, Lichtenstein and Cuchel 6 ) observed, that although VLDL apo B-100 residence time was not affected by age, the production rate of VLDL apo B-100 was correlated with age. The age-related increase in body fat and elevated plasma free fatty acids were attributed to this increase in VLDL apo B-100 production rate.
Cholesterol metabolism and diet
Herron et al.( Reference Herron, Vega-Lopez and Conde 69 ) examined the effect of about 640 mg/d cholesterol feeding on men aged 18–57 years, and determined that 37·5 % of subjects behaved as hyper-responders, with an increase of ≥0·06 mm/l in TC, while 62·5 % behaved as hypo-responders, with an increase in TC of <0·05 mm/l. Hyper-responders exhibited a significant 23·0, 7·8 and 18·0 % increase in LDL-cholesterol and HDL-cholesterol and LDL:HDL ratio, respectively, whereas changes to LDL-cholesterol, HDL-cholesterol, TG and LDL:HDL ratio were NS for hypo-responders. Interestingly, hyper-responders exhibited elevated lecithin-cholesterol acyltransferase and CETP activity, suggesting the up-regulation of reverse cholesterol transport as a compensatory mechanism, to reduce the risk of atherosclerosis. Quintao et al.( Reference Quintao, Grundy and Ahrens 70 ) propose tissue pools of cholesterol may rapidly expand in response to cholesterol feeding, even in the absence of aberrations to plasma cholesterol levels. Typically, there are two main mechanisms to compensate for an increase in dietary cholesterol; elevated cholesterol excretion and decreased cholesterol synthesis. It has been suggested a reduction in cholesterol intake should be considered unnecessary for individuals who have already reduced SFA intake, and increased PUFA:SFA ratio( Reference Edington, Geekie and Carter 71 ). Edington et al.( Reference Edington, Geekie and Carter 71 ) determined a 2-fold increase or decrease in dietary cholesterol in participants who also reduced dietary fat with an increased PUFA:SFA ratio had no effect on TC or LDL-cholesterol after 8 weeks. This is due to the significant impact SFA had on serum LDL-cholesterol, by influencing several regulatory mechanisms. Firstly, it has been observed there is a reduction in LDLr; resulting in reduced LDL-cholesterol clearance and increased LDL-cholesterol( Reference Mustad, Etherton and Cooper 72 ). Mustad et al.( Reference Mustad, Etherton and Cooper 72 ) demonstrated an 8-week reduction in SFA resulted in a 10·5 % increase in LDLr and subsequent 11·8 % decrease in LDL-cholesterol. It has been estimated for every 1 % increase in LDLr, there is a 0·74 % reduction in LDL-cholesterol. Secondly, SFA may influence cholesterol synthesis( Reference Glatz and Katan 73 ). Glatz and Katan( Reference Glatz and Katan 73 ) determined a low PUFA:SFA ratio diet resulted in increased cholesterol synthesis, compared with a high PUFA:SFA ratio diet (1·86 v. 1·55 mm/d). Additionally, Jones et al.( Reference Jones, Lichtenstein and Schaefer 74 ) demonstrated maize oil increased absolute cholesterol synthesis from 13·9 mg/kg per d at baseline, to 21·3 mg/kg per d. Thirdly, it has been demonstrated SFA influences the concentration of CETP. For example, Jansen et al.( Reference Jansen, López-Miranda and Castro 75 ) observed that CETP concentrations were significantly elevated by 12 and 11 %, in individuals on a high SFA diet, compared with individuals on the National Cholesterol Education Program StepI diet and MUFA diet, respectively. Additionally, an elevation of SFA from 8·4 to 11 % decreased lecithin-cholesterol acyltransferase activity from 56 to 74 nm/ml per h, which may result in decreased reverse cholesterol transport and influence CVD risk( Reference Berard, Dabadie and Palos-Pinto 76 ).
Cholesterol metabolism and disease
The age-related accumulation of both TC and LDL-cholesterol has been associated with the pathogenesis of several diseases( Reference Sjogren and Blennow 77 – Reference Sharrett, Ballantyne and Coady 79 ). In a meta-analysis of sixty-two studies, a 17·5 % reduction in relative risk for all-cause mortality for every 1 mm/l decrease in TC was reported, while each 1 mm/l decrease in LDL-cholesterol was associated with a 15·6 % relative risk reduction for all-cause mortality( Reference Gould, Davies and Alemao 4 ).
CHD has been associated with significantly elevated TC, LDL-cholesterol, TAG, apo B and Lp(a) and reduced HDL-cholesterol and apo A–I in both American males and females( Reference Sharrett, Ballantyne and Coady 79 ). Gould et al.( Reference Gould, Davies and Alemao 4 ) reported a 24·5 and 29·5 % reduction in relative risk for CHD-related mortality and CHD event respectively for every 1 mm/l decline in TC. Additionally, each 1 mm/l decline in LDL-cholesterol was associated with a 28·0 and 26·6 % decline in relative risk for CHD-related mortality and CHD event, respectively. Sharrett et al.( Reference Sharrett, Ballantyne and Coady 79 ) found that each 1 mm/l increase in LDL-cholesterol was associated with a relative risk for CHD of between 1·36 and 1·44 for males and 1·19 and 1·32 for females, while a 0·4 mm/l increase in HDL-cholesterol was associated with a 0·64–0·72 and 0·64–0·76 relative risk for CHD for males and females, respectively. Interestingly, the decreased risk for mortality with reduced TC has been observed to decline with age. For example, a meta-analysis conducted by Lewington et al.( Reference Lewington, Whitlock and Clarke 80 ) concluded a 1 mm/l reduction in TC was associated with an about 50 % reduction in IHD mortality for 40–49 year olds, which decreased to an about 33 % reduction for 50–69 year olds and about 17 % for 70–89 year olds.
Intriguingly, several studies have described an inverse relationship between cholesterol and mortality( Reference Ravnskov, Diamond and Hama 81 ). Lv et al.( Reference Lv, Yin and Chei 82 ) observed a 19 % decrease in relative risk for each 1 mm/l increase in LDL-cholesterol in Chinese ≥80 year olds. Additionally, a 40 % lower risk for mortality was associated with those with abnormally high LDL-cholesterol (≥3·37 mm/l), compared with those with a lower plasma concentration of LDL-cholesterol. Similarly, Takata et al.( Reference Takata, Ansai and Soh 83 ) observed a 0·8 % decrease in mortality for each 1 mg/dl (0·026 mm/l) increase in LDL-cholesterol in Japanese ≥80 year olds. In addition, each 1 mg/dl increase in TC was associated with a 0·9 % reduction in mortality.
Targeting cholesterol metabolism as a therapeutic strategy
There are many strategies utilised in the treatment of hypercholesterolaemia. These can be used singularly or in combination, and include pharmacological intervention, changes to diet and exercise regimens, and dietary supplementation( Reference Guo, Liu and Zhang 9 , Reference Mozaffarian, Micha and Wallace 11 , Reference Cannon, Blazing and Giugliano 84 , Reference Ras, Geleijnse and Trautwein 85 ).
Pharmacological intervention
Lipid-lowering drugs are often utilised in the treatment of CVD (Fig. 6). Statins are often recommended for those diagnosed with CVD, or those with a high risk of developing the disease, due to their tolerability and efficacy( Reference Weng, Yang and Lin 86 ). Statins act by competitively inhibiting HMG CoA reductase, the rate-limiting enzyme of cholesterol synthesis in order to lower serum cholesterol, thus reducing CVD risk( Reference Law, Wald and Rudnicka 87 ). Simivastatin reduced LDL-cholesterol by 33 % after 12 weeks, in patients with a history of myocardial infarction, while atorvastatin exhibited a 49 % reduction( Reference Pedersen, Faergeman and Kastelein 88 ). A meta-analysis of four trials demonstrated standard-dose statin therapy reduced LDL-cholesterol by 22 %, to a mean of 2·59 mm/l (101 mg/dl), while high-dose statin therapy reduced LDL-cholesterol by 42 %, to a mean of 1·92 mm/l (75 mg/dl)( Reference Cannon, Steinberg and Murphy 89 ). The standard-dose therapy was associated with a coronary death or myocardial event rate of 9·4 %, whereas high-dose therapy was associated with an 8·0 % event rate. Significantly, overall statin therapy resulted in a 16 % odds reduction in coronary death or myocardial infarction( Reference Cannon, Steinberg and Murphy 89 ). Ridker et al.( Reference Ridker, Cannon and Morrow 90 ) observed that patients whose statin therapy reduced LDL-cholesterol to <70 mg/dl (<1·81 mm/dl), had a reduced rate of recurrent myocardial infarction or death from coronary events (2·7 events per 100 person-years), than individuals whose statin therapy did not reach this goal (4·0 events per 100 person-years). However, it has also been demonstrated non-statin therapy (diet, bile acid sequesterants and ileal bypass surgery) reduced CHD risk equally to that of statins, with each 1 % reduction in LDL-cholesterol corresponding to a 1 % decrease in CHD risk( Reference Robinson, Smith and Maheshwari 10 ). Statins can also be used in combination with drugs such as Ezetimbibe, which targets NPC1L1 to lower cholesterol absorption. Cannon et al.( Reference Cannon, Blazing and Giugliano 84 ) observed that combination therapy lowered LDL-cholesterol by a further 24 % than statin therapy alone. Combination therapy also significantly reduced myocardial infarction and ischaemic stroke risk.

Fig. 6. (Colour online) UK prescription drugs for CVD. Data from British Heart Foundation( Reference Townsend, Bhatnagar and Wilkins 134 ).
Dietary intervention
A reduced intake of SFA and an increase in MUFA and PUFA may be useful in maintaining a healthy lipid profile, and reducing CHD risk( Reference Mozaffarian, Micha and Wallace 11 ). An 8-week reduction in SFA was observed to reduce TC and LDL-cholesterol by 9·3 and 11·8 %, respectively, in healthy males and females aged between 20 and 65 years( Reference Mustad, Etherton and Cooper 72 ). Additionally, Berry et al.( Reference Berry, Eisenberg and Haratz 91 ) reported a 12-week MUFA or PUFA diet reduced TC by about 10 and 16 %, respectively, while reducing LDL-cholesterol by 14 and 21 %. A meta-analysis of 13 614 participants participating in diets where SFA was replaced with PUFA, found that on average there was a 0·76 mm/l (29 mg/dl) reduction in TC, and that each 1 mm/l reduction in TC was associated with 24 % reduced risk for a CHD event. Moreover, each additional year of diet was related to a further 9·2 % risk reduction( Reference Mozaffarian, Micha and Wallace 11 ).
Additionally, the use of phytosterols is recommended as a therapeutic strategy for the treatment of hypercholesterolaemia. It is estimated a 2 g/d dose of phytosterols lowers LDL-cholesterol by 10 %( Reference Katan, Grundy and Jones 92 ). A meta-analysis of 124 studies concluded 0·6–3·3 g/d phytosterols reduced LDL-cholesterol by 6–12 %, with stanols and sterols showing similar LDL-cholesterol lowering efficacies( Reference Ras, Geleijnse and Trautwein 85 ). The effect of phytosterols diminishes above about 2–3 g/d( Reference Katan, Grundy and Jones 92 ), consequently 2 g/d is generally accepted as the daily amount required to help treat hypercholesterolaemia( 93 ).
Furthermore, meta-analysis of sixty-seven trials revealed that soluble fibre could lower TC and LDL-cholesterol by 0·045 and 0·057 mm/l per g soluble fibre, respectively( Reference Brown, Rosner and Willett 94 ). There are a variety of mechanisms that dietary fibre could influence; for example, bile acid micelle formulation, fat excretion, intestinal motility, SCFA production and absorption of macronutrients( Reference Weickert and Pfeiffer 95 – Reference Slavin 97 ). Interestingly, diet may not only impact cholesterol metabolism directly, and may also interact with the gut microbiome( Reference Gibson, Beatty and Wang 98 ). For example, David et al.( Reference David, Maurice and Carmody 99 ) describe that short-term changes to diet could significantly influence both the microbial community structure and bacterial gene expression. For instance, a 5 d animal-based-diet significantly altered the abundance of twenty-two clusters, elevated faecal bile acid concentration, and increased BSH expression( Reference David, Maurice and Carmody 99 ). Everard et al.( Reference Everard, Lazarevic and Gaia 100 ) describe that high-fat feeding significantly affected twenty genera. This may have been influenced by a decline in the antimicrobial peptide regenerating islet-derived protein three gamma. Interestingly, prebiotic treatment increased regenerating islet-derived protein three gamma about 6-fold within the colon. Targeting the digestive microbiota with pre- and probiotics is a potential avenue for the treatment of diseases including hypercholesterolaemia.
Targeting the gut microbiome
There is emerging evidence indicating the important role for the gut microbiome in regulating many biological systems, including cholesterol metabolism( Reference Cénit, Matzaraki and Tigchelaar 101 , Reference Kinross, Darzi and Nicholson 102 ). Ageing is associated with the alteration of the gut microbiome. These changes differ between individuals and populations, as diet, lifestyle, host health and antibiotic use likely play a role in composition( Reference Claesson, Jeffery and Conde 103 , Reference O'Sullivan, Coakley and Lakshminarayanan 104 ). However, it has been observed Lactobacillus and Bifidobacterium species diversity and total count decline with age( Reference Hopkins and Macfarlane 8 , Reference Woodmansey, McMurdo and Macfarlane 66 ). Supplementation with probiotics may have the ability to partially ameliorate changes associated with ageing, such as immunosenescence, neurodegeneration, carcinogenic transformation and hypercholesterolaemia( Reference Patel, Singh and Panaich 105 , Reference Moroti, Souza Magri and de Rezende Costa 106 ).
With regard to hypercholesterolaemia treatment, supplementation with probiotics has been associated with a decline in TC and LDL-cholesterol( Reference Guo, Liu and Zhang 9 ). A meta-analysis of probiotic administration studies concluded probiotic administration resulted in a mean 6·40 mg/dl (0·17 mm/l) reduction in TC and 4·90 mg/dl (0·13 mm/l) reduction in LDL-cholesterol. Additionally, probiotics were observed to have beneficial effects on TC and LDL-cholesterol for individuals with high, borderline high and normal cholesterol levels( Reference Guo, Liu and Zhang 9 ). Probiotics have also been an effective treatment strategy for individuals with underlying conditions. For example, Bernini et al.( Reference Bernini, Simão and Alfieri 107 ) observed ingestion of 80 ml probiotic milk containing 3·4 × 108 colony-forming units/ml Bifidobacterium lactis HN019 for 45 d, significantly lowered TC from 209 to 194 mg/dl (5·40 and 5·02 mm/l), and LDL from 128·5 to 111 mg/dl (3·32–2·87 mm/l) in patients with metabolic syndrome, in addition to lowering BMI. Moreover, the proinflammatory cytokines TNF-α and IL-6 were significantly reduced with probiotic treatment.
There are several proposed mechanisms to explain the lipid-lowering ability of probiotics( Reference Ishimwe, Daliri and Lee 108 ). For example, some probiotics produce the bile acid deconjugating enzyme BSH, which may increase bile acid excretion, and up-regulate the conversion of cholesterol to bile acids to replace those lost( Reference Tsai, Lin and Hsieh 109 ). It has also been observed that probiotics increase SCFA, which inhibit the hepatic rate-limiting enzyme in cholesterol synthesis, HMG CoA reductase( Reference Ishimwe, Daliri and Lee 108 ). Furthermore, there may be a reduction in cholesterol absorption due to the assimilation and possible incorporation of cholesterol into the bacterial cell membrane( Reference Brashears, Gilliland and Buck 110 ). Additionally, some bacteria may act by destabilising cholesterol micelles resulting in the coprecipitation of cholesterol with deconjugated bile acids( Reference Brashears, Gilliland and Buck 110 ).
Applying systems biology to our understanding of cholesterol metabolism and ageing
It is apparent from our discussion of cholesterol metabolism, that it is an exceptionally complex system with a multitude of interacting mechanisms( Reference Mc Auley and Mooney 111 , Reference Mooney and Mc Auley 112 ). Many of these mechanisms interact via positive and negative feedback regulators, the dynamics of which is not trivial. Our understanding of this complexity is further compounded by ageing which alters the behaviour of these regulatory mechanisms, thus modifying the overall dynamics of whole-body cholesterol metabolism. Moreover, changes to extrinsic factors such as diet exert a significant influence on the overall behaviour of this system. Thus, it is imperative if we are to gain a more in-depth appreciation of cholesterol metabolism and its interaction with ageing, that we investigate this system in a more integrated manner. The systems biology paradigm contrasts with the more traditional reductionist approach, commonly used in nutrition research, and offers a more integrated way to study this multifaceted system( Reference Mc Auley, Proctor and Corfe 14 , Reference Mc Auley, Choi and Mooney 113 , Reference Mooney, Morgan and Mc Auley 114 ). A fundamental element of this paradigm shift is the close coupling of computational modelling with experimental work( Reference Enrique Salcedo-Sora and Mc Auley 115 – Reference Mc Auley, Mooney and Angell 118 ). Our group has used computational systems biology to investigate cholesterol metabolism and ageing( Reference Mc Auley and Mooney 119 ). Firstly, we constructed a whole-body model of cholesterol metabolism( Reference Mc Auley, Jones and Wilkinson 120 , Reference Mc Auley, Wilkinson and Jones 121 ). The model is defined by the key components that characterise whole-body cholesterol balance, namely cholesterol ingestion, excretion and synthesis together with LDLr turnover and reverse cholesterol transport. Using in silico experiments we explored the impact of ageing on these fundamental elements of cholesterol metabolism. We investigated the influence of ageing on cholesterol absorption and it was found that for every 10 % increment in the rate of cholesterol absorption, there was a concomitant increase of 12·5 mg/dl in LDL-cholesterol. The model also revealed that increasing cholesterol absorption from 50 to 80 % by age 65 years, resulted in a 34 mg/dl increase in plasma LDL-cholesterol. In addition, the model found that reducing the number of hepatic LDLr had a significant impact on the system. An increase of 116 mg/dl in LDL-cholesterol was observed by 65 years in response to a reduction of 50 % in their numbers; thus emphasising LDLr maintenance as a key component in maintaining cholesterol metabolism during ageing. This model is coded in the Systems Biology Markup Language and archived in the BioModels Database, a repository for models encased in Systems Biology Markup Language exchange framework (http://www.ebi.ac.uk/biomodels-main/BIOMD0000000434). This means the model is straightforward to update and adapt, a feature exploited by other groups working in this area( Reference Mishra, Somvanshi and Venkatesh 122 ). We recently implemented significant updates to the model described earlier for the following reasons( Reference Morgan, Mooney and Wilkinson 123 ). The model was lacking several key mechanisms which recent experimental evidence has stressed are central to the regulation of cholesterol metabolism. Briefly, these processes included plasma membrane receptors, and in vivo and intestinal microfloral enzymes. Therefore, it was deemed cogent that these ninety-six additional mechanisms were incorporated into the original model( Reference Morgan, Mooney and Wilkinson 5 ). We found that our updated model behaved as a hypo-responder to excessive cholesterol feeding. Moreover, the model was utilised to investigate the effects of ageing coupled with three different CETP genotypes. Ageing in the presence of a genotype conferring low CETP activity resulted in a 0·6 % decrease in LDL-cholesterol after 1000 h. In comparison, ageing with a genotype indicative of high CETP activity, provoked a 1·6 % increase in LDL-cholesterol levels (Fig. 7). Thus, our new model consolidates experimental findings, which emphasise the significance of CETP genotypes in healthspan( Reference Morgan, Mooney and Wilkinson 123 ).

Fig. 7. Model simulation of ageing in the presence of cholesteryl ester transfer protein genotypes. Taken from Morgan et al.( Reference Morgan, Mooney and Wilkinson 123 ).
Complementing computational models with simulated digestive tracts
To complement our in silico studies of cholesterol metabolism, we aim to develop a simulator of the human digestive tract. The digestive track simulator will be used to refine and inform our computational model. One example is the simulator of the human intestinal microbial ecosystem reactor, which represents the length of the human digestive system, with several closed reactors used to represent the differing conditions (microbial ecosystem, pH, enzymes, etc.) of each section of the digestive tract( Reference Van de Wiele, Van den Abbeele, Ossieur, Verhoeckx, Cotter and Lopez-Exposito 124 ). The flexible nature of the simulator of the human intestinal microbial ecosystem reactor means that it can be easily adapted. This may include additional reactors to more accurately represent the human digestive tract( Reference Nollet, Pereira and Verstraete 125 , Reference Molly, Vande Woestyne and Verstraete 126 ). Simulated digestive tracts, such as the simulator of the human intestinal microbial ecosystem reactor provide a suitable test environment to supplement experimental data on potential therapeutic strategies. For example, De Smet et al.( Reference De Smet, De Boever and Verstraete 127 ) determined administration of Lactobacillus to pigs for 4 weeks decreased TC and LDL-cholesterol by 15 and 24 %, respectively. Additionally, TC and LDL-cholesterol reduced a further 18 and 34 % 3 weeks after treatment. Follow-up experimentation using the simulator of the human intestinal microbial ecosystem reactor was able to provide details of the intestinal bacterial population and overall metabolic activity associated with this treatment( Reference Nollet, Pereira and Verstraete 125 ). Recently, a continuous gut adhesion model was developed using mucin-alginate beads to immobilise bacteria, which may more effectively represent the gut lining and intestinal lumen. This system was also used to examine bacterial colonisation following probiotic treatment( Reference Rodes, Coussa-Charley and Marinescu 128 ). The TNO gastrointestinal model can be used to examine compound absorption( Reference Minekus, Verhoeckx, Cotter, López-Expósito, Kleiveland, Lea, Mackie, Requena, Swiatecka and Wichers 129 ). This model simulates ingestion of food and water, digestive enzyme and bile salt release, peristalsis, body temperature, and transit through the stomach and small intestine (TNO gastrointestinal model-1) and large intestine (TNO gastrointestinal model-2) under computer control. The model can be altered to represent a different species, such as a human or pig, or can represent varying age, such as infant, adult and older people( Reference Minekus, Verhoeckx, Cotter, López-Expósito, Kleiveland, Lea, Mackie, Requena, Swiatecka and Wichers 129 ). This is particularly significant for the augmentation and refinement of our computational model. It is hoped that utilising both these approaches in tandem will help to significantly deepen our understanding of cholesterol metabolism and its relationship with ageing.
Conclusions
We have outlined how the age-related dysregulation of cholesterol metabolism is influenced by many factors. Specifically, (i) an increase in LDL-cholesterol residence time due to reduction in LDLr and concomitant decline in LDL-cholesterol clearance;, (ii) a decline in CYP7AI activity which reduces bile acid synthesis, one pathway for cholesterol removal; (iii) a reduction in digestive bacteria which modify bile acids that reduce reabsorption and promotes excretion; (iv) a potential increase in cholesterol absorption due to an increase in NPC1L1 and decrease in ABCG5/G8, as observed in murine models. Cholesterol metabolism is also influenced by diet and gut microbial dynamics, therefore studying the mechanisms underpinning these complex interactions is challenging. Due to its ability to handle this complexity computational modelling has been used to study the intricacies associated with ageing and cholesterol metabolism. Computational modelling is a key component of the systems biology paradigm which nutrition research is beginning to adopt. It is possible nutrition research will benefit significantly from this approach in the coming years. For instance, computational modelling will be needed to deepen our understanding of the role the gut microbiome has to play in host health( Reference Christley, Cockrell and An 130 ). In tandem with computational modelling, experimental approaches are a key part of systems biology. An important experimental method which dovetails with computational modelling is the use of artificial digestive tracts. These can be used to simulate the activities of the digestive tract under a wide variety of conditions, something which is difficult to achieve in vivo ( Reference de Wiele, Boon and Possemiers 131 ). Thus, utilising these methodologies as part of a systems biology framework, provides a means of investigating the dynamics of the gut microbiome together with its interaction with cholesterol metabolism and ageing. In order for this approach to be successful it is imperative for nutritionists to work closely with scientists from the systems biology community. This novel way of conducting nutrition research will in turn facilitate the discovery of nutrient-based strategies to treat hypercholesterolaemia, which so often accompanies the ageing process.
Acknowledgement
A. E. M. was funded by a University of Chester PhD scholarship.
Financial Support
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of Interest
None.
Authorship
A. E. M. and M. T. M. A. drafted the manuscript and conceived the idea. K. M. M. provided advice on the nutrition focused sections of the manuscript. N. A. P. provided advice on the microbiology components.