Nutrition in chronic pancreatitis has been described as a problem area( Reference Lankisch 1 ). Progressive exocrine and endocrine impairment occur, presenting an escalating challenge for nutritional management. While there has been much research into nutrition in acute pancreatitis in recent years, traditionally there was less interest in chronic pancreatitis. Therefore, there was minimal mention of the nutritional management of chronic pancreatitis in international practice guidelines. Faced with the clinical dilemma of patients with chronic pancreatitis with considerable nutrient deficiencies and undernutrition, and without the benefit of guidelines to aid practice, we set about investigating various aspects of nutrition in chronic pancreatitis, with a strong emphasis on clinical management. Specifically, we were interested in exocrine and endocrine deficiency, nutrient deficiency, osteoporosis, bone metabolism, malabsorption and in the epidemiology of chronic pancreatitis. Simultaneously, in the past 5–10 years, there has been a considerable increase in nutrition-related publications in chronic pancreatitis internationally, including from research groups in New Zealand, the USA, the Netherlands, Germany, Spain, Denmark and others. This has been reflected in the rising number of recently published consensus guidelines on key nutrition-related topics in chronic pancreatitis over the past 5 years( Reference Frulloni, Falconi and Gabbrielli 2 – Reference Hoffmeister, Mayerle and Beglinger 6 ). This review aims to describe the specific nutrition-related problems in chronic pancreatitis, to summarise recent studies, as well as to discuss recent guidelines and hot topics.
Chronic pancreatitis is defined as a chronic inflammatory disease of the pancreas, characterised by irreversible morphological changes and typically causing pain and/or permanent loss of function. In chronic pancreatitis, there is a progressive atrophy of pancreatic tissue, with normal tissue being replaced by fibrous tissue, followed by gland enlargement. The main duct of the pancreas becomes dilated and strictured, and ultimately, the pancreas shrinks and there may be significant calcification throughout the gland. The dominant aetiology for chronic pancreatitis in western countries is excess alcohol consumption( Reference Spanier, Dijkgraaf and Bruno 7 ). Other causes include pancreatic duct obstruction, pancreas divisum, cystic fibrosis, hypercalcaemia, autoimmunity, genetic mutations and hypertryglyceridaemia. About a fifth of cases are deemed to be idiopathic( Reference Spanier, Dijkgraaf and Bruno 7 ). Tropical pancreatitis describes the type of chronic pancreatitis that arises in tropical/subtropical countries, associated with relatively young, undernourished patients, with early development of diabetes( Reference Midha, Singh and Sachdev 8 ). Heavy smoking increases the risk of developing chronic pancreatitis( Reference Imoto and DiMagno 9 – Reference Pitchumoni 11 ).
Epidemiology
Prevalence data are scarce for chronic pancreatitis worldwide. We conducted the first nationwide epidemiological study of chronic pancreatitis in Ireland( Reference Ní Chonchubhair, Bashir and McNaughton 12 ). In a retrospective study of inpatient discharges from acute hospitals, International Classification of Diseases-10 codes for ‘alcohol-related’ aetiology and ‘other’ aetiology were searched between 2009 and 2013. Chronic pancreatitis prevalence in Ireland was 11·6–13 per 100 000, which was similar to international studies using similar methodological techniques (i.e. exact counts from administrative databases, rather than surveys of centres or health professionals). Consistent with the literature, most patients were middle-aged males (between 40 and 64 years). Surprisingly, alcohol-aetiology discharges were half that of ‘other’ aetiology discharges. There were notable geographical variations with higher activity in the Northwest of Ireland. Comparable variations in geography were reported in a study from the Czech Republic by Díte et al. ( Reference Díte, Starý and Novotný 13 ), which was attributed to regional variations in alcohol consumption. However, there are no per county alcohol consumption data for Ireland that would allow a similar analysis. A comparison between the geographical patterns for chronic pancreatitis activity to that of cystic fibrosis in Ireland showed that while chronic pancreatitis was more prevalent in the Northwest, cystic fibrosis was more prevalent in the South and Southwest. This study provides the first large-scale, nationwide epidemiological data for either Ireland or the UK, and will therefore provide valuable data regarding a disease about which relatively little is known. Data of this nature will allow the relevant bodies and policy-makers to make informed, logical decisions on resource allocation and service planning( Reference Ní Chonchubhair, Bashir and McNaughton 12 ).
Malabsorption
Destruction of the pancreatic acinar cells results in a decrease in the production and secretion of pancreatic enzymes. This leads to malabsorption of macronutrients and micronutrients, severe and distressing gastrointestinal symptoms, and if untreated, nutrient deficiency and undernutrition. Lipase is particularly vulnerable to destruction, and therefore malabsorption of fat is especially problematic and clinically evident. Malabsorption is exacerbated by precipitation of bile acids and impaired gastric emptying. Excretion of abnormal amounts of fat in the faeces (steatorrhoea) occurs, and fat loss of >15 g/d is considered severe. Exocrine impairment (termed pancreatic exocrine insufficiency (PEI)) may be measured directly by intubating the duodenum and measuring lipase output following hormonal stimulation. However, this invasive technique is rarely performed outside of specialist research centres. Therefore, indirect methods of measuring PEI are required, including measurement of faecal fat, measurement of the enzyme faecal elastase-1 or in some cases, evidence of pathological pancreatic function along with clinical signs of malabsorption. Symptoms of PEI include fatty diarrhoea (pale, bulky stools that are difficult to flush), bloating, abdominal cramping, flatulence and abdominal pain with dyspepsia. However, PEI may still exist even in the absence of obvious clinical symptoms. The gold standard treatment of PEI is the administration of exogenous enzymes (pancreatic enzyme replacement therapy, (PERT)), which reduce fat malabsorption thereby helping to achieve normal nutritional status( Reference de la Iglesia-García, Huang and Szatmary 14 – Reference Whitcomb, Lehman and Vasileva 16 ).
It is believed that 90 % of the pancreas must be destroyed before malabsorption occurs, and that fat digestion is not impaired until lipase secretion drops to <10 % of normal. This belief is based on a study published in 1973 reporting on seventeen patients with chronic pancreatitis and thirty-three healthy controls( Reference DiMagno, Go and Summerskill 17 ). Enzyme output was measured in response to duodenal perfusion with essential amino acids and administration of intravenous cholecystokinin and pancreaozymin. Values were presented as a percentage of normal, which was derived from thirty-three healthy controls. However, the interpretation of data and graphs from the original paper has been challenged. Firstly, as expected, the controls had normal lipase secretion and did not have fat malabsorption. Of the patients, sixteen of seventeen had severe PEI (with a lipase output of <10 % of normal), and all patients exhibited fat malabsorption of varying degrees. The authors concluded that fat malabsorption only occurs when lipase secretion falls below 10 %. However, based on these data, this study should only have concluded that those with severe PEI (and therefore severe chronic pancreatitis) exhibit fat malabsorption of >7 g/d. Only one patient had moderately impaired lipase secretion (and that one patient had normal fat excretion); however, there is insufficient evidence that patients with >10 % pancreatic exocrine function have normal fat excretion. Secondly, the range of fat loss among those with clearly minimal lipase secretion (<10 % lipase secretion) was extraordinarily broad (ranging from just over 7 g/d to almost 100 g/d), indicating that the study methodology may be open to debate( Reference Duggan, Ní Chonchubhair and Lawal 18 ). Specifically, the study did not account for the action of gastric lipase, which may have (for some patients) acted to reduce fat malabsorption to near normal levels. A second study from Germany in 1986 described a similarly conducted study with slight larger sample size( Reference Lankisch, Lembcke and Wemken 19 ). Results from that study, while apparently consistent with the 1973 study, showed in fact that some patients with mild/moderate reductions in lipase secretion did exhibit excess fat loss, as well as amylase and trypsin. These two studies remain the only two papers to have investigated this topic, and based on these data, there is insufficient evidence to state that the pancreas must be virtually destroyed before fat malabsorption occurs, as has been the narrative for many years( Reference Duggan, Ní Chonchubhair and Lawal 18 ). In fact clinically, fat malabsorption (and that of carbohydrate and protein) occurs even in mild or moderate chronic pancreatitis. However, when trusted, guidelines continue to state that PEI is not a concern except in the most severe cases, there will be delays in prescribing PERT, with consequences including adverse gastrointestinal symptoms, undernutrition, nutrient deficiency and the effects of deficiency, such as osteoporosis and atraumatic fracture. A study from the Netherlands found that a sizeable number of chronic pancreatitis patients were undertreated, with 70 % still experiencing steatorrhoea-related symptoms, and 42 % reporting weight loss( Reference Sikkens, Cahen and van Eijck 20 ). Clearly PERT, the mainstay of treatment for PEI, is underused and underprescribed. For some patients, PERT alone is insufficient in relieving gastrointestinal symptoms associated with chronic pancreatitis. The dose may be titrated, or acid-suppression medication may be required in some cases to prevent denaturation of both endogenous and administered enzymes( Reference Imrie, Connett and Hall 21 ). This is due to a reduction in the production of bicarbonate by the pancreas in chronic pancreatitis, and therefore a higher than normal acidity in the stomach and duodenum. In some cases, other additive causes of malabsorption should be considered.
Bacterial overgrowth and dysbiosis
Small intestinal bacterial overgrowth (SIBO) is a condition whereby there is increased bacterial load in the small bowel, resulting in excessive fermentation and inflammation, and leading to adverse clinical symptoms. Symptoms include malabsorption, abdominal discomfort, diarrhoea, constipation, flatulence and bloating. SIBO may complicate chronic pancreatitis in up to 92 % of patients according to a recent systematic review by Capurso et al., and its symptoms may be confused with PEI( Reference Capurso, Signoretti and Archibugi 22 ). It occurs more frequently in those with prior surgical history and heavy smokers. In a case-matched study, we found that SIBO occurred in up to 14 % of chronic pancreatitis patients with no surgical history and was more likely to occur in those with PEI, and concurrent diabetes( Reference Ni Chonchubhair, Dobson and Ryan 23 ). In this controlled study (matched for age, sex and smoking status), no healthy controls tested positive for SIBO. Treatment for SIBO may require several courses of antibiotics, and it may recur; therefore, SIBO should be considered in patients with chronic pancreatitis even if they have not had prior surgery, where PERT appears to insufficiently treat gastrointestinal symptoms.
SIBO could be related to microbial imbalance (dysbiosis) in the large bowel; however, the latter may occur even without overgrowth into the small bowel as seen in SIBO. Microbes in the human gut play a vital role in the balance between health and disease. Dysbiosis has been linked to the activation of inflammatory cytokines in several inflammatory-mediated diseases. Associations between dysbiosis and coeliac disease( Reference Verdu, Galipeau and Jabri 24 ), irritable bowel disease( Reference Distrutti, Monaldi and Ricci 25 ) and inflammatory bowel disease( Reference Sheehan, Moran and Shanahan 26 ) have been described; however, there are few studies for chronic pancreatitis, or for pancreatic disease in general. Only a few studies have examined a link between alterations in intestinal microbiota and chronic pancreatitis( Reference Farrell, Zhang and Zhou 27 – Reference Manasa, Harish and Madhulika 31 ). However, in these limited studies, some tentative patterns are apparent. A higher level of Bacteroidetes and a lower level of Faecalibacteria were found in diabetic chronic pancreatitis patients v. non-diabetic patients( Reference Manasa, Harish and Madhulika 31 ). Lower levels of Bifidobacteria were found in diabetic patients with PEI, compared with those with intact exocrine function. One study found lower levels of Bifidobacteria and Lactobacillus in patients with chronic pancreatitis, along with higher levels of Escherichia coli, Enterococcus faecalis and Enterococcus faecium ( Reference Savitskaya, Melnikova and Vorobyev 29 ). Another study also reported decreased levels of Lactobacillus and Bifidobacterium ( Reference Gorovits, Tokareva and Khlynova 30 ). Bifidobacteria and Lactobacilli belong to the lactic acid bacteria group and are considered to be health enhancing, with the former believed to relieve diarrhoea and malabsorption, produce SCFA (including acetate, propionate and butyrate), reduce luminal pH (inhibiting pathogenic micro-organisms) and increase the absorption of some nutrients. Therefore, based on a limited number of studies, decreased levels of Bifidobacteria in chronic pancreatitis may be clinically relevant. However, it is not known if this decrease in health-enhancing gut bacteria is reactive or causal. Nevertheless, these findings may potentially present avenues for therapeutic interventions (such as probiotics, targeted antibiotics, dietary modification and possibly faecal microbial transplantation); however, this topic has been essentially unexplored to date.
Nutrient deficiency
The prevalence of biochemical vitamin deficiency in chronic pancreatitis differs widely between studies and between countries, and individual studies vary greatly regarding quality. Vitamin A deficiency in chronic pancreatitis was reportedly as low as 3 % (the Netherlands)( Reference Sikkens, Cahen and Koch 32 ) and as high as 40 % (Japan)( Reference Nakamura, Takebe and Imamura 33 ). For vitamin E deficiency, which should be measured as a ratio of blood lipid, prevalence varied from 25 % (Ireland)( Reference Duggan, Smyth and O'Sullivan 34 ) to about 75 % (South Africa) of patients with chronic pancreatitis( Reference Marotta, Labadarios and Frazer 35 ). There is some evidence that vitamin E deficiency is more prevalent in chronic pancreatitis patients with steatorrhoea. Regarding vitamin K, no study has measured this vitamin using the recommended methods; either undercarboxylated osteocalcin or proteins of vitamin K absence( Reference Jaganath, Fedorowicz and Thoher 36 ). Measurement of serum vitamin K or prothrombin time is inaccurate; therefore, the occurrence of vitamin K deficiency in chronic pancreatitis is unknown. Of the fat-soluble vitamins, serum 25-hydroxyvitamin D (25OHD) deficiency has been the most studied; however, prevalence varies greatly, largely depending on the definitions of deficiency used. When defined as <50 nmol/l, 25OHD deficiency in chronic pancreatitis varies between 41 and 86 %( Reference Dujsikova, Dite and Tomandl 37 ). Deficiencies of zinc( Reference Girish, Rajesh and Vaidyanathan 38 ), vitamin B12 ( Reference Glasbrenner, Malfertheiner and Büchler 39 ) and magnesium( Reference Papazachariou, Martinez-Isla and Efthimiou 40 ) have rarely been reported for chronic pancreatitis.
It should be noted that while biochemical deficiencies, particularly of the fat-soluble vitamins, have been reported, studies describing overt, clinically relevant consequences of deficiency are less common and generally confined to case reports or case series. Serious neurological manifestations of vitamin E deficiency in chronic pancreatitis were described in one case series( Reference Yokota, Tsuchiya and Furukawa 41 ), while a condition known as brown bowel syndrome was reported in a further case report. Brown bowel syndrome is a rare disorder characterised by a brown pigmentation of the bowel wall( Reference Reynaert, Debeuckelaere and De Waele 42 ). This syndrome occurs in malabsorption syndromes and is specifically associated with vitamin E deficiency. While the exact pathway is not known, it is thought that vitamin E protects the mitochondrial membrane from damage by free radicals. In vitamin E deficiency, the free radicals are not oxidised by vitamin E, but by the phospholipids of the mitochondrial membrane, which degradate. The result is formation of lipofuscin in the tunica muscularis causing a brown colour( Reference Reynaert, Debeuckelaere and De Waele 42 ). Severe visual defects, including ulcerative keratitis and necrotising stromal ulceration with hyphema, associated with vitamin A deficiency have been described in two chronic pancreatitis case reports( Reference Benítez Cruz, Gómez Candela and Ruiz Martín 43 , Reference Ruiz-Martín, Boto-de-los-Bueis and Romero-Martín 44 ). In the case of vitamin D deficiency, there have been several case reports of osteomalacia in patients with chronic pancreatitis( Reference Kurtulmus, Yarman and Tanakol 45 , Reference Kaur, Gupta and Minocha 46 ). In all reports, the clinical manifestations of deficiency appeared to take years to develop, and tended to occur in patients who had other contributing conditions, such as coeliac disease, surgical history, or in patients who were severe alcoholics, or had restrictive diets, such as veganism. In all cases, deficiencies were resolved following treatment including targeted supplementation of nutrients, implementation of adequate PERT and improvement of oral diet. Therefore, the clinical relevance of biochemical or subclinical deficiencies (without overt symptoms of deficiencies) is unclear. Nevertheless, where measured levels are lower normal reference range, it seems prudent to replace the relevant vitamin to prevent overt deficiencies from developing. However, there are no intervention studies to inform the replacement of vitamins A, E or K in chronic pancreatitis, and therefore a case-by-case approach is warranted. For the treatment of 25OHD deficiency, both oral and intramuscular supplementation appears to be effective. In a study by Bang et al., oral supplementation of 38 μg (1520 IU)/d compared with ultraviolet radiation effectively increased serum 25OHD by 32 nmol/l (12·8 ng/ml) in patients with chronic pancreatitis over a 10-week period( Reference Bang, Matzen and Benfield 47 ). Another study by Reddy et al. demonstrated that a once-off, high-dose intramuscular injection of 15 000 μg (600 000 IU) effectively increased serum 25OHD in patients with chronic pancreatitis with no adverse events, such as hypercalcaemia( Reference Reddy, Ramesh and Bhatia 48 ). For all patients with chronic pancreatitis, attention should be given to optimisation of diet and ensuring appropriate and adequate PERT( Reference Duggan, O'Sullivan and Feehan 49 , Reference Duggan and Conlon 50 ).
Chronic systemic inflammation
Irrespective of the aetiology of chronic pancreatitis, the end result is the same. Inflammation destroys pancreatic tissue leading to pancreatic fibrosis, and ultimately, impairment of pancreatic function( Reference Rasch, Valantiene and Mickevicius 51 ). Chronic pancreatitis is also associated with accelerated biological ageing, with premature death (patients typically die 8 years earlier than age- and sex-matched controls). Death is due to a higher occurrence of diseases such as diabetes, cerebrovascular disease, pulmonary disease, ulcer disease and renal disease. Rasch et al. described how the phenomenon ‘inflamaging’ applies to chronic pancreatitis( Reference Rasch, Valantiene and Mickevicius 51 ). Inflamaging is characterised by elevated proinflammatory cytokines (IL6, IL4, TNF, T-cell factor-β, IL8) and lower anti-inflammatory cytokines (including IL10). Systemic inflammation in chronic pancreatitis (exacerbated by a chronically poor diet, smoking and malabsorption) results in an aged phenotype, specifically osteoporosis and sarcopenia( Reference Rasch, Valantiene and Mickevicius 51 ). To date, there has been little research on sarcopenia in chronic pancreatitis.
Body composition and sarcopenia
The BMI of chronic pancreatitis patients varies by country from (mean) 19 kg/m2 in India( Reference Regunath, Shivakumar and Kurien 52 ) to 25·9/25·5 kg/m2 (male/female) in Ireland( Reference Duggan, Smyth and O'Sullivan 34 ). The differences may be explained by regional or aetiological variations (e.g. idiopathic or ‘tropical’ chronic pancreatitis in Asia v. alcohol-induced pancreatitis in Europe/USA), or by temporal changes in BMI generally, with more recent studies reporting a higher BMI( Reference Duggan, Smyth and O'Sullivan 34 ). Several studies have reported that muscle and fat stores (measured by crude anthropometric means such as mid arm muscle circumference and triceps skinfold) were lower in chronic pancreatitis than in healthy controls, including a study from India( Reference Regunath, Shivakumar and Kurien 52 ), one from Italy( Reference Vaona, Armellini and Bovo 53 ) and our study from Ireland( Reference Duggan, Smyth and O'Sullivan 34 ). In the latter study, handgrip strength was also lower in male patients with chronic pancreatitis than in age-matched male controls, as well as being lower in patients who were weight losing (v. those who were weight stable or weight gaining in the past year) indicating that measurements of handgrip strength may be a useful indicator of deteriorating nutritional status( Reference Duggan, Smyth and O'Sullivan 34 ). Only two studies to date have investigated sarcopenia using computerised tomography scans read by sophisticated specialised software. Computerised tomography is the gold standard imaging method for the measurement of body composition at tissue–organ level. Using methods devised for oncology patients, regional analysis of the L3 lumbar vertebrae has shown a high correlation with whole body muscle volume. In a study of twenty-nine patients with chronic pancreatitis, we found that over half (56·7 %) were sarcopenic, and vitally more than half of sarcopenia patients were either overweight or obese( Reference O'Connor, Kok and Christina 54 ). Sarcopenic obesity is a phenomenon where excess body weight and reduced muscle mass or strength occur simultaneously. A second study by Shintakuya et al. aimed to determine if various nutritional parameters were associated with PEI in several pancreatic diseases, including chronic pancreatitis( Reference Shintakuya, Uemura and Murakami 55 ). They found that L3 muscle mass was the only nutrition-related factor associated with PEI using multivariable analysis. Therefore, these limited studies suggest that sarcopenia and sarcopenic obesity are relevant conditions in chronic pancreatitis and may be associated with exocrine dysfunction. How sarcopenia impacts outcome is unknown, but intuitively one would expect it to be predictive of increased morbidity and possibly mortality. By extension, whether or not sarcopenia in chronic pancreatitis may be reversed by nutritional, physical or pharmacological means is a compelling question. Clearly, the lack of studies on this topic represents a significant research gap.
Bone health and osteoporosis
Recent studies have shown that osteoporosis is a major concern in chronic pancreatitis. Bone demineralisation is caused by poor dietary intake, malabsorption, 25OHD deficiency, low physical activity, heavy smoking and is likely to be driven by chronic systemic inflammation. The first study that reported osteoporosis in this disease was an uncontrolled study published in 1997, reporting on just fourteen patients( Reference Morán, Sosa and Martinez 56 ). Since then, there have been many studies (most in the past few years) showing that osteoporosis and osteopenia occur frequently and prematurely in chronic pancreatitis( Reference Sikkens, Cahen and Koch 32 , Reference Dujsikova, Dite and Tomandl 37 , Reference Duggan, O'Sullivan and Hamilton 57 – Reference Joshi, Reddy and Bhatia 60 ). We performed a systematic review and meta-analysis of ten studies and reported that in a pooled sample of 513 patients, a quarter had osteoporosis and 65 % had either osteoporosis or osteopenia( Reference Duggan, Smyth and Murphy 61 ). However, only two studies from the meta-analysis provided usable control data, with an osteoporosis prevalence among healthy controls of 8·6–10·2 %( Reference Duggan, O'Sullivan and Hamilton 57 , Reference Joshi, Reddy and Bhatia 60 ). This review showed that there was some association with PEI with some (but not all) studies reporting an association between low bone mineral density and fat malabsorption. There was no apparent relationship between osteoporosis and serum 25OHD in chronic pancreatitis, likely to be due to the variable definitions for vitamin D deficiency, as well as seasonal differences in measurement. Since then, several studies have shown that low bone mineral density translates into a real risk of atraumatic fracture. In a population-based study from Denmark, there was a higher fracture rate among chronic pancreatitis than controls, with an adjusted hazard ratio of 1·7 (95 % CI 1·6, 1·8)( Reference Bang, Benfield and Bendtsen 62 ). A US study similarly found that the risk of fracture in chronic pancreatitis (4·8 %) was similar, or higher, than in comparable gastrointestinal disorders such as coeliac disease (5 %), Crohn's disease (3 %), post-gastrectomy (5·4 %) and cirrhosis (4·8 %)( Reference Tignor, Wu and Whitlock 63 ). The rate for healthy controls was 1·1 %. Notably patients with chronic pancreatitis do not have the additional risk factors of long-term steroid use or hypogonadism that drive bone demineralisation in other gastrointestinal conditions; therefore, the comparably high rates of osteoporosis and fracture in this disease are unexpected.
Bone metabolism
Bone metabolism is a complex process, summarised as follows. Bone remodelling occurs throughout life in a coordinated interaction between osteoclasts and osteoblasts to remove bone and replace it with a new matrix. Osteoclasts resorb bone by releasing acid and enzymes, while osteoblasts lay down organic ostoid, which then mineralises. Osteoblasts produce osteoprotegerin, which inhibits osteoclastogenesis (and therefore has a bone protection role). Fig. 1(a) illustrates bone metabolism under normal physiological conditions. In this process receptor activator of nuclear factor-κB (RANK) is the pivotal cytokine receptor for osteoclastogenisis. RANK ligand is produced by osteoblasts( Reference Ghishan and Kiela 64 ), and interacts with its receptor RANK on osteoclast precursor cells and mature osteoclasts. The ligation of RANK with RANK ligand results in the commitment of macrophage precursor cells to the osteoclast lineage, and the activation of mature osteoclasts leading to increased bone resorption( Reference Ghishan and Kiela 64 ). Importantly, RANK ligand signalling is accelerated by proinflammatory cytokines IL-1, IL-6 and TNF-α, which potently induce RANK ligand production by osteoblasts, and directly contribute to the bone destruction process.
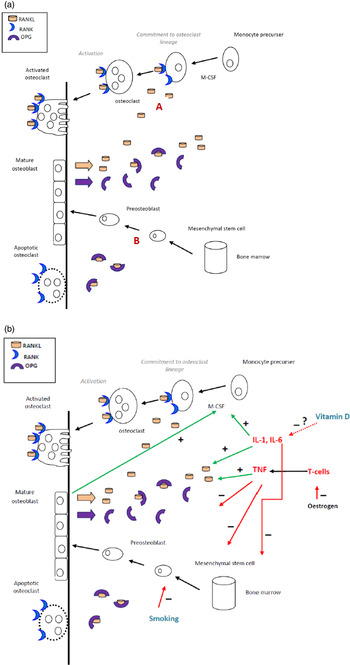
Fig. 1. (Colour online) The suggested role of proinflammatory cytokines, smoking and vitamin D (deficiency) in promoting bone resorption in chronic pancreatitis. (a) Schematic of bone metabolism under normal physiological conditions. Receptor activator of nuclear factor-κB (RANK) ligand produced by osteoblasts, stromal cells or T cells are required for the differentiation of osteoclasts. The binding of RANK to RANK ligand commits monocyte precurser cells to the osteoblastic lineage. Osteoblasts regulate osteoclastic development by secreting osteoprotegerin (OPG), a decoy receptor that competes with RANK for RANK ligand. (b) Proinflammatory cytokines, along with TNF, enhance the production of RANK, suppress the production of OPG, and suppress osteoblastic differentiation. A vitamin D deficient state results in elevated inflammation. Smoking negatively affects osteoblastic development and reduces oestrogen.
In chronic pancreatitis, the chronic inflammatory state, characterised by high levels of proinflammatory cytokines is likely to lead to increased bone turnover (Fig. 1(b)). We conducted the first study investigating bone turnover by measuring novel bone metabolism markers, specifically osteocalcin, aminoterminal propeptide of type-1 collagen and carboxy-terminal telopeptide of type 1 collagen( Reference Duggan, Purcell and Kilbane 65 ). We found an association between abnormal bone turnover and chronic systemic inflammation in chronic pancreatitis. Moreover, we demonstrated an apparent direct association between bone mineral density and sensitive serum inflammatory markers, high-sensitivity C reactive protein and IL-6. We identified an association between serum 25OHD deficiency and lower bone mineral density. These factors may be related, as vitamin D is known to have anti-inflammatory properties( Reference Gatti, Idolazzi and Fassio 66 ), including evidence of an immunomodulatory effect in inflammatory bowel disease( Reference O'Sullivan 67 ). Furthermore, we found that serum 25OHD deficiency was associated with heavy smoking, which itself poses an independent threat to bone integrity( Reference Duggan, Purcell and Kilbane 65 ). Table 1 summarises the putative risk factors for osteoporosis in chronic pancreatitis.
Table 1. Modifiable and non-modifiable risk factors for osteoporosis and osteopenia in chronic pancreatitis

25OHD, 25 hydroxyvitamin D.
*Nutrition related factors.
Bone health guidelines
Given the considerable risk of developing osteoporosis, and the economic and quality of life-associated burden associated with fracture, bone-related guidelines for this group are warranted. However, until recently, there were few guidelines on this topic. The HaPanEU chronic pancreatitis guidelines( Reference Löhr, Dominguez-Munoz and Rosendahl 68 ) published this year have specified that bone density testing by dual X-ray absoptiometry should be extended to chronic pancreatitis patients who are post-menopausal women, those with previous low trauma fractures, men over 50 years and those with malabsorption( Reference Löhr, Dominguez-Munoz and Rosendahl 68 , Reference Duggan and Conlon 69 ). However arguably, given the high risk, baseline bone density assessment should be performed in all patients with chronic pancreatitis, as well as ensuring that basic preventative measures are implemented as part of routine clinical practice. Basic preventative measures include adequate diet, particularly calcium and vitamin D intake, ensuring regular weight-bearing exercise and smoking/alcohol cessation. Following a diagnosis of osteopenia, as well as implementing basic preventative measures, dual X-ray absoptiometry should be repeated every 2 years. Patients with confirmed osteoporosis (or those who have vertebral fractures) should commence suitable medication, be screened for other causes and/or be referred to a bone specialist( Reference Löhr, Dominguez-Munoz and Rosendahl 68 , Reference Duggan and Conlon 69 ).
Endocrine insufficiency
The subgroup of diabetes mellitus that occurs in conjunction with diseases of the exocrine pancreas is termed type 3c diabetes (or pancreatogenic diabetes)( 70 , 71 ). Type 3c diabetes tends to be misclassified, usually as type 2 diabetes; however, there are important clinical and metabolic factors, which distinguish type 3c diabetes from other diabetes types( Reference Ewald, Kaufmann and Raspe 72 – Reference Duggan, Ewald and Kelleher 74 ). Due to its association with pancreatic disease, patients with type 3c diabetes tend to be undernourished and have nutrient deficiencies. Management is complicated by malabsorption, excess alcohol intake (for some) and poor dietary intake (due to chronic abdominal pain, anorexia, heavy smoking and/or symptom avoidance)( Reference Duggan, Ewald and Kelleher 74 ). As well as low insulin levels, patients with type 3c diabetes will have reduced glucagon secretion from the pancreatic α-cells and lower levels of pancreatic polypeptide( Reference Cui and Andersen 73 , Reference Rickels, Bellin and Toledo 75 ). A reduction in pancreatic polypeptide can contribute to decreased hepatic insulin sensitivity and unsuppressed hepatic glucose production. Together, these factors lead to the characteristically ‘brittle’ diabetes that is difficult to control, with erratic swings in blood glucose levels from hypoglycaemia to hyperglycaemia( Reference Duggan, Ewald and Kelleher 74 ). Research into type 3c diabetes is lacking in general, and in fact patients with this diabetes subgroup tended to be specifically excluded from major diabetes studies. Several recent publications have drawn attention to type 3c diabetes; however, clinical management guidelines remain scarce, those that exist have tended to draw from studies on type 1 and type 2 diabetes (in the absence of type 3c-specific data)( Reference Cui and Andersen 73 – Reference Ewald and Bretzel 77 ). Nevertheless, type 3c diabetes is not uncommon. It is thought that about 8 % of all diabetes cases may be type 3c diabetes( Reference Ewald, Kaufmann and Raspe 72 ). In chronic pancreatitis, the prevalence is higher in heavy smokers, those who have had a distal pancreatectomy (due to the high concentration of islets cells in the tail), those with longer duration of disease and those with pancreatic calcifications.
An individualised medical nutrition therapy programme is vital for patients with type 3c diabetes. Emphasis should be placed on regular monitoring and recording of blood glucose levels, along with alcohol avoidance to pre-empt and prevent hypoglycaemic events, and the implementation of patient-specific meal plans, which aim to reduce the frequency and extent of hyperglycaemia( Reference Duggan and Conlon 50 , Reference Duggan, Ewald and Kelleher 74 , 78 ). While patients with type 3c diabetes require a different approach compared with those with types 1 and 2 diabetes, it should be noted that not all patients with pancreatic disease have type 3c diabetes. The prevalence of diabetes following acute pancreatitis is high, with a recent systematic review reporting that almost one in four patients had diabetes or prediabetes (25 % had frank diabetes). However, it may be that some of these patients have a diabetes type that is more consistent with type 2 and hence will behave differently, with different management priorities. In fact, type 2 and type 3c diabetes can co-exist, and there could be a degree of overlap, but there is a dearth of research into this topic. We designed a study to investigate the occurrence and nature of diabetes following severe acute pancreatitis in patients with no prior diagnosis of diabetes or history of chronic pancreatitis( Reference Duggan, Mohammed and Lawal 79 ). In a study of almost thirty patients who survived severe acute pancreatitis, we found that the prevalence of undiagnosed endocrine insufficiency was high at 48 % (30 % diabetes, 17 % impaired fasting glucose or impaired glucose tolerance).
However, only a minority with endocrine insufficiency were insulin sensitive indicating an exclusively pancreatogenic aetiology. In the majority of subjects, insulin resistance appeared to be the contributing factor, which is therefore more consistent with type 2 diabetes. This area of pancreatic disease constitutes a substantial research gap.
Conclusions
Our understanding of the nutritional status and management of patients with chronic pancreatitis has increased considerably in a short timeframe. Studies on nutrient deficiency, PEI and PERT, osteoporosis and bone metabolism, and type 3c diabetes have allowed for the development of much-needed evidence-based approach to nutritional management that did not previously exist. However, many unanswered questions remain and there is a requirement for continued research into many aspects of the nutritional management of this disease. Topics that warrant immediate attention include type 3c diabetes (nature of the disease and dietary management), sarcopenia (prevalence, impact and intervention studies to prevent or reduce muscle depletion), nutrient supplementation (intervention studies on the effectiveness and safety of supplementation), osteoporosis (dietary and pharmacological strategies for prevention and treatment) and diet (dietary intake, nutritional requirements, intervention studies). In the medium term, efforts should be made to understand the potential relationship between the microbiome and inflammation in chronic pancreatitis and to explore the potential for therapeutic agents that may modulate the disease process and improve outcomes. Ultimately, the potential to ameliorate the inflammatory process by dietary or pharmacological means may open up new therapeutic avenues. By addressing these substantial research gaps through the establishment of well-funded, high-quality, multidisciplinary, multicentre studies, we may begin to achieve real clinical benefits for patients with this neglected disease.
Acknowledgments
The author wishes to acknowledge all past and current members of the Centre for Pancreatico-Biliary Disease (Tallaght Hospital, and The University of Dublin, Trinity College,) along with the Departments of Surgery, Nutrition & Dietetics, Gastroenterology, Endocrinology, and Radiology of Tallaght Hospital, Dublin. Thank you also to the Department of Endocrinology St Vincent's University Hospital, Dublin, The Department of Public Health and Primary Care, University of Dublin, Trinity College, and The Medical Library at the Trinity Centre for Health Sciences, Tallaght Hospital, Dublin. Special thanks to Professor Maria O'Sullivan (The University of Dublin, Trinity College), Ms Sinead Feehan (Tallaght Hospital, Dublin), and Ms Hazel Ni Chonchubhair, Mr Robert Memba, Mr Donal O'Connor, Ms Niamh Murray, Ms Marie Egan, and Professor Paul Ridgway, all of the Centre for Pancreatico-Biliary Disease. The author is indebted to Professor Kevin C Conlon, Director of the Centre for Pancreatico-Bilary Disease, and Professor of Surgery at The University of Dublin, Trinity College.
Financial Support
Financial support was received primarily from Health Research Board, Ireland by means of a Health Professional's Fellowship. Research grants were also received from The Meath Foundation of Tallaght Hospital (two grants), and Mylan, Ireland.
Conflicts of Interest
None.
Authorship
This review was solely authored by Sinead N Duggan based on The Cuthbertson Medal Lecture (London, 2016).





