Introduction
Major depressive disorder (MDD), further referred to as depression, due to its high prevalence, early onset, chronic nature, and low treatment success, causes a major disease burden (Murray et al., Reference Murray, Barber, Foreman, Ozgoren, Abd-Allah, Abera and Abu-Raddad2015, Reference Murray, Vos, Lozano, Naghavi, Flaxman, Michaud and Abdalla2012). Depression not only affects mental health, it also affects physical health, as it has been shown to increase the risk for somatic morbidities, such as diabetes and cardiovascular disease (Penninx, Milaneschi, Lamers, & Vogelzangs, Reference Penninx, Milaneschi, Lamers and Vogelzangs2013). As depression is predicted to become the primary cause of global disease burden by 2030, the World Health Organization states that depression prevention is an area that requires attention (Smit, Cuijpers, Duivis, & Petrea, Reference Smit, Cuijpers, Duivis and Petrea2013).
Recently, there has been interest in the role of diet in the onset of depression. Evidence from observational studies has emerged to suggest an association between healthy dietary patterns and less depressive symptoms even over time, but reverse causation or hidden confounding remains possible (Lassale et al., Reference Lassale, Batty, Baghdadli, Jacka, Sánchez-Villegas, Kivimäki and Akbaraly2019; Nicolaou et al., Reference Nicolaou, Colpo, Vermeulen, Elstgeest, Cabout, Gibson-Smith and Mishra2019). Prospective studies further indicated that adherence to a healthy diet can reduce the risk for the onset of depressive symptoms, although not all studies supported a predictive role of diet quality in the development of depression (Molendijk, Molero, Sánchez-Pedreño, Van der Does, & Martínez-González, Reference Molendijk, Molero, Sánchez-Pedreño, Van der Does and Martínez-González2018). Overall, these findings point toward the potential benefits of dietary modification in the prevention of depression.
Nevertheless, results from the MooDFOOD depression prevention study, the largest dietary randomized prevention trial published thus far, do not indicate that dietary interventions [food-related behavioral activation (F-BA) therapy and multi-nutrient supplementation] prevent depression onset, nor do they reduce depressive symptoms during one year in overweight individuals with subsyndromal depression symptoms (Bot et al., Reference Bot, Brouwer, Roca, Kohls, Penninx, Watkins and Gili2019). While this study intended to target a sample at high risk of depression, the observed depression incidence was however relatively low (~10%), which lowered the statistical power to find significant effects. Follow-up analyses show that the F-BA therapy did improve food intake in line with the Mediterranean dietary pattern, although this effect was of limited clinical relevance (Grasso et al., Reference Grasso, Olthof, van Dooren, Roca, Gili, Visser and Kohls2020; Paans et al., Reference Paans, Bot, Brouwer, Visser, Gili, Roca and Penninx2020). In addition, F-BA led to favorable changes in unhealthy eating styles (e.g. less emotional and uncontrolled eating) (Paans et al., Reference Paans, Bot, Brouwer, Visser, Gili, Roca and Penninx2020), which was in turn associated with a reduction in depressive symptoms (Owens et al., Reference Owens, Watkins, Bot, Brouwer, Roca, Kohls and Visserin press). In contrast to Bot et al. (Reference Bot, Brouwer, Roca, Kohls, Penninx, Watkins and Gili2019), a recent meta-analysis did report a significant effect of ‘whole of food’ dietary interventions on the reduction of depressive symptoms, although the pooled effect size was small and there was substantial heterogeneity between studies (Firth et al., Reference Firth, Marx, Dash, Carney, Teasdale, Solmi and Jacka2019a).
A key issue in the field of depression research is the tendency to conceptualize depression as a unidimensional construct rather than as a collection of different phenotypes. Although depression is considered as a distinct disorder, its symptoms are diverse (Fried & Nesse, Reference Fried and Nesse2015), which complicates the examination of intervention effects on combined symptom rating scores (Fried, Reference Fried2017). A number of studies have examined such effects on depressive symptom profiles and found that different treatment modalities for depression differentially impact the severity of specific symptom profiles. For example, some antidepressants have shown to be more effective in reducing mood and cognitive symptoms compared to somatic symptoms (Green et al., Reference Green, Goldstein-Piekarski, Schatzberg, Rush, Ma and Williams2017; Uher et al., Reference Uher, Maier, Hauser, Marušič, Schmael, Mors and Rietschel2009).
So far, there has been little consensus about the classification of individual depressive symptoms into symptom profiles, although common depression scales generally distinguish between mood, cognitive, somatic, and sleep symptom clusters (Shafer, Reference Shafer2006; Wardenaar et al., Reference Wardenaar, van Veen, Giltay, den Hollander-Gijsman, Penninx and Zitman2010). Dietary interventions may improve somatic symptoms more than mood/cognitive symptoms of depression, as somatic depressive symptoms reflect, among other symptoms, alterations in appetite and weight. Apart from this, another reason for the potential higher efficacy of dietary interventions for somatic symptoms is that somatic symptoms, and not mood/cognition symptoms, have been previously associated with body weight status (Baldofski et al., Reference Baldofski, Mauche, Dogan-Sander, Bot, Brouwer, Paans and Hegerl2019) and emotional and external eating (Paans et al., Reference Paans, Bot, Brouwer, Visser, Roca, Kohls and Penninx2018a).
A third interesting symptom profile to study in relation to dietary interventions is the atypical, energy-related symptom profile, including both a number of somatic and mood/cognition symptoms. This symptom profile is based on data-driven analyses that partly confirmed the DSM specifier of atypical depression (Alexandrino-Silva et al., Reference Alexandrino-Silva, Wang, Viana, Bulhões, Martins and Andrade2013; Lamers et al., Reference Lamers, de Jonge, Nolen, Smit, Zitman, Beekman and Penninx2010; Rodgers et al., Reference Rodgers, Ajdacic-Gross, Müller, Hengartner, Holtforth, Angst and Rössler2014; Sullivan, Prescott, & Kendler, Reference Sullivan, Prescott and Kendler2002). This data-driven atypical symptom profile was characterized by symptoms reflecting altered energy intake/expenditure balance, driven primarily by increased appetite and weight as well as by leaden paralysis and hypersomnia, and not by mood reactivity which is considered a core symptom of the DSM-5 atypical specifier (Lamers et al., Reference Lamers, de Jonge, Nolen, Smit, Zitman, Beekman and Penninx2010). A growing body of literature has demonstrated that these atypical, energy-related symptoms are associated with immuno-metabolic dysregulations, including elevated levels of inflammatory markers, increased body mass index (BMI), and dysregulated levels of leptin and insulin (as reviewed in Milaneschi, Lamers, Berk, & Penninx, Reference Milaneschi, Lamers, Berk and Penninx2020). The link between this atypical, energy-related symptom profile and poorer immuno-metabolic health was subsequently confirmed in a longitudinal study by Lamers et al. (Reference Lamers, Milaneschi, Vinkers, Schoevers, Giltay and Penninx2020). Due to the role of diet on energy homeostasis and other relevant immuno-metabolic pathways, dietary interventions may be particularly effective for this atypical, energy-related symptom profile.
The aim of this study was to investigate the effects of two dietary interventions (F-BA therapy and multi-nutrient supplementation) on three symptom profiles of depression: (1) mood/cognition; (2) somatic; (3) atypical, energy-related, using data from the MooDFOOD depression prevention study. Although previous analyses on these data did not find preventive effects of both interventions on depressive symptoms (Bot et al., Reference Bot, Brouwer, Roca, Kohls, Penninx, Watkins and Gili2019), considering the clinical heterogeneity of depression, it is still possible that the interventions had a more beneficial impact on selected specific depression symptom profiles. It was hypothesized that dietary interventions had the strongest impact on atypical, energy-related depressive symptoms, and the least on mood/cognitive symptoms of depression.
Methods
Study design
The Multi-country cOllaborative project on the role of Diet, Food-related behavior, and Obesity in the prevention of Depression (MooDFOOD) depression prevention study is a 2 × 2 factorial randomized controlled trial, conducted in four European countries (Germany, Spain, The Netherlands, and the UK) between July 2015 and October 2017. For a complete description of the study design, see Roca et al. (Reference Roca, Kohls, Gili, Watkins, Owens, Hegerl and Brouwer2016), and for the main results, see Bot et al. (Reference Bot, Brouwer, Roca, Kohls, Penninx, Watkins and Gili2019). Written informed consent was obtained from all participants and the study was approved by the Ethical Review Boards of the four study sites.
Eligibility and randomization
Participants were recruited from websites, social media and local newspaper advertisements, mailings to registered persons in the general practice or in other registers (e.g. city registers), and via other studies conducted at the four sites. Eligible participants were adults between 18 and 75 years old with subsyndromal depressive symptoms (⩾5 on the Patient Health Questionnaire) and a self-reported BMI between 25 and 40 kg/m2. Exclusion criteria were an episode of MDD according to DSM-IV criteria at least 6 months up to baseline as assessed with the structured MINI International Neuropsychiatric Interview 5.0 (MINI 5.0; Sheehan et al., Reference Sheehan, Lecrubier, Sheehan, Amorim, Janavs, Weiller and Dunbar1998), current eating disorder and a history of severe psychiatric disorders (e.g. psychosis, bipolar disorder, or substance dependence), a history of or planned bariatric surgery, severe physical morbidity or cognitive impairment, pregnancy or breastfeeding, use of antidepressants or psychological interventions in the past 6 months, and an unwillingness to refrain from supplements that interfere with the trial supplements.
All in- and exclusion criteria were checked during a telephone screening. A total of 1025 participants underwent a baseline assessment, including a clinical interview, physical measurements, blood sampling, and self-report questionnaire. Participants were then randomized (in a 1:1:1:1 ratio, in permuted blocks of 8–12) to one out of four groups: (1) placebo supplements only, (2) placebo supplements with FB-A therapy, (3) multi-nutrient supplements only, (4) or multi-nutrient supplements with FB-A. Randomization was stratified by recruitment site and history of depression (yes/no). Follow-up assessments were done at 3 (T3), 6 (T6), and 12 months (T12) after baseline by researchers blind to randomization. In the current study, data of 993 participants who completed the 30-item Inventory of Depressive Symptom Severity Self-Reported (IDS-SR; Rush, Gullion, Basco, Jarrett, & Trivedi, Reference Rush, Gullion, Basco, Jarrett and Trivedi1996) at baseline were used (at some time points, IDS-SR data were unavailable for some participants: see below). Participants excluded from the analyses (n = 32) did not significantly differ from our sample in sex, age, BMI at baseline, history of depression, and randomization status.
Intervention components
Food-related behavioral activation (FB-A)
FB-A therapy consisted of up to 21 protocolled sessions (15 individual sessions of 30 minutes and six group sessions of 1 hour) over a period of 12 months. The therapy is based on behavioral activation principles and incorporates therapeutic techniques, such as self-monitoring, functional analysis, and activity scheduling, with the aim to change unhealthy (mood-related) eating styles and habits (e.g. emotional eating), reinforce healthy food behaviors (e.g. eating regular meals), and implement a healthy Mediterranean diet. For example, participants self-monitored their dietary intake and food-related behaviors, functional analysis was applied to identify contingencies that sustain these behaviors, and activity scheduling was used to plan desired actions to improve mood and promote healthy dietary behaviors (for details, see Roca et al., Reference Roca, Kohls, Gili, Watkins, Owens, Hegerl and Brouwer2016). As there was a strong emphasis on changing behaviors following the behavioral activation framework, F-BA was delivered by trained clinicians familiar with behavioral activation principles (e.g. psychologists) and were able to consult a dietician over the course of the therapy.
Supplements
Multi-nutrient supplementation consisted of a capsule of omega-3 fatty acids in the form of 1412 mg eicosapentaenoic acid and docosahexaenoic acid (ratio 3:1) and a supplement composed of 100 mg of calcium, 30 μg of selenium, 400 μg of folic acid, and 20 μg of vitamin D3. The nutrient composition of these supplements was based on prior research available at the time the study was designed, demonstrating associations between deficiency in these nutrients and depression risk (for details see Roca et al., Reference Roca, Kohls, Gili, Watkins, Owens, Hegerl and Brouwer2016). Placebo supplements were a sunflower oil capsule and a pill containing microcrystalline cellulose, corn starch, polyvinylpyrrolidone, cross-linked carboxymethylcellulose, sodium, magnesium stearate, and magnesium silicate. To ensure blinding of participants, placebos were provided in identical capsules and containers as the multi-nutrient supplements. The supplements were provided in two pills per day taken on a daily basis for a duration of 12 months.
Measures
Depressive symptom profiles
The 30-item IDS-SR was used to assess the severity of depressive symptoms in the past week, measured on a four-point scale from 0 to 3 (higher scores indicating higher symptom severity). In line with the scoring instructions, for the items on appetite/weight change, either increase or decrease (not both) was scored. The items not filled out were recoded as 0. For example, if a participant reported increased appetite, the item on decreased appetite was recoded as 0. The IDS-SR was assessed at T0, T3, T6, and T12.
Depressive symptoms were classified into three symptom profiles. An atypical, energy-related symptom profile (further referred to as energy-related) was created that included all five atypical symptoms essential for energy homeostasis: sleeping too much; increased appetite; increased weight; low energy level/fatigue; leaden paralysis, as used before (Lamers et al., Reference Lamers, Milaneschi, Vinkers, Schoevers, Giltay and Penninx2020). We also created a mood/cognition and a somatic symptom profile based on the results of a principal component analysis on IDS data in ~3000 patients with depressive disorders and healthy controls (Wardenaar et al., Reference Wardenaar, van Veen, Giltay, den Hollander-Gijsman, Penninx and Zitman2010) and in line with other classifications (Baldofski et al., Reference Baldofski, Mauche, Dogan-Sander, Bot, Brouwer, Paans and Hegerl2019; Paans et al., Reference Paans, Bot, Brouwer, Visser, Roca, Kohls and Penninx2018a; Schaakxs, Comijs, Lamers, Beekman, & Penninx, Reference Schaakxs, Comijs, Lamers, Beekman and Penninx2017). The item on diurnal variation of mood was removed from classification as this item has previously been found to associate poorly with either mood/cognition or the somatic symptom profile (Rush et al., Reference Rush, Gullion, Basco, Jarrett and Trivedi1996; Wardenaar et al., Reference Wardenaar, van Veen, Giltay, den Hollander-Gijsman, Penninx and Zitman2010). The mood/cognition symptom profile consisted of 16 items: feeling sad; feeling irritable; feeling anxious or tense; mood reactivity; quality of mood; concentration/decision-making problems; self-criticism and blame; future pessimism; suicidal thoughts; diminished interest in people/activities; low energy level/fatigue; diminished capacity for pleasure/enjoyment; reduced interest in sex; psychomotor retardation; interpersonal sensitivity; leaden paralysis. The 13 items of the somatic symptom profile were: problems falling asleep; problems sleeping during the night; early morning awakenings; sleeping too much; decreased appetite; increased appetite; increased weight; decreased weight; psychomotor agitation; aches and pains; other bodily symptoms; panic/phobic symptoms; constipation/diarrhea. An overview of the three symptom profiles is provided in online Supplementary Table S1.
Sum scores were calculated for each symptom profile. Due to the unequal number of symptoms in each profile, sum scores divided by the number of items in each profile were used in the analyses. The number of participants with available data on the symptom profiles was 933 at baseline, 808 (somatic and energy-related) and 807 (mood/cognition) at T3, 746 (somatic and energy-related) and 740 (mood/cognition) at T6, and 748 for all symptom profiles at T12.
Sociodemographic, clinical, and lifestyle characteristics
Age in years, sex, level of education, smoking status, and alcohol consumption were assessed with the baseline interview. Educational level was recoded into low (no education, primary education, or lower secondary education), middle (upper secondary education, postsecondary non-tertiary education, short-cycle tertiary education), and high (bachelor's degree or higher or equivalent level). History of depression, i.e. the presence or absence of a lifetime MDD diagnosis, and number of previous depressive episodes were assessed with the MINI 5.0. Measured height and body weight were used to calculate BMI (weight in kilograms divided by the square of the height in meters). Physical activity was measured with the Short Questionnaire to ASsess Health-enhancing physical activity (SQUASH; Wendel-Vos, Schuit, Saris, & Kromhout, Reference Wendel-Vos, Schuit, Saris and Kromhout2003) and quantified as the number of hours per day actively commuting (e.g. walking, cycling), physical activity at work or school, household activities, and leisure time activities (e.g. sports, gardening).
Statistical analysis
Sample characteristics at baseline for the four groups were described using descriptive statistics (mean and standard deviation, median and interquartile range and frequencies). Differences in symptom profile scores at each time point between the four intervention groups with placebo as reference were examined using t tests. The four intervention groups were: (1) placebo supplements only, (2) placebo supplements with F-BA therapy, (3) multi-nutrient supplements only, or (4) multi-nutrient supplements with F-BA.
According to methods outlined in Bot et al. (Reference Bot, Brouwer, Roca, Kohls, Penninx, Watkins and Gili2019), the effects of the two intervention components on symptom profiles were jointly modeled based on the presence v. absence of each component. Briefly, the effect of F-BA therapy was assessed by comparing the groups receiving supplements only (no intervention) to the groups receiving F-BA therapy. For multi-nutrient supplementation, the groups receiving placebo supplements with and without F-BA (placebo) were compared to the groups receiving multi-nutrient supplements with and without F-BA (supplements). Analyses were performed on an intention-to-treat basis.
To examine intervention effects on depression symptom profiles over all follow-up measurements while adjusting for within-person dependency across the repeated measures, Generalized Estimating Equations (GEE) longitudinal analyses of covariance with an exchangeable working correlation structure were conducted. Separate models were run for the three symptom profiles. Predictors were dummy-coded intervention variables and the outcome was a depressive symptom profile score modeled over 3, 6, and 12 months. To test the combined effects of the two components, a supplements by F-BA interaction term was added in the second step to the model. Models were adjusted for time (coded as 3, 6, and 12), recruitment site, history of depression, and symptom profile scores at baseline. Cohen's d values were calculated for significant results from adjusted means for the two intervention components and pooled standard deviations (derived from the standard errors of the adjusted means).
To explore whether intervention effects on symptom profiles could be explained by some symptoms more than others, the same GEE analyses were performed using individual depressive symptoms as outcomes. Covariates included time, recruitment site, history of depression, and baseline symptom scores. The 30 obtained p values for each intervention variable were together adjusted for multiple testing using the false discovery rate (FDR) correction (Benjamini & Hochberg, Reference Benjamini and Hochberg1995).
We tested for effect modification by age, sex, and baseline BMI by adding interaction terms with the two interventions in the GEE models. Sensitivity models were run in a subset of participants with complete IDS data at all follow-up measures and on subsets of participants with good adherence to the interventions (per-protocol analyses). Good adherence to the interventions was defined a priori as receiving ⩾8 of 21 therapy sessions and ⩾70% of the supplements over a period of 12 months (Roca et al., Reference Roca, Kohls, Gili, Watkins, Owens, Hegerl and Brouwer2016). The latter was based on the weights of provided v. returned supplement jars or, if not available, self-reported supplement use.
Two-tailed statistical tests were used and statistical significance was set at an α of 5%. Analyses were conducted in R version 3.5.1.
Results
Description of participants
Sociodemographic, clinical, and lifestyle characteristics of participants in each of the four intervention groups at T0 are shown in Table 1. The mean age of the sample (n = 993) was 48.6 (standard deviation = 13; range = 18–75) years and most participants were female (75.2%). On average, at the baseline interview participants had a BMI of 31.4 kg/m2 (standard deviation = 4.0, range = 23.9–45.1). About one-third of the sample (33.4%) had a history of depression.
Table 1. Sample characteristics at baseline (n = 933)

F-BA, food-related behavioral activation therapy; MDD, major depressive disorder.
Not-normally distributed variables are presented as medians with interquartile ranges (IQR).
Effects of the interventions on depressive symptom profiles
Unadjusted mean depressive symptom profile scores for the four intervention groups at T0, T3, T6, and T12 are presented in Fig. 1. At T0, the multi-nutrient supplements without F-BA group had a significantly higher somatic symptom profile score than the placebo-only group (p = 0.027), which was maintained at T3 (p = 0.030) and T6 (p = 0.020).

Fig. 1. Depressive symptom profile scores by intervention groups at baseline and follow-up (unadjusted means ± 1 standard error). Asterisks indicate t test comparisons of the dietary interventions to the placebo without F-BA group for each time point: ***p < 0.001, **p < 0.01, *p < 0.05.
To examine the intervention effects on depression symptom profiles over all follow-up measurements adjusted for time, recruitment site, history of depression, and baseline symptom scores, GEE models were conducted. As shown in Table 2, F-BA therapy was significantly associated with decreased severity of the somatic profile [B = −0.03, p = 0.014, d = −0.10 (95% CI −0.18 to −0.02)], and the energy-related profile [B = −0.08, p = 0.001, d = −0.13 (95% CI −0.21 to −0.05)], but not with the mood/cognition profile (B = −0.01, p = 0.476) during follow-up. Conversely, multi-nutrient supplementation was significantly associated with increased severity of the mood/cognition profile [B = 0.05, p = 0.022, d = 0.09 (95% CI 0.01–0.17)] and the energy-related profile [B = 0.07, p = 0.002, d = 0.12 (95% CI 0.04–0.20)], but not with the somatic profile (B = 0.02, p = 0.207) during follow-up. No significant combined effects were found for the two interventions on any of the symptom profiles (p > 0.05). Results did not show consistent effect modification by age, sex, and baseline BMI (see online Supplementary Table S2). Sensitivity analyses after the exclusion of 246 participants with incomplete IDS data on any of the follow-up measurements returned similar results as the main analyses (see online Supplementary Table S3). Results of the per-protocol sensitivity analyses including only participants with good adherence were also similar to those of the main analyses (see online Supplementary Table S4.1–4.3).
Table 2. Individual and combined effects of supplements and F-BA therapy on depressive symptom profiles (n = 993, n observations = 2979)

Intervention effects are modeled over 3, 6, and 12-month follow-up measurements. Intervention variables are dummy-coded (supplements/F-BA = 1; placebo/no intervention = 0). Model 1: adjusted for time, baseline symptom profile score, recruitment site, and history of major depressive disorder. Model 2: adjusted for model 1, F-BA therapy, and supplements. p values < 0.05 are highlighted in bold. s.e. = robust standard error obtained from the Generalized Estimating Equation model.
Effects of the interventions on individual depressive symptoms
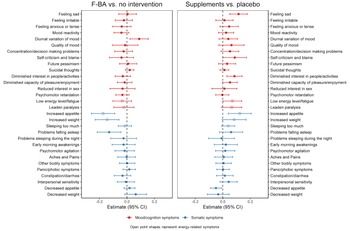
To further investigate the intervention effects, we explore the effects of the interventions on all 30 individual depression symptoms over all follow-up measurements. As shown in Fig. 2, the association of F-BA therapy with decreased severity of energy-related symptom profile appeared to be driven by the inverse effects on increased appetite (B = −0.17, p = 0.0001) and increased weight (B = −0.14, p = 0.001). Apart from increased appetite and weight, the association of F-BA therapy with decreased severity of the somatic symptom profile seemed especially present through the effect on problems of sleep during the night (B = −0.13, p = 0.003).

Fig. 2. Generalized Estimated Equation estimates for the overall follow-up effects of the two interventions on depressive symptoms. Models were adjusted for time, baseline symptom profile scores, recruitment site, and history of major depressive disorder. CI= confidence interval.
The association of multi-nutrient supplementation with increased severity of the mood/cognition profile was suggested to be primarily driven by the effects on two core symptoms of depression diminished interest in people/activities (B = 0.09, p = 0.003) and feeling sad (B = 0.12, p = 0.001). Besides these two mood/cognition symptoms, multi-nutrient supplementation was also associated with increased severity of self-criticism and blame (B = 0.12, p = 0.047) and low energy level or fatigue (B = 0.07, p = 0.043), although these associations did not survive FDR correction. The association of multi-nutrient supplementation with increased severity of the energy-related profile was suggested to be strongly driven by the effects on increased appetite (B = 0.13, p = 0.004).
Discussion
In a large sample of overweight adults with subsyndromal depressive symptoms, this study investigated whether two dietary intervention components have a differential impact on the severity of specific depressive symptom profiles (mood/cognition; somatic; energy-related). F-BA therapy alleviated somatic and energy-related, but not mood/cognition symptoms of depression. Daily intake of multi-nutrient supplements was inferior to placebo in reducing mood/cognition and energy-related symptoms but had no effect on somatic symptoms of depression. Since these effects would have been disregarded when using only combined total depressive symptom rating scales, our findings strengthen the idea that depression should not be considered as a homogeneous construct in relation to diet and eating behavior (Paans et al., Reference Paans, Bot, Brouwer, Visser, Roca, Kohls and Penninx2018a, Reference Paans, Bot, van Strien, Brouwer, Visser and Penninx2018b).
Previous analyses of these data indicated that F-BA therapy did not prevent depression onset nor overall depressive symptoms (Bot et al., Reference Bot, Brouwer, Roca, Kohls, Penninx, Watkins and Gili2019). In the current study, however, we showed that F-BA therapy (v. no intervention) had a significant but small beneficial effect on somatic and energy-related symptom profiles. Although depression and obesity are two separate clinical entities, there is overlap in specific somatic symptoms which may have a pleiotropic genetic basis (Milaneschi et al., Reference Milaneschi, Lamers, Berk and Penninx2020), especially in symptoms belonging to the energy-related symptom profile. Improvement in these symptoms, regardless of their origin, is likely to contribute to better mental and physical health and is therefore, from a prevention point of view, a relevant target for interventions. As stated earlier, the outcome of Bot et al. (Reference Bot, Brouwer, Roca, Kohls, Penninx, Watkins and Gili2019) is contrary to that of a meta-analysis which concluded that overall a beneficial effect of whole-diet interventions on the reduction of depressive symptoms can be found, although large heterogeneity in effect sizes was observed (Firth et al., Reference Firth, Marx, Dash, Carney, Teasdale, Solmi and Jacka2019a). Our findings strongly suggest that dietary interventions may differentially impact different depression symptom profiles in high-risk groups, highlighting that consideration of clinical heterogeneity in outcomes is of great importance and may thus help explain inconsistencies in previous research.
The beneficial effect of F-BA therapy on somatic and energy-related symptoms corroborates with the findings of previous work in MooDFOOD (Paans et al., Reference Paans, Bot, Brouwer, Visser, Roca, Kohls and Penninx2018a) and another large-scale study (Paans et al., Reference Paans, Bot, van Strien, Brouwer, Visser and Penninx2018b) which showed that specific somatic, here also regarded as energy-related, symptoms such as ‘increased appetite’ and ‘increased weight’ are more strongly associated with unhealthy eating styles than mood symptoms are. In line with this, somatic symptoms but not mood/cognitive symptoms were related to anthropometric measures of obesity in MooDFOOD and other studies (Baldofski et al., Reference Baldofski, Mauche, Dogan-Sander, Bot, Brouwer, Paans and Hegerl2019; Wiltink et al., Reference Wiltink, Michal, Wild, Zwiener, Blettner, Münzel and Beutel2013). Recent work shows that, by identifying individual triggers and functions of food-related behaviors and learning to substitute these with healthier alternatives, F-BA therapy improved participants' diet quality and eating styles but not weight status (Paans et al., Reference Paans, Bot, Brouwer, Visser, Gili, Roca and Penninx2020). Importantly, among many potential mediators (e.g. avoidance and rumination, adherence to the Mediterranean diet, weight status), only improvements in emotional and uncontrolled eating significantly explained the effects of F-BA on depressive symptoms (Owens et al., Reference Owens, Watkins, Bot, Brouwer, Roca, Kohls and Visserin press). In other words, the F-BA therapy resulted in a lower tendency to engage in emotional and uncontrolled eating which was subsequently linked to less depressive symptoms. Based on the results of the current study, it can be hypothesized that this may be especially true for specific somatic symptoms, particularly those related to altered energy intake/expenditure balance.
Emerging evidence shows that the energy-related symptom burden is linked to immuno-inflammatory and metabolic dysregulations and may be indicative of an immuno-metabolic depression (IMD) (Lamers et al., Reference Lamers, Milaneschi, Vinkers, Schoevers, Giltay and Penninx2020; Milaneschi et al., Reference Milaneschi, Lamers, Berk and Penninx2020). We hypothesize that persons with IMD features – which include atypical, energy-related symptoms, metabolic dysregulation such as high BMI, and low-grade inflammation – will specifically benefit from interventions, such as F-BA, that are likely to have an impact on immuno-metabolic dysregulations. It is possible that the more IMD features are present in a person, the more this person will benefit from interventions targeting the immuno-metabolic systems, and that reduction in symptoms is accompanied by a reduction in inflammatory marker levels. Specifically, evidence from observational studies and clinical trials shows a relation between the adoption of Mediterranean dietary pattern, one of the main components of F-BA therapy, and reduced biomarkers of low-grade inflammation, reduced insulin resistance, and improved lipid profiles (Kastorini et al., Reference Kastorini, Milionis, Esposito, Giugliano, Goudevenos and Panagiotakos2011; Martínez-González et al., Reference Martínez-González, Salas-Salvadó, Estruch, Corella, Fitó and Ros2015; Schwingshackl & Hoffmann, Reference Schwingshackl and Hoffmann2014; Sureda et al., Reference Sureda, Bibiloni, Julibert, Bouzas, Argelich, Llompart and Tur2018). The impact of diet on these immuno-metabolic pathways provides a biological mechanism underlying the effect of F-BA therapy on the energy-related symptom profile linked to IMD. Exploratory analyses on individual symptoms did indeed suggest that the effect of F-BA therapy was present through the inverse effects on two energy-related symptoms ‘increased appetite’ and ‘increased weight’. As these symptoms are a subset of somatic symptoms, they may specifically drive the effect of F-BA therapy on the somatic symptom profile. F-BA therapy also alleviated the somatic symptom ‘problems sleeping during the night’. This finding, while exploratory, fits with previous associations between insomnia symptoms, obesity, and metabolic syndrome components (Chan, Levsen, & McCrae, Reference Chan, Levsen and McCrae2018; Lamers, Milaneschi, De Jonge, Giltay, & Penninx, Reference Lamers, Milaneschi, De Jonge, Giltay and Penninx2018) and suggests that diet may influence metabolic pathways that, besides being implicated in IMD, may also be associated with insomnia.
With regards to multi-nutrient supplements, it was found that multi-nutrient supplementation (v. placebo) was associated with a higher severity on somatic and energy-related symptom profiles. Earlier observations in MooDFOOD showed that multi-nutrient supplementation was not associated with a lower depression onset but associated with adverse effects on depression severity. Our results add that supplements have a small adverse effect on specific symptoms. Supplements are proposed to exert their effect through the correction of specific nutrient deficiencies. Although nutrient deficiencies more often occur in overweight and obese than in normal weight individuals (Kaidar-Person, Person, Szomstein, & Rosenthal, Reference Kaidar-Person, Person, Szomstein and Rosenthal2008), whether vitamin and mineral consumption was in a normal range before supplementation in the current sample is not known. In the absence of deficiencies, excess nutrient intake through supplementation could disrupt normal homeostasis, which potentially has an effect on certain depression symptoms more than others. Taken together, these findings suggest that multi-nutrient supplementation is most likely not an effective strategy for the reduction of depressive symptom profiles among overweight individuals with subsyndromal depressive symptoms. It should however be noted that present findings do not support a previous meta-analysis of studies in non-clinical samples demonstrating beneficial effects of multi-nutrient supplements on aspects of mood (e.g. ‘feeling happier’) and cognition (e.g. ‘clear-headedness’), as well as on energy levels and fatigue (Long & Benton, Reference Long and Benton2013). This inconsistency is likely to be due to variations in sample characteristics (e.g. weight status) and in nutrient compositions and dosages of the supplements, which complicates comparisons between studies.
The dose and ratio of the multi-nutrient supplements used in MooDFOOD were based on several systematic reviews and meta-analyses of observational and treatment studies in clinically depressed patients available at that time (published between 2006 and 2016; for details see Roca et al., Reference Roca, Kohls, Gili, Watkins, Owens, Hegerl and Brouwer2016). According to the updated meta-review by Firth et al. (Reference Firth, Teasdale, Allott, Siskind, Marx, Cotter and Carvalho2019b) of treatment studies and the International Society for Nutritional Psychiatry Research Practice (ISNPR) guidelines (Guu et al., Reference Guu, Mischoulon, Sarris, Hibbeln, McNamara, Hamazaki and Jacka2019), the indicated dosage for omega-3 supplementation is higher than the amount used in the current study. Based on these findings and in the absence of results from prevention studies, perhaps in hindsight a higher dosage – particularly of EPA – could have been considered for the omega-3 supplement. However, factors that contribute to the development of a disorder in high-risk groups may differ from those that play a role in the progression of disorder in patient groups and the evidence to date for omega-3 supplementation to prevent depression in high-risk groups is limited (Thesing, Lamers, Bot, Penninx, & Milaneschi, Reference Thesing, Lamers, Bot, Penninx and Milaneschi2019). Whether not finding a reduction in depressive symptom profiles in the multi-nutrient supplementation group can be explained by the dose of the omega-3 supplement thus remains unclear.
A central issue in the clinical relevance of our findings is that effect sizes were quite small. Compared to the effect sizes for F-BA therapy on the somatic and energy-related symptom profiles in the current study, previous studies involving non- or subclinical populations generally report larger effect sizes for psychotherapy (g = 0.35) (Cuijpers et al., Reference Cuijpers, Koole, van Dijke, Roca, Li and Reynolds2014), smartphone-based mental health interventions (g = 0.38) (Firth et al., Reference Firth, Torous, Nicholas, Carney, Pratap, Rosenbaum and Sarris2017), and exercise (d = 0.37–0.52) (Conn, Reference Conn2010) on combined measures of depressive symptoms. Nevertheless, learning how to effectively manage dietary intake and behaviors can lay the foundation for lifelong healthy eating. This could ultimately affect health outcomes that extend beyond the reduction of specific depressive symptoms, for instance by preventing additional weight gain and related somatic conditions (e.g. cardiovascular disease, diabetes). In such a way, they can have long-lasting effects.
The strength of the current study is the large sample size involving individuals from four European countries. The findings have, however, to be considered in light of some limitations. A first limitation is the reliance on self-report data, which is potentially influenced by social desirability and recall biases. Second, as a result of the unexpectedly low incidence of depression, the MooDFOOD depression prevention study may have been underpowered. Third, the absence of an active control comparison for F-BA therapy makes ruling out potential effects of non-specific attention impact impossible. Specifically, the social interaction component in the F-BA group relative to ‘no F-BA’ might have inflated the effects of F-BA. Studies using active comparators exposing control participants to similar therapeutic experiences matched in time and attention to the dietary intervention (e.g. social support, counseling, exercise) generally report smaller effect sizes (Firth et al., Reference Firth, Marx, Dash, Carney, Teasdale, Solmi and Jacka2019a). However, the common practice regarding the control condition to a psychological intervention is to not participate in an intervention, reflecting typical usual care. Fourth, it has been shown that dietitians are more effective in delivering dietary interventions to people with severe mental illness than other healthcare professionals (Teasdale, Ward, Rosenbaum, Samaras, & Stubbs, Reference Teasdale, Ward, Rosenbaum, Samaras and Stubbs2017), although it is unclear whether these findings generalize to the current (non-clinical) sample and to dietary interventions with a strong behavioral component such as F-BA.
To conclude, decomposing the clinical heterogeneity of depression at the level of symptom profiles provided better insight into the efficacy of dietary interventions for the prevention of depression. Notwithstanding the relatively small effect sizes, this study shows that promoting healthy dietary patterns and behaviors is particularly effective for the reduction of somatic symptoms and the atypical energy-related symptoms profile linked to an immuno-metabolic form of depression. However, future research on the efficacy of F-BA therapy compared to other interventions such as exercise are necessary before more definitive conclusions about its value for clinical practice can be made. In order to personalize the selection of prevention or treatment programs, further studies should also be conducted to determine whether symptom profiles or biological (e.g. immuno-metabolic) profiles at baseline predict response to dietary interventions or whether changes in these features correlate with better outcomes. Results also showed that multi-nutrient supplementation had no beneficial effect on the severity of depressive symptom profiles but was rather linked to poorer outcomes. Even though the precise mechanism underlying these effects remains to be elucidated, careful use of multi-nutrient supplements for prevention purposes at this point is recommended. These findings suggest that clinical heterogeneity of depression plays a role in the efficacy of dietary interventions.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291721000337
Acknowledgements
The authors would like to thank all participants for their participation in the trial and all members of the MooDFOOD prevention trial investigators (for a complete list see www.moodfood-vu.eu).
Financial support
Funding for this work was provided by ZonMw: The Netherlands Organization for Health Research and Development, research program GGZ (project number: 636310017). The MooDFOOD Project ‘Multi-country cOllaborative project on the rOle of Diet, FOod-related behaviour, and Obesity in the prevention of Depression’ was funded by the European Union FP7 (grant agreement no. 613598). This work is supported in the UK by the National Institute for Health Research (NIHR), through the Primary Care Research Network and the NIHR Exeter Clinical Research Facility.
Conflict of interest
Dr Penninx has received (non-related) research grants from Boehringer Ingelheim and Jansen Research. Dr Roca reported receiving grants from the European Union and research funding from Janssen and Lundbeck outside the submitted work. Other authors declare no conflicts of interest.
Ethical standards
The trial was performed in accordance with the principles of Good Clinical Practice. Institutional review board approval was obtained from the Research Ethics Committee Govern de les Illes Balears, Palma, Spain, the Ethics Committee of the University of Leipzig, Germany, VU Medical Center Amsterdam, the Netherlands and the NHS National Research Ethics Service Committee, SouthWest, UK for University of Exeter.