Dementia is a major global public health challenge. There are 46·8 million people living with dementia in the world, a number that is predicted to rise to 131·5 million by 2050( Reference Prince, Wimo and Guerchet 1 ). There is no known cure for dementia and thus more efforts have been made to investigate its risk or protective factors for prevention. Previous studies showed that eating fish was related to reduced risks of CVD (e.g. CHD( Reference He, Song and Daviglus 2 ), stroke( Reference Larsson and Orsini 3 )), respiratory disease( Reference Yang, Xun and He 4 ) and depression( Reference Li, Liu and Zhang 5 ). There are also some studies suggesting that fish consumption could improve cognitive function across the life course( Reference Prince, Albanese and Guerchet 6 ), mainly in young people( Reference Eilander, Hundscheid and Osendarp 7 ).
Since fish fatty acids are important constituents for proper brain functioning and neurocognitive development( Reference Salem, Litman and Kim 8 ), there has been an increase in research investigating whether fish consumption could reduce the risk of dementia( Reference Lopez, Kritz-Silverstein and Barrett-Connor 9 ). However, the findings from those studies are not consistent( Reference Morris, Evans and Tangney 10 , Reference van Gelder, Tijhuis and Kalmijn 11 ). Some studies suggested that fish consumption was associated with a reduced risk of dementia( Reference Barberger-Gateau, Letenneur and Deschamps 12 , Reference Morris, Evans and Bienias 13 ), while others did not show such an association( Reference van de Rest, Spiro and Krall-Kaye 14 , Reference Engelhart, Geerlings and Ruitenberg 15 ). Previous studies on the association of fish consumption with dementia are predominantly from high-income countries, where the characteristics of the populations would make difficulties in dealing with confounding effects including high levels of CVD and risk factors on the association between fish consumption and dementia risk, and the findings could not be generalized to other countries. There is lack of data( Reference Zhang, Chen and Qiu 16 ) from low- and middle-income countries (LMIC), where people have high risk of dementia but low level of fish consumption( Reference Prince, Wimo and Guerchet 1 ). Although there were meta-analyses published previously( Reference Zhang, Chen and Qiu 16 – Reference Cao, Tan and Wang 18 ) to investigate the association of fish consumption with risk of dementia, inferences from those meta-analysis studies were hindered by several potential limitations, for instance missing relevant key publications( Reference Lopez, Kritz-Silverstein and Barrett-Connor 9 ). In the present work, we examined data from a large-scale household health survey in China and carried out a new comprehensive systematic worldwide literature review and meta-analysis to investigate the association of fish consumption with risk of dementia and its dose–response relationship, and to examine any differences in the association between high-income countries and LMIC.
Methods
Multi-province health survey study of older people in China
We analysed data from a multi-province health survey study of dementia in China. The methods of the study, populations and interview outcomes have been fully reported before( Reference Chen 19 , Reference Chen, Hu and Chen 20 ). In brief, during 2007–2010, we carried out a large-scale health survey study of older people in the provinces of Guangdong, Heilongjiang, Shanghai, Shaanxi, Anhui and Hubei in China to investigate the prevalence, risk factors and care of dementia and other chronic conditions( Reference Chen, Hu and Chen 20 , Reference Chen, Zhang and Chen 21 ).
The four-province study
In 2008–2009 we selected one rural and one urban community from each of four provinces (Guangdong, Heilongjiang, Shanghai, Shaanxi) as the study fields. We tried to recruit no fewer than 500 participants in each community and employed a cluster-randomized sampling method to choose residential communities (the district in urban areas and the village in rural areas) from each of the four provinces. The target population consisted of residents aged ≥60 years living in the area for at least 5 years. Based on the residency lists of the committees of the villages and the districts, we recruited a total of 4314 participants with an overall response rate of 93·8 %. The local survey team interviewed the participants at home. The main interview included a general health and risk factors record, the Geriatric Mental State (GMS) questionnaire( Reference Copeland, Prince and Wilson 22 ) and other components of the 10/66 algorithm dementia research package( Reference Prince, Acosta and Chiu 23 ). We carried out a two-phase interview to save our research resources. In phase one, we completed the general health and risk factors record, the GMS, the Community Screening Instrument for Dementia (CSI-D) cognitive test and the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Using three of the four constituent components of the 10/66 algorithm (i.e. data of GMS-AGECAT, the CSI-D cognitive test and CERAD interview), we calculated a probability of possible dementia for each participant. In phase two, we selected the top 15 % of the population who had the highest probability of having ‘dementia’ as ‘probable cases’ and a random sample of 5 % of the rest as ‘probable non-cases’ for subsequent interviews in each province. The interview team completed the CSI-D informant interview for the selected participants.
The Anhui study
Using the same interview approach as that in the four-province study, we completed interviews of 1757 older people from the third wave survey of the Anhui cohort( Reference Chen, Hu and Chen 20 ), the initial number of which was 3336 participants at baseline aged ≥60 years who were randomly recruited in 2001 and 2003, respectively.
The Hubei study
In 2010–2011 we extended the project to include Hubei province( Reference Chen, Hu and Chen 20 ). We used the same protocol and interview materials as in the four-province study but interviewed all participants in a one-stage phase using the full 10/66 methods. We recruited 1001 participants aged ≥60 years and achieved a response rate of 91·8 %.
Risk factors
In the general health and risk factors questionnaire interview, we recorded details relating to sociodemographic characteristics, lifestyle, social networks and support, histories of chronic diseases and risk factors( Reference Chen, Wei and Hu 24 ). We measured height, weight, waist circumference and blood pressure for all participants. In the interview, we asked each participant for details of dietary intakes, including rice, wheat flour, meat, fish, eggs, fresh vegetables, fruits, chilli peppers, garlic, ginger and different types of vegetable oil. All participants were required to provide the answer to the frequency of fish consumption in the past two years: (i) never eat; (ii) ≤once weekly; (iii) >once per weekly and <daily; (iv) once daily; and (v) ≥twice daily.
Diagnosis of dementia
The GMS data were analysed by a computer program-assisted diagnosis, the Automated Geriatric Examination for Computer Assisted Taxonomy (AGECAT), to assess the principal mental disorders in the study participants( Reference Copeland, Prince and Wilson 22 ). We employed the 10/66 dementia algorithm to diagnose dementia, which included data from the GMS-AGECAT diagnostic output, the CSI-D cognitive test score (COGSCORE), the CSI-D informant interview (RELSCORE) and the CERAD ten-word list learning task with delayed recall( Reference Prince, Acosta and Chiu 23 , Reference Prince, De Rodriguez and Noriega 25 ). We used a cut-off point of probability (≥0·25) derived from the full 10/66 algorithm to diagnose dementia, which has been validated in China( Reference Rodriguez, Ferri and Acosta 26 ). Three hundred and twenty-six participants were diagnosed to have dementia.
Data analysis
We employed a binary logistic regression model to calculate OR and their 95 % CI of dementia in participants with different levels of fish consumption in comparison to those with no fish consumption over the past two years. In the model, we adjusted for age, sex, province, urban/rural areas, education level, smoking status and stroke. The data analysis was conducted using the statistical software package IBM SPSS Statistics version 20.
Systematic literature review
Four authors (A.T.B., R.C., I.M.D. and W.Z.) independently searched and re-searched literature from the MEDLINE, PubMed, CINAHL, PsychINFO, and Psychology and Behavioural Sciences Collection databases. The strategy for the database search was developed using the PEO (Population, Exposure and Outcome) framework( Reference Moher, Liberati and Tetzlaff 27 ). The search terms were (‘dementia’ OR ‘Alzheimer’s disease’) AND (‘fish’). The literature was searched from the earliest date of each of the databases to 30 November 2016. The search for relevant articles included all studies with no language restriction. We read the title and abstract of the searched studies. The studies selected were appropriate for the current review if they investigated an association between fish consumption and dementia (or Alzheimer’s disease (AD)) in the population. Alongside the electronic database search, a manual reference search was also conducted to find additional articles missed by the online search. If two articles were published from the same cohort data but in different follow-up durations( Reference Kalmijn, Launer and Ott 28 , Reference Devore, Grodstein and van Rooij 29 ), we used the article from the longest follow-up study for review( Reference Devore, Grodstein and van Rooij 29 ). Figure 1 shows the study selection process. We identified eleven original studies eligible for review. Following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)( Reference Moher, Liberati and Tetzlaff 27 ) guidelines, four authors (A.T.B., I.M.D., W.Z. and G.Q.) conducted a systematic review. Each of the articles was reviewed by two reviewers and assessed independently using a predesigned data extraction form to extract the necessary information from the chosen studies. Differences in reviewing literature and extracting data between the two reviewers were resolved through face-to-face discussion; if differences remained, a third reviewer discussed with them to reach agreement. The quality assessment of the articles was achieved by employing the Newcastle–Ottawa scale( Reference Wells, Shea and O’Connell 30 ) and the AXIS tool( Reference Downes, Brennan and Williams 31 ).
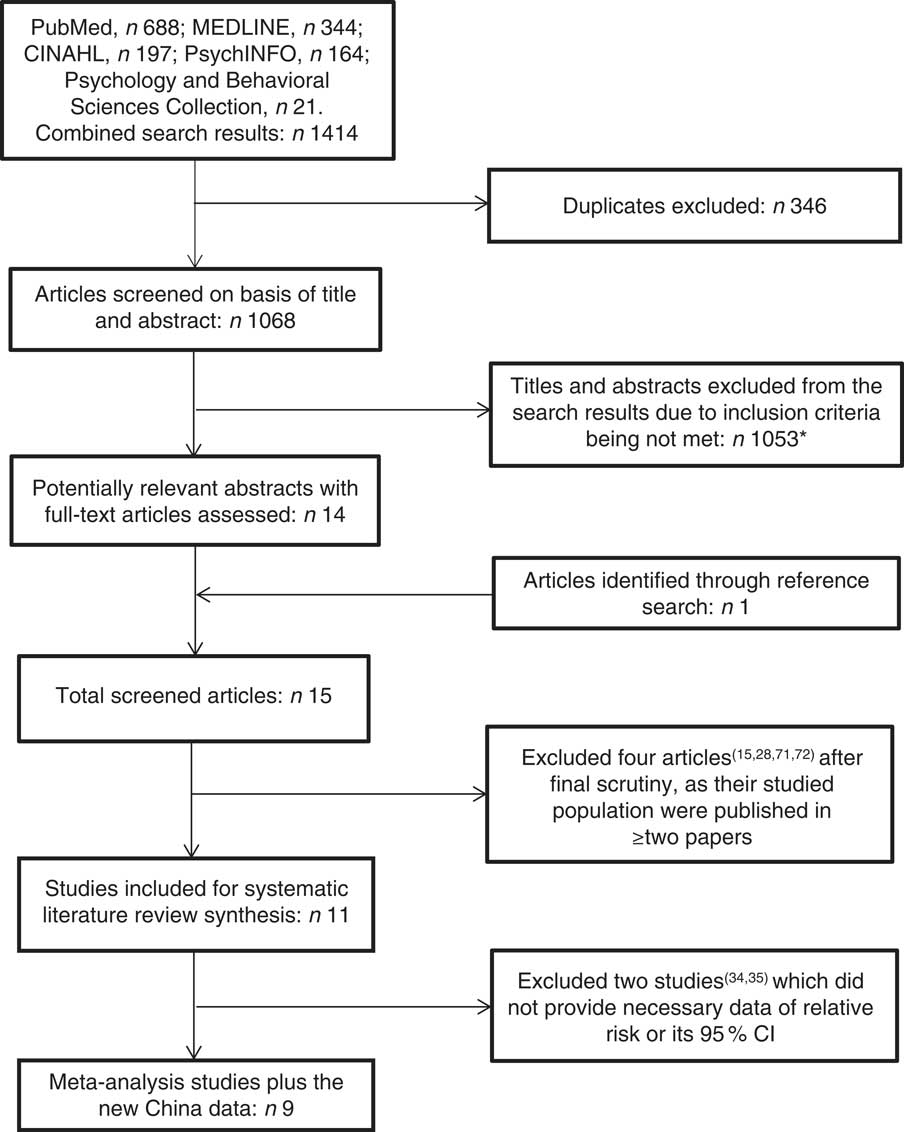
Fig. 1 Flowchart showing the literature search technique. *Reasons for exclusions: appropriate outcome not reported; randomized control trial; assessed another exposure other than fish; assessed another outcome other than dementia or Alzheimer’s disease; articles on importance of fish to dementia and brain development; news briefs; articles on elderly nutrition; literature review/meta-analysis; presentation
Meta-analysis
Data (odds ratios, rate ratios or hazard ratios and their 95 % CI) were pooled from published studies and the new study. All these measures and their 95 % CI were pooled together as a relative risk (RR) with the assumption of achieving a common unit of comparison. We analysed the data grouped by studied population in each of the studies which we selected to investigate all types of dementia in relation to fish consumption. The studied population was defined as each individual sample in the study according to its place (country, region), time (years) and person (e.g. ethnicity) where applicable. A random-effect model was employed if the heterogeneity of the within- and between-study variations was significant; otherwise, a fixed-effect model was used. Publication bias was evaluated using Egger’s regression( Reference Egger, Davey Smith and Schneider 32 ). First, we tried to assess an overall RR of dementia in participants who consumed fish in comparison with those who did not. If the article only gave the RR in different levels of fish consumption, we took the figure from the highest fish consumption group for analysis. If the article only gave the figure from the continuous data analysis of fish consumption or from just high v. low levels of fish consumption, we took them in the meta-analysis. Second, we stratified the identified studies for meta-analysis according to the number of groups of fish consumption measured at differing levels. This would help to examine differences in the RR among studies with different levels of fish consumption in their data analysis. Third, we investigated a dose–response association between fish consumption and risk of dementia according to low, middle and high consumption v. no/rare consumption. Where an article only gave the figure from the continuous data analysis of fish consumption or from just two groups of fish consumption (high v. low level), we took it in the middle level of fish consumption for the meta-analysis. If the article only provided the data of RR and 95 % CI from the middle and high levels of fish consumption v. no/rare consumption, we took them in the middle and high group levels for pooling the data. We examined any differences in the impact of fish consumption on the risk of dementia among LMIC and high-income countries. We also investigated any influence of the study design (cases–control studies, cross-sectional studies, cohort studies) and duration of the cohort follow-up on the association. We repeated above analyses for AD, where the data were available. All analyses were performed using the statistical software package STATA version 14.2.
Results
The six-provinces study in China
Of 7072 participants, 6981 (98·7 %) provided information on fish consumption. Their mean age was 72·2 (sd 7·6) years and 55·6 % were women. In total, 1528 participants (21·9 %) did not eat fish over the past two years, 2631 (37·7 %) consumed fish once weekly, 1938 (27·8 %) ≥twice weekly and 884 (12·7 %) ≥once daily. We examined the demographic characteristics of participants in each of these four groups (data not shown). Table 1 shows numbers, percentages and OR of dementia in participants with different levels of fish consumption. The risk of dementia decreased with increased consumption of fish, although participants who consumed fish ≥once daily had the highest prevalence of dementia. After adjusting for age, sex, stroke and other confounding factors, we found that participants with different levels of fish consumption had a reduced risk of dementia (details of OR shown in Table 1), but there seemed no significant ‘dose–response’ relationship. Participants with any level of fish consumption had a 27 % significant reduction in the risk of dementia (adjusted OR=0·73, 95 % CI 0·64, 0·99) in comparison with those who did not consume fish over the past two years.
Table 1 Numbers, percentages and OR (with 95 % CI) for dementia according to level of fish consumption: the six-province health survey in China conducted among 6981 Chinese adults aged ≥60 years, 2007–2011
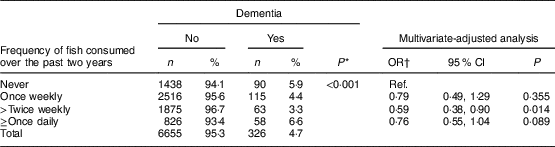
* P value from χ 2 test.
† Adjusted for age, sex, province, urban/rural areas, education level, smoking status and stroke.
Systematic literature review
In the eleven identified articles, we found that all were from high-income countries, except for one study led by the UK( Reference Albanese, Dangour and Uauy 33 ) which included seven studied populations from LMIC. They were published between 2002 and 2011. One of the studies was cross-sectional( Reference Albanese, Dangour and Uauy 33 ), three were case–control( Reference Conquer, Tierney and Zecevic 34 – Reference Kim, Nam and Oh 36 ) and seven were cohort( Reference Lopez, Kritz-Silverstein and Barrett-Connor 9 , Reference Barberger-Gateau, Letenneur and Deschamps 12 , Reference Morris, Evans and Bienias 13 , Reference Devore, Grodstein and van Rooij 29 , Reference Barberger-Gateau, Raffaitin and Letenneur 37 – Reference Schaefer, Bongard and Beiser 39 ). These articles included seventeen studied populations (one study( Reference Albanese, Dangour and Uauy 33 ) covered seven populations). Their sample size varied from fifty-seven to 14 956, with a total of 33 964 participants, and the minimum age in these studies’ populations varied from 55 to 76 years. An FFQ was used in four of the studies( Reference Barberger-Gateau, Letenneur and Deschamps 12 , Reference Morris, Evans and Bienias 13 , Reference Barberger-Gateau, Raffaitin and Letenneur 37 , Reference Huang, Zandi and Tucker 38 ), a semi-quantitative FFQ (SFFQ) was used in the other three( Reference Lopez, Kritz-Silverstein and Barrett-Connor 9 , Reference Kim, Nam and Oh 36 , Reference Schaefer, Bongard and Beiser 39 ). A meal-based check list alongside an SFFQ was used in one( Reference Devore, Grodstein and van Rooij 29 ), and the remaining one used a face-to-face standard method of assessment to evaluate the participant’s fish intake( Reference Albanese, Dangour and Uauy 33 ). Four of the studied populations reported a statistically significant association of fish consumption with reduced risk of dementia, although two of them( Reference Conquer, Tierney and Zecevic 34 , Reference Tully, Roche and Doyle 35 ) did not present the effect sizes. Data from eleven studied populations showed an association but a non-statistically significant reduction, while two exhibited no association (or increased risk)( Reference Devore, Grodstein and van Rooij 29 , Reference Albanese, Dangour and Uauy 33 ). Supplemental Tables 1 and 2 (see online supplementary material) document the details of the studies’ characteristics and outcomes. We examined the quality of each of these studies and found that the quality of these articles was in general good (Supplemental Table 3).
Meta-analysis
After excluding two studies that did not present the effect sizes( Reference Conquer, Tierney and Zecevic 34 , Reference Tully, Roche and Doyle 35 ), we took data from fifteen studied populations reported within nine published studies, and the data from the new study in China, for the meta-analysis. Figure 2 shows a forest plot of the findings of the association between fish consumption and dementia risk. In total, 3139 dementia cases in 40 668 participants were analysed. Data from these studied populations suggested little variability in the associated effects between studies, with only one study showing an increased risk (albeit not statistically significant) of dementia associated with higher fish consumption. The fixed-effect model analysis showed that there was a 20 % reduction in the risk of dementia in participants who consumed fish (or consumed fish at a higher level) compared with those who did not eat fish (or who consumed fish at a lower level). There was little evidence of publication bias; the Egger method of bias estimate showed a P value of 0·597 (see online supplementary material, Supplemental Fig. 1).
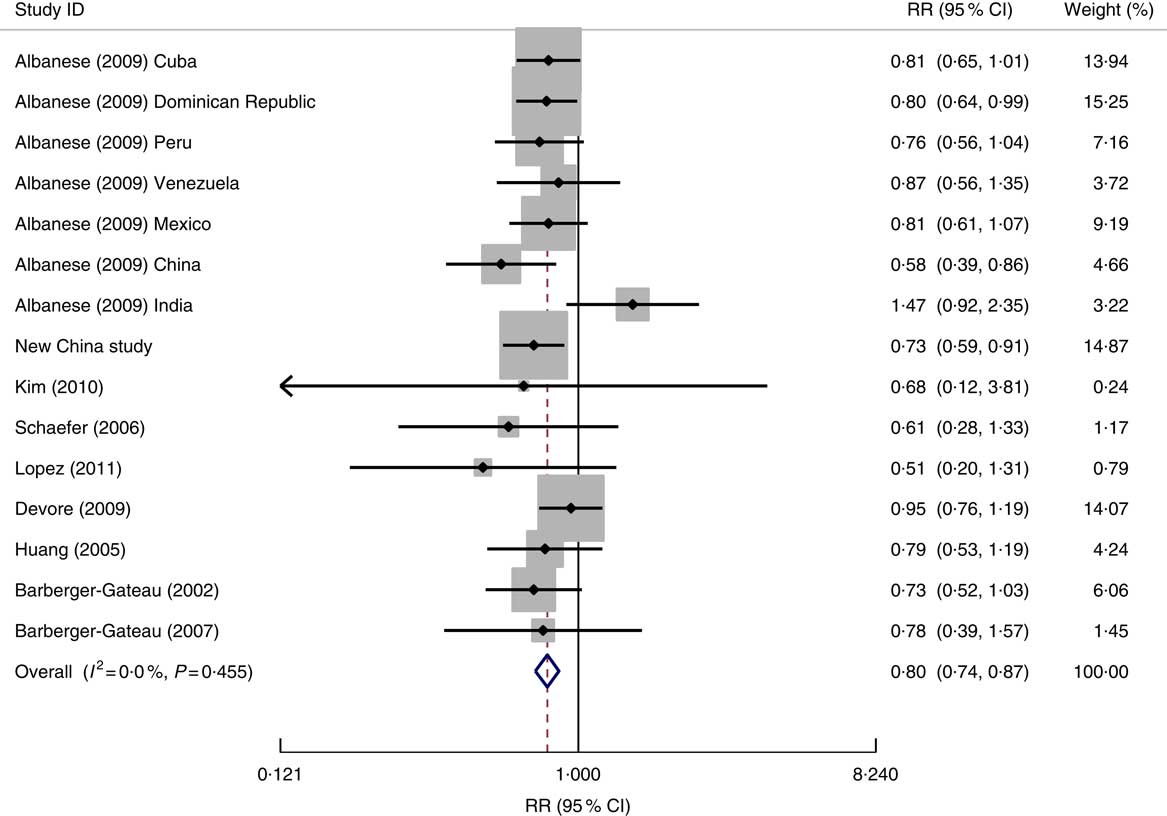
Fig. 2 Forest plot for the pooled relative risk (RR) of fish consumption and dementia* risk. The study-specific RR and 95 % CI are represented by the black diamond and the horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the open diamond and the vertical dashed line represent the pooled RR and the width of the open diamond represents the pooled 95 % CI. *One of the nine studies used for the meta-analysis (Morris (2003)( Reference Morris, Evans and Bienias 13 )) provided the RR result for Alzheimer’s disease only and therefore it was not included in the above analysis
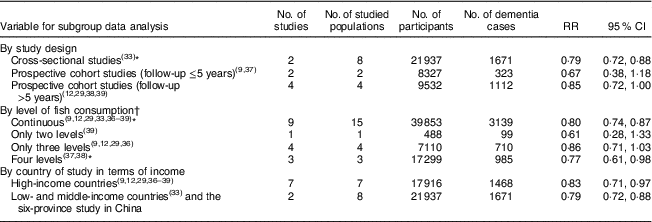
Data from different study designs or from different measures of fish consumption level showed no significant differences in RR for dementia risk in relation to fish consumption (Table 2). The association of fish consumption with dementia risk was similar between high-income countries (RR=0·83; 95 % CI 0·71, 0·97) and LMIC (RR=0·79; 95 % CI 0·72, 0·88; Table 2).
Table 2 Pooled analysis results for dementia risk in people with fish consumption v. those with no or lower levels of fish consumption, by study design, level of fish consumption and country of study in terms of income
RR, relative risk.
* Including the new community-based cross-sectional study of the six-province health survey in China.
† Fish consumption level: ‘continuous’ means that the authors analysed data of fish consumption for the results presentation; ‘only two levels’ means that the authors analysed the data of fish consumption in two levels, based on the questionnaire record or grouping them into two; ‘only three levels’ means that the authors analysed the data of fish consumption in three levels; and ‘four levels’ means that the authors analysed the data of fish consumption in four levels.
Of sixteen studied populations from nine articles and the new study in China for the meta-analysis, two( Reference Albanese, Dangour and Uauy 33 , Reference Barberger-Gateau, Raffaitin and Letenneur 37 ) showed a significant trend for a dose–response relationship. The pooled data showed a reduced RR of 0·84 (95 % CI 0·72, 0·98) for dementia in participants with a low level of fish consumption, RR of 0·78 (95 % CI 0·68, 0·90) with a middle level of fish consumption and RR of 0·77 (95 % CI 0·61, 0·98) with a high level of fish consumption (Table 3).
In all seven studied populations which examined the risk of AD specifically in relation to fish consumption( Reference Lopez, Kritz-Silverstein and Barrett-Connor 9 , Reference Barberger-Gateau, Letenneur and Deschamps 12 , Reference Morris, Evans and Bienias 13 , Reference Devore, Grodstein and van Rooij 29 , Reference Barberger-Gateau, Raffaitin and Letenneur 37 – Reference Schaefer, Bongard and Beiser 39 ), the pooled data (in total 1105 cases of AD) showed a significant impact of fish consumption on reduced risk of AD (RR=0·73; 95 % CI 0·65, 0·82; the forest plot is shown in Fig. 3). All studies were undertaken in high-income countries and were of cohort design. The patterns for the impact of fish consumption on reduced risk of AD (see online supplementary material, Supplemental Table 4) were similar to those in all dementia, and it may have a stronger dose–response relationship in comparison with those in dementia (see Table 3).
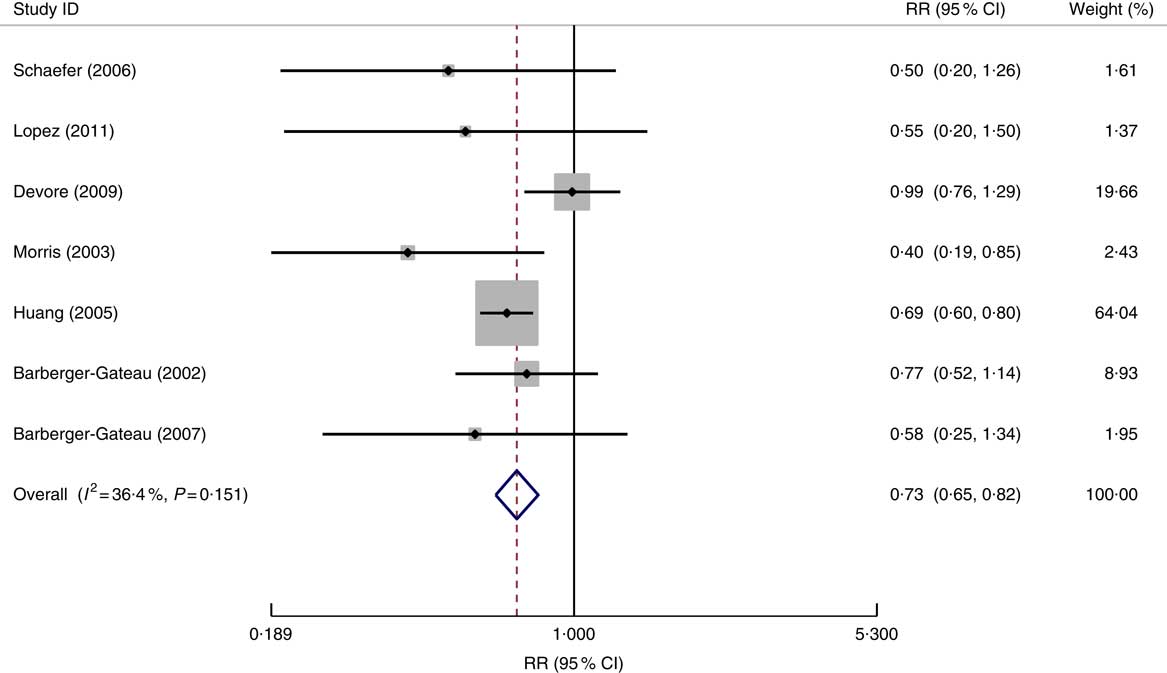
Fig. 3 Forest plot for the pooled relative risk (RR) of fish consumption and Alzheimer’s disease risk. The study-specific RR and 95 % CI are represented by the black diamond and the horizontal line, respectively; the area of the grey square is proportional to the specific-study weight to the overall meta-analysis. The centre of the open diamond and the vertical dashed line represent the pooled RR and the width of the open diamond represents the pooled 95 % CI
Table 3 Dose–response relationship between fish consumption and risk of dementia and Alzheimer’s disease (AD)Footnote *
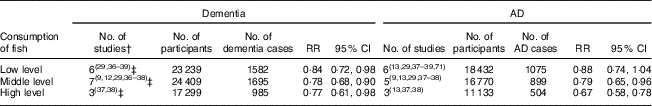
RR, relative risk.
* Each of low, middle and high levels of fish consumption v. no or lowest consumption of fish.
† The same number of studied populations.
‡ Including the new community-based cross-sectional study of the six-province health survey in China.
Discussion
Our study examined the data from a large-scale health survey of dementia prevalence and risk factors in China and completed a systematic worldwide literature review and meta-analysis to assess the association of fish consumption with dementia and AD risks in countries with different levels of income. We found that increased consumption of fish was significantly associated with a reduced risk of dementia and there was a stronger dose–response relationship between fish consumption and a reduced risk of AD.
The observed inverse association between the risk of dementia and fish consumption is biologically plausible. Fish is the major dietary source of n-3 PUFA, which comprise DHA and EPA, being collectively called the ‘fish fatty acids’( Reference Uauy and Dangour 40 , Reference Connor and Connor 41 ). Previous studies have suggested the preventive effect of fish consumption and its constituent n-3 fatty acids on CVD through inflammation reduction, blood pressure reduction and endothelial function enhancement( Reference Larsson and Orsini 3 ). Fish consumption has been shown to have a preventive effect on reducing the risks of CHD (RR=0·62; 95 % CI 0·46, 0·82)( Reference He, Song and Daviglus 2 ) and stroke (RR=0·94; 95 % CI 0·89, 0·99)( Reference Larsson and Orsini 3 ). These are co-morbidities associated with dementia( Reference Newman, Fitzpatrick and Lopez 42 ). Therefore, reducing these diseases may be one of the pathways for the preventive impact of fish consumption on dementia.
Strengths and limitations of the study
The main strength of the present paper is the inclusion of both original data from a large-scale health survey in China and data from all other relevant studies worldwide based on a systematic search and review. Older Chinese citizens have higher levels of socio-economic deprivation, but low levels of cardiovascular risk factors (e.g. obesity) and depression( Reference Chen, Wei and Hu 24 ). These special population characteristics of older Chinese residents helped to assess the association of fish consumption with the risk of dementia. Our systematic literature review and meta-analysis focused on determining the association between fish consumption and risk of dementia worldwide. The previous meta-analysis papers( Reference Zhang, Chen and Qiu 16 – Reference Cao, Tan and Wang 18 ) investigated the associations of both fish and n-3 PUFA with combined mild and severe cognitive impairment (e.g. mild cognitive impairment (MCI), Parkinson disease, all-type dementia and AD), not specifying exposure or outcomes, and failed to include some relevant studies( Reference Lopez, Kritz-Silverstein and Barrett-Connor 9 ). In comparison with those previous reviews and meta-analyses( Reference Zhang, Chen and Qiu 16 – Reference Cao, Tan and Wang 18 ), our systematic review and meta-analysis elaborated specifically on the impact that the consumption of fish has on dementia and AD development. Our findings were based on a literature search without any limited selection and identified all eligible studies, further including a new study from China (LMIC) which compensated for the scarce data from LMIC generally. Adding in the new community-based cross-sectional study from China made our meta-analysis findings more robust and generalizable.
Our study has several potential limitations. First, the six-province health survey data were cross-sectional, and the causal relationship between fish consumption and dementia risk could not be assessed. However, the findings of the six-province study were similar to those in the cohort studies( Reference Lopez, Kritz-Silverstein and Barrett-Connor 9 , Reference Barberger-Gateau, Letenneur and Deschamps 12 , Reference Morris, Evans and Bienias 13 , Reference Devore, Grodstein and van Rooij 29 , Reference Barberger-Gateau, Raffaitin and Letenneur 37 – Reference Schaefer, Bongard and Beiser 39 ). Second, like the majority of previous studies, in the six-province survey we did not have information on different types (lean, fatty fish, fried fish and seafood) and amounts of fish consumed, which may hinder our inferences on specific types of fish and dementia. But overall, total fish consumption was significantly and inversely associated with dementia risk. We need further studies on specific types of fish consumption in relation to reduced dementia risk to warrant making more informative recommendations to the public. Third, the identified studies used different levels of fish consumption for data analysis, making it difficult to assess the presence of a dose–response relationship between fish consumption and dementia risk. Using the RR data from the group with the highest level of fish consumption in some studies may be overestimating the overall effect of fish consumption on dementia risk. However, when stratifying the articles for meta-analysis according to the number of groups of fish consumption level, we did not find that there was a trend of reduced risk of dementia or AD with increased number of fish consumption level groups (Table 2 and online supplementary material, Supplemental Table 4). If we included all RR from different levels of fish consumption to pool the data (Supplemental Fig. 2), the finding of the overall impact was not substantially changed (RR=0·80; 95 % CI 0·75, 0·87).
In the current systematic literature review, we noted that these eleven identified articles plus the new study in China had various study designs, different locations and various types of FFQ to measure their fish intake. As the studies included in our meta-analysis were observational, the outcome of the current study was examined using the review guidelines of Bradford Hill( Reference Hill 43 ) to provide evidence of a direct and causal relationship between fish consumption and risk of dementia and/or AD.
How strong are the associations?
The majority of the identified studies showed a moderate to high association of fish consumption with reduced risk of dementia( Reference Lopez, Kritz-Silverstein and Barrett-Connor 9 , Reference Barberger-Gateau, Letenneur and Deschamps 12 , Reference Morris, Evans and Bienias 13 , Reference Kim, Nam and Oh 36 – Reference Schaefer, Bongard and Beiser 39 ) after adjusting for possible confounders. Only one study showed a weak or no association between fish consumption and the risk of dementia( Reference Devore, Grodstein and van Rooij 29 ). Our pooled data analysis showed a 20–30 % increase in the risk of dementia and AD in people who did not eat fish in comparison with those who did. The magnitude of the association between fish consumption and the risk of dementia is similar to the impacts of environmental tobacco smoke on the incidence of CHD (25 % increased risk( Reference He, Vupputuri and Allen 44 )) and on lung cancer (27 % increased risk( Reference Taylor, Najafi and Dobson 45 )), and both have been taken as having a causal relationship with environmental tobacco smoke exposure.
How consistent are the reported studies?
Of the seventeen studied populations in the current review, fifteen reported a reduction in the risk of dementia with a moderate to high intake of fish after adjusting for possible confounders( Reference Lopez, Kritz-Silverstein and Barrett-Connor 9 , Reference Barberger-Gateau, Letenneur and Deschamps 12 , Reference Morris, Evans and Bienias 13 , Reference Conquer, Tierney and Zecevic 34 , Reference Tully, Roche and Doyle 35 , Reference Barberger-Gateau, Raffaitin and Letenneur 37 – Reference Schaefer, Bongard and Beiser 39 ). Two of the studies also showed a significant inverse association of fish consumption with the risk of mild to severe dementia and AD development, when the plasma phospholipids and the serum of the AD participants were assessed for their DHA and EPA levels( Reference Conquer, Tierney and Zecevic 34 , Reference Tully, Roche and Doyle 35 ). A significant reduction was also observed in the six-province study from China. A consistent inverse association between fish consumption and dementia risk was observed in all seven countries that took part in the 10/66 dementia research group study, except India( Reference Albanese, Dangour and Uauy 33 ). Our meta-analysis for these reviewed studies showed a high level of homogeneity, suggesting their consistent data.
Moreover, there are similar findings of the impact of fish consumption on cognitive function in children. Cohen et al.( Reference Cohen, Bellinger and Connor 46 ) analysed the data of a randomized control trial (RCT) and demonstrated a 0·13-point increase in the IQ (intelligence quotient) of children when mothers received a DHA supplement of 100 mg/d. A review by Eilander et al. ( Reference Eilander, Hundscheid and Osendarp 7 ) established enhanced cognitive development in infants and children after maternal supplementation with long-chain n-3 PUFA during pregnancy and lactation, although they had inadequate evidence for an association with children over 2 years old. Ryan et al. ( Reference Ryan, Astwood and Gautier 47 ) also indicated in their review that neurocognitive development during childhood is enhanced when pregnant and lactating mothers are supplemented with DHA. These would support our findings of the impact of fish consumption on reduced risk of dementia.
How specific are the proposed fish consumption and the response to outcome?
Of these identified articles, a few studies( Reference Barberger-Gateau, Letenneur and Deschamps 12 , Reference Barberger-Gateau, Raffaitin and Letenneur 37 , Reference Huang, Zandi and Tucker 38 ) investigated the fish intake based on fatty, lean, fried fish and seafood. The varying consumption of these types of fish might have affected the outcome of these studies. Huang et al. ( Reference Huang, Zandi and Tucker 38 ) revealed a 28 % reduction in the risk of developing dementia with the intake of fatty fish, while the consumption of lean fried fish produced no significant beneficial effect. The two major fish fatty acid constituents (DHA and EPA) were associated with a reduced risk of developing dementia and cognitive decline( Reference Wu, Ding and Wu 17 , Reference Dangour, Allen and Elbourne 48 ). The dose–response impact of fish consumption on specific dementia, i.e. AD, seemed to be stronger.
Is there a temporal relationship between exposure and response?
The observed association between fish consumption and dementia was prominent in all of the prospective cohort studies( Reference Lopez, Kritz-Silverstein and Barrett-Connor 9 , Reference Barberger-Gateau, Letenneur and Deschamps 12 , Reference Morris, Evans and Bienias 13 , Reference Devore, Grodstein and van Rooij 29 , Reference Barberger-Gateau, Raffaitin and Letenneur 37 – Reference Schaefer, Bongard and Beiser 39 ), demonstrating a temporal association which signified that an exposure preceded the outcome. In the USA, Huang et al. ( Reference Huang, Zandi and Tucker 38 ) followed up 2233 participants for 5·4 years and identified 378 new cases of dementia; the RR in participants with fish consumption was 0·79 (95 % CI 0·53, 1·20). The Rotterdam Study followed up 5395 participants for 9·6 years and observed that 465 dementia cases developed, showing an RR of 0·95 (95 % CI 0·76, 1·19) for dementia in relation to fish consumption( Reference Devore, Grodstein and van Rooij 29 ). The pooled data of RR between short- and long-term follow-up studies were similar (Table 2).
Is there an exposure–response relationship?
An exposure–response relationship was identified between different levels of fish consumption and risks of dementia and AD in our meta-analysis. The majority of identified studies( Reference Lopez, Kritz-Silverstein and Barrett-Connor 9 , Reference Barberger-Gateau, Letenneur and Deschamps 12 , Reference Morris, Evans and Bienias 13 , Reference Devore, Grodstein and van Rooij 29 , Reference Albanese, Dangour and Uauy 33 , Reference Kim, Nam and Oh 36 – Reference Schaefer, Bongard and Beiser 39 ) showed this, with non-statistical significance. Morris et al. ( Reference Morris, Evans and Bienias 13 ) demonstrated a non-significant dose–response relationship of AD with fish consumption; RR=0·6 (95 % CI 0·3, 1·3) in participants who consumed fish 1–3 times monthly, RR=0·4 (95 % CI 0·2, 0·9) in those who consumed fish once weekly and RR=0·4 (95 % CI 0·2, 0·9) in those consuming ≥twice weekly (trend P=0·07). However, other cohort studies( Reference Barberger-Gateau, Letenneur and Deschamps 12 , Reference Barberger-Gateau, Raffaitin and Letenneur 37 , Reference Schaefer, Bongard and Beiser 39 ) showed that the reduced risk of dementia was not significant in the highest level of fish consumption. This may be due to the small number of patients in these groups. Nevertheless, the pooled data in our meta-analysis (Table 3) across all the different levels of fish intake from the included studies showed a significant reduction in the risk of dementia (online supplementary material, Supplemental Fig. 2) and AD.
Is the association biologically plausible?
The biological mechanism exhibited by fish consumption in relation to the prevention of dementia may result from the presence of n-3 fatty acids as part of their constituents. n-3 Fatty acids are a major component of neuronal membranes, with cardioprotective, anti-inflammatory, antioxidant and anti-atherogenic properties( Reference Uauy and Dangour 40 , Reference Innis 49 , Reference Calder 50 ). They have the capability to display a beneficial effect on the risk of developing dementia and AD, particularly vascular dementia( Reference Engelhart, Geerlings and Ruitenberg 15 , Reference Kalmijn, Launer and Ott 28 , Reference Barberger-Gateau, Raffaitin and Letenneur 37 ). Fish is a beneficial source of essential amino acids, micronutrients and vitamins, thus increasing the protective effect they exhibit on the risk of developing all-cause dementia and cognitive impairment( Reference Chandra 51 ). Fatty fish are known to be richer sources of DHA and EPA, which are naturally found in trout, tuna, salmon, sardine, herring( Reference Mohanty, Ganguly and Mahanty 52 ) and mackerel, but minimal sources are found in lean fishes, such as cod, haddock and halibut. An increase in the intake of fatty fish may also be positively associated with a decrease in the level of consumption of saturated fat, thus reducing the risk of stroke( Reference Larsson and Orsini 3 ). This might be as a result of the anti-inflammatory, antithrombotic, antioxidant and anti-amyloid properties of its n-3 fatty acid components( Reference Connor and Connor 41 , Reference Innis 49 , Reference Calder 50 ).
Is the evidence coherent with knowledge of the natural history of disease?
Dietary fatty acids have displayed a significant effect on the risk of developing CVD( Reference Connor and Connor 41 , Reference Connor 53 , Reference Nestel 54 ) and depression( Reference Grosso, Micek and Marventano 55 ) and in children’s cognitive impairment( Reference Eilander, Hundscheid and Osendarp 7 , Reference Gould, Smithers and Makrides 56 ). This association involves the higher consumption of saturated fat and cholesterol and the lower consumption of PUFA (n-3 fatty acids). Intake of n-3 fatty acids has been associated with reduced risk of cognitive impairment and dementia through several possible mechanisms. They display a cardioprotective property that makes them protective over several cardiovascular risk factors such as stroke, atherosclerosis and inflammation through influence on brain development and proper membrane function( Reference Kalmijn, Launer and Ott 28 , Reference Huang 57 ). They have exhibited their cognitive-enhancing effect during infancy, childhood, old age and among adults with neurocognitive impairments in some clinical trials( Reference Huang 57 , Reference Luchtman and Song 58 ). This beneficial effect was supported by the outcome of the Chicago Health and Aging 6-year prospective cohort study (CHAP) that involved fish intake and cognitive impairment( Reference Morris, Evans and Tangney 10 ), and in the result revealed in the Zutphen Elderly 5-year prospective cohort study of fish consumption, n-3 fatty acids and cognitive decline( Reference van Gelder, Tijhuis and Kalmijn 11 ). The China Health and Nutrition Survey also maintained that an adequate intake of fish does lower cognitive decline( Reference Qin, Plassman and Edwards 59 ).
Is there experimental evidence?
Numerous animal studies have demonstrated the positive role that n-3 fatty acids (a fish constituent) play on brain development. They increase neurotransmission( Reference Horrocks and Farooqui 60 ), enhance memory capabilities( Reference Hashimoto, Tanabe and Fujii 61 ), enhance the excitability regulation of neuronal membranes( Reference Xiao and Li 62 ), decrease neurons’ ischaemic damage( Reference Okada, Amamoto and Tomonaga 63 ) and increase the cerebral flow of blood( Reference Tsukada, Kakiuchi and Fukumoto 64 ). Experimental studies showed that rats that had a reduced level of DHA in their diet exhibited an impaired cognitive function, while those animals that had a prolonged administration of DHA demonstrated an enhanced gain in memory( Reference Gamoh, Hashimoto and Sugioka 65 ). These studies confirmed that the exposure of animal models to the intake of DHA positively influenced their neurological status.
Does the evidence accord by analogy with that from other fields?
Previous studies showed a significant beneficial effect of intake of n-3 fatty acids as a supplement on dementia and cognitive impairment( Reference Morris, Evans and Tangney 10 , Reference van Gelder, Tijhuis and Kalmijn 11 ). Findings from an RCT that involved supplementing the treatment group with arachidonic acid and DHA, components of fish fatty acids, did exhibit a significant beneficial effect on cognitive function in the treatment MCI group, while the placebo group showed no significant beneficial effect( Reference Kotani, Sakaguchi and Warashina 66 ). A similar beneficial effect was observed among an MCI group in an RCT of forty-six participants (twenty-three with mild or moderate AD and twenty-three with MCI) who were randomized to receive either an n-3 PUFA treatment or olive oil (placebo)( Reference Chiu, Su and Cheng 67 ). In a 1-year RCT that investigated the effects of fish oil supplementation on cognitive function in older adults, Lee et al. ( Reference Lee, Shahar and Chin 68 ) found a significant beneficial effect within a short term and after a 12-month period on participants’ working memory, immediate verbal memory and in the delayed recall ability among the treatment group that was supplemented with fish oil. The results of the current study are thus consistent with the findings of these studies, thereby acknowledging the positive influence that fish and its constituents has on cognitive function.
Implication of the study findings
Our study demonstrated a significant beneficial effect of eating fish on reducing dementia. The epidemic of dementia has become a public health problem worldwide. As the world population is continuing to age, the number of people with dementia will continue to rise. The vast majority of the increment is expected to be in LMIC, which currently hold 58 % of people living with dementia, with a further increment by the year 2050( Reference Prince, Wimo and Guerchet 1 ). In China, there is a growing number of people living with dementia due to the population of older people with mixed characteristics (e.g. low level of education but rapidly increased income)( Reference Chen, Ma and Wilson 69 ). Our study demonstrated a significant association of higher fish consumption with reduced risk of dementia, which further indicates the potential importance of consuming fish in preventing dementia worldwide. At present, global per capita fish consumption is estimated to be on average 20 kg/year( 70 ), and is lower in LMIC (18·8 kg/year) than in high-income countries (26·8 kg/year). Our study demonstrated consistent findings of the impact of fish consumption on the risk of dementia between LMIC and high-income countries. People should thus increase their level of fish consumption, especially in areas where the consumption is quite low such as LMIC, to reduce the burden of dementia. Also, people living in high-income countries, including the UK, should be informed of the beneficial impact of fish consumption to increase its intake further.
Acknowledgements
Acknowledgements: The authors thank the participants and all who were involved in the six-province study in China. Financial support: R.C. and L.W. thank the BUPA Foundation and Alzheimer’s Research UK for providing research grants for the Research Programme of Dementia in China, collecting the six-province health survey data. R.C. and A.T.B. thank the Faculty of Education, Health and Wellbeing, University of Wolverhampton for the provision of QR travel grants to support their presentation of parts of the study at the 4th International Conference on Epidemiology and Public Health, London, 3–5 October 2016 and the Alzheimer’s Research UK Conference, Aberdeen, UK, 14–15 March 2017. The funders had no role in the design, analysis or writing of this article. Conflict of interest: The authors have no competing interests to declare. Authorship: A.T.B.: searching and reviewing the topic-related literature, carrying out the meta-analysis, and drafting and revising the manuscript. R.C.: study concept and design, study supervision, and drafting and revising the manuscript. J.W.: study concept, coordination and data collection and analysis design of the Chinese study. L.W. and J.N.: analysing the data of the Chinese study, interpreting the findings, and commenting on the manuscript. G.Q., I.M.D. and W.Z.: literature search and reviewing the identified articles. R.K., T.S., P.S., A.C., A.V. and C.Z.: critically reviewing the manuscript, interpreting the data, and revising the manuscript. All authors checked and interpreted the results and approved the final version. R.C. is the guarantor for the study. Ethics of human subject participation: Ethical approval for the six-province study was obtained from the Research Ethics Committee of Anhui Medical University and the local governments in China, from the Research Ethics Committee of University College London, and the Research Ethics Committee of the Faculty of Education, Health and Wellbeing, University of Wolverhampton, UK.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S136898001800037X









