Introduction
Pregnancy is associated with profound changes in thyroid function and the requirement for iodineReference Glinoer1. Under the influence of a rise in the concentration of oestrogen, there is a marked increase in the concentration of serum thyroxine-binding globulin, which begins during early gestation, reaches a plateau at mid-gestation and is maintained thereafter. Also, starting in early gestation, there is an increase in renal blood flow and glomerular filtration, which leads to an increased iodide clearance from the plasma and thus to an obligatory loss of iodine. Occurring transiently near the end of the first trimester, there is direct stimulation of the thyroid gland by an increase in the concentration of human chorionic gonadotrophin that may lead temporarily to a slightly increased concentration of free thyroxine. Finally, significant changes occur in the peripheral metabolism of maternal thyroid hormones during the second half of gestation, mainly under the influence of placental type 3 iodothyronine deiodinaseReference Bianco, Salvatore and Gereben2.
Together, these events represent profound metabolic changes associated with the first half of gestation that constitutes a transition from a preconception steady-state thyroid gland to a pregnancy steady-state thyroid glandReference Glinoer3. In order for such metabolic changes to happen, this needs an increase in hormone production by the maternal thyroid gland of about 50%. As Fig. 1 shows, once the new equilibrium has been reached, the increased demand for hormones during pregnancy is sustained until full term. For a healthy pregnant woman with a sufficient iodine intake before conception of about 150 μg day− 1, the challenge for the thyroid gland is to adjust its hormonal output to achieve a new equilibrium and then maintain it until pregnancy is completed: this corresponds to the physiological adaptation of the thyroid economy to the pregnant state. However, when pregnancy takes place in healthy women who live in areas with an inadequate iodine intake of approximately 50–75 μg day− 1, physiological adaptation is progressively replaced by pathological alterations. Pregnancy typically acts therefore to reveal the underlying lack of iodine: the more severe the iodine deficiency, the more pronounced are the consequences for the maternal and foetal thyroid glandsReference Glinoer4.

Fig. 1 A conceptual model of the changes in hormones that occur during pregnancy. E2, oestrogens; hCG, human chorionic gonadotrophin; TBG, thyroxine-binding globulin; T4, thyroxine; GA, gestational age.
Metabolism of iodine during pregnancy
After being reduced to iodide, dietary iodine is rapidly absorbed from the gut. Iodide of dietary origin then mixes rapidly with iodide derived from the peripheral catabolism of iodothyronines and together they constitute the extra-thyroid pool of plasma inorganic iodide (PII), which exists in a dynamic equilibrium with two organs, the thyroid gland and the kidneys. A normal adult uses approximately 80 μg iodide a day to produce thyroid hormones and the system is balanced to fulfil these daily needs. When the iodine intake by non-pregnant women is adequate (~150 μg day− 1), a kinetic balance is achieved by thyroid uptake of 35% of the available iodine. Of the 80 μg hormonal iodide produced daily by thyroid hormone catabolism, 15 μg iodide is lost in the faeces, leaving 65 μg to be redistributed between the thyroid gland and irreversible urinary losses. In these conditions, the metabolic balance remains in equilibrium and the body is able to maintain a plentiful store of iodine in the thyroid ranging from 10 to 20 mg. In contrast, when iodine intake is restricted before the onset of a pregnancy to approximately 70 μg day− 1 or less, the body must increase iodide trapping through the pituitary–thyroid feedback mechanism in order to maintain the necessary absolute iodine intake. In such conditions, there is a shortfall of iodine of approximately 10 μg day− 1 and the thyroid gland uses stored iodine, which is therefore progressively depleted to low amounts of 2–5 mg stable iodine. Over time, if the nutritional situation remains unchanged, the metabolic balance of iodine becomes negative.
Two fundamental changes take place during pregnancy. There is a significant increase in renal iodide clearance by approximately 30–50% and, concomitantly, a sustained increase in thyroid hormone production by 50%, from 80 to 120 μg hormonal iodide a day. Since the renal iodide clearance already increases in the first weeks of gestation and persists thereafter, this constitutes an obligatory iodine ‘leakage’ which tends to lower the circulating PII concentration and, in turn, induces a compensatory increase in the thyroidal clearance of iodide. These mechanisms underscore the increased physiological activity of the thyroid gland during the first half of pregnancy. Calculations show that in such conditions, there is a shortfall of about 20 μg iodine a day. As Fig. 2 shows, in order to sustain an increased production of thyroid hormones, the glandular machinery must draw iodine from already depleted thyroid stores. This is the rationale for the excessive stimulation of the thyroid gland observed during a pregnancy that takes place in iodine-deficient conditions. The consequences of this are relative hypothyroxinaemia, preferential secretion of tri-iodothyronine, an increased concentration of serum thyroid-stimulating hormone as well as serum thyroglobulin and, finally, increase in thyroid volume leading to goitreReference Glinoer5.
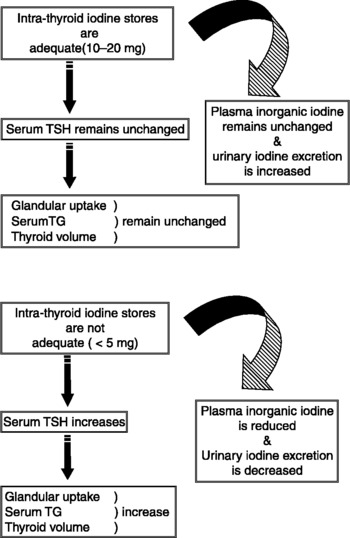
Fig. 2 A conceptual model of iodine nutrition and thyroid function when iodine stores are adequate (upper diagram) or not adequate (lower diagram). TSH, thyroid-stimulating hormone; TG, thyroglobulin.
Goitre during pregnancy and iodine deficiency
In countries such as the United States of America (USA), Japan and in some European countries where efficient national programmes of dietary iodine supplementation have been in place for many years, iodine deficiency disorders are believed not to present a problem. This view, however, is probably too optimistic. For instance, a recent survey in the USA showed that the average iodine intake had decreased markedly during the period from 1988 to 1994 compared with a similar survey carried out two decades earlier, between 1971 and 1974: the median urinary iodine excretion in the most recent survey was 145 μg l− 1, compared with over 300 μg l− 1 beforehandReference Hollowell, Staehling, Hannon and Flanders6 (see paper by Hollowell and Haddow, this issue). Even though this level of iodine excretion in the USA may be considered to represent an almost ideal intake, the survey showed that 15% of women of child-bearing age and approximately 4–8% of pregnant women had a urinary iodine concentration of less than 50 μg l− 1, indicating a moderate iodine deficiency.
An important epidemiological consequence is that the risk of iodine deprivation during pregnancy needs to be assessed locally and monitored over time, because mild to moderate iodine deficiency may occur in areas that are not immediately recognised as iodine deficient. The population of the city of Toulouse in south-west France was not known to be particularly iodine deficient because of their relative proximity to the sea and their fish-eating habits. Nevertheless, a study performed in a cohort of pregnant women in this city clearly showed that urinary iodine excretion was too low, with over 75% of pregnant women having an iodine concentration in their urine of less than 100 μg per lReference Caron, Hoff, Bazzi and Dufor7.
Another important epidemiological concept relates to the notion of unexpected geographical variation in iodine intake within a given country, because iodine deficiency in general, mild to moderate iodine deficiency more specifically, may frequently show significant variations from one place to another. In a study carried out in Denmark, it was shown that pregnant women in Copenhagen, who were not taking iodine supplements, had a median urinary iodine concentration of 62 μg g− 1 creatinine compared with only 33 μg g− 1 creatinine in similar women in JutlandReference Nohr, Laurberg, Borlum and Pedersen8. Furthermore, these striking differences were not alleviated in pregnant women from the same areas who had received iodine supplements, indicating that the supplementation was not sufficient, presumably because the iodine supplements were entirely taken up by the women's iodine-deprived thyroid glandsReference Nohr, Laurberg, Borlum and Pedersen8.
Iodine deficiency during pregnancy typically becomes significant when the iodine intake falls below 100 μg daily. The most recent recommended dietary allowance for pregnant and lactating women is 250 μg per day9. The degree of iodine deficiency should therefore be assessed in each specific locality and the situation correctly evaluated before embarking on medical recommendations for iodine supplementation programmesReference Dunn and Delange10, Reference Delange11.
Goitre formation is the most directly visible consequence of iodine deprivation, and pregnancy should therefore be viewed as an environmental factor that triggers the glandular machinery and induces functional and anatomical abnormalities of the thyroid gland in localities with a low iodine intake. Several studies in Europe have indicated that significant changes in thyroid volume occur in association with pregnancy (reviewed in 1). These studies showed that pregnancy was associated with goitre formation in places where people were known to have a low iodine intake, with increases in thyroid volume ranging between 20 and 35%Reference Glinoer1.
It is also important to note that it is not only the mother but also the foetus who is at risk of developing glandular hyperplasia when an infant is born to a mother who has not been supplemented with iodine during gestationReference Glinoer, De Nayer, Delange and Lemone12. Thus, pregnancy represents a strong goitrogenic stimulus for both the mother and the foetus, even in areas where there is only a moderate iodine deficiency. Maternal goitre formation is correlated with the degree and duration of glandular stimulation that takes place during gestation. In addition, a goitre formed during gestation may only partially regress after parturition, so that pregnancy is one of the factors that may help explain the higher prevalence of goitre and thyroid disorders in women compared with men. Finally and perhaps most importantly, since goitre formation also takes place in the progeny, this emphasises the exquisite sensitivity of the foetal thyroid gland to the consequences of maternal iodine deprivation. In conditions of iodine deficiency, the process of goitre formation may start during the earliest stages of foetal thyroid gland development.
Prevention of gestational goitre formation by iodine supplementation
To prevent gestational goitrogenesis, women should ideally be provided with an adequate iodine intake of 150 μg day− 1 long before they become pregnant, because it is only by reaching a long-term steady state with plentiful iodine stored in the thyroid gland, that the triggering of the thyroid can be avoided once gestation begins. To achieve such a goal, national public health authorities need to develop iodine supplementation programmes for the diet of the whole population. In the mean time, the most appropriate preventive and therapeutic approach to avoid gestational goitrogenesis is to systematically increase the iodine supply as soon as possible during gestation and to continue after parturition, particularly for mothers who breast-feed. This can be achieved using iodised salt (albeit obviously limited during gestation) or by multivitamin pills containing appropriate amounts of iodine. How much supplementary iodine should be given to prevent goitre formation remains a local decision, since it depends mainly on the extent of the pre-existing iodine deprivationReference Delange and Lecomte13, Reference Glinoer14. The ultimate goal is rapidly to restore and maintain a balanced iodine status: this goal can be reached in most instances by giving 100–200 μg iodine daily as a supplement during pregnancy.
Pregnancy and severe iodine deficiency
In many regions of the world, iodine deficiency is not only overtly present, but also often severe. People in large parts of Central Africa and Asia still have iodine intakes today that are below 25 μg per dayReference Hetzel15, Reference Delange16. In such regions, the thyroid status of pregnant women and their offspring is frequently impaired. In addition, other factors, such as selenium deficiency and thiocyanate excess, combine with severe iodine deficiency to complicate the situation even further. In terms of thyroid function, the adult populations usually exhibit a mixed pattern encompassing subjects with a normal thyroid function and others who present with various degrees of hypothyroidism. In women of child-bearing age, a severe iodine deficiency and hypothyroidism play a role in reducing fertility and increasing the rate of spontaneous abortions. When these women become pregnant, their thyroid function tends to deteriorate even further as gestation progresses. Thus, the stress to the thyroid associated with pregnancy that was discussed above in conditions where there is mild or moderate iodine deficiency, such as frequently observed in Europe, cannot be quantitatively compared with the repercussions for the thyroid gland observed in countries with severe iodine deficiency. Owing to the obvious difficulties inherent in doing careful field studies in most areas with a severe iodine deficiency, there have been only a few studies of thyroid function and no systematic study to assess changes in goitre size in pregnant women. Until a few years ago, it was not feasible to obtain echographic measurements of the thyroid gland on a large and representative sample; it was even more difficult to investigate prospectively goitrogenic changes during pregnancy. This situation is now rapidly changing because of the possibility of adapting ‘Thyromobil’ technology to large field studies in remote areasReference Djokomoeljanto, Setyawan, Dramaix and Hadisaputro17, Reference Rossi, Tomimori, Camargo and Medeiros-Neto18. In women of child-bearing age and during pregnancy, iodine supplements have been administered in the form of iodised salt, potassium iodide drops or in the form of iodised oil given intramuscularly or orally as an emergency prophylactic and therapeutic approach in areas with severe iodine deficiency complicated by endemic cretinism. Several such programmes have conclusively demonstrated remarkable efficiency in treating endemic goitre, as well in eradicating endemic cretinism. The results of these studies have proved that the pregnancies of women who live in regions that are severely iodine deficient can be managed adequately using iodine supplements. Except for such emergency situations, there is presumably no need to use supra-physiological amounts of iodine to improve thyroid function parameters significantly.
Foetal–neonatal consequences of maternal iodine deficiency and thyroid impairment
Iodine is required for the synthesis of thyroid hormones, and thyroid hormones are crucial for the development of the brain both during foetal and early postnatal life. When severe enough, iodine deficiency may induce maternal and foetal hypothyroxinaemia from early gestation onwardsReference Glinoer and Delange19. An impairment to the availability of thyroid hormones during critical periods of brain development may lead to irreversible brain damage, with mental retardation and neurological abnormalities, such as cretinism, that ultimately depend upon the timing and severity of insult to the brainReference Morreale de Escobar, Obregon and Escobar del Rey20, Reference Calvo, Jauniaux, Gulbis and Asuncion21. The characteristic neurological picture of endemic cretinism is presumably directly due to damage to the developing brain, some of which occurs during the first trimester, but mostly during the second. Endemic cretinism therefore constitutes an extreme in the range of abnormalities that occur in the physical and intellectual development of children, as well as the diminished functional capacity of the thyroid gland that is observed in inhabitants of areas with severe iodine deficiency. In a meta-analysis of 18 studies conducted in areas with severe iodine deficiency, it was estimated that iodine deficiency was responsible for a deficit in IQ of 13.5 pointsReference Bleichrodt, Born and Stanbury22.
Neurological and intellectual deficits associated with iodine deficiency are not limited to people known to be severely affected by iodine deficiency and, in recent years, the impact of mild to moderate iodine deficiency on the foetus has also been recognised. In studies conducted in areas with only moderate or even mild iodine deficiency, mainly in southern Europe, developmental abnormalities have been shown to occur in clinically euthyroid school-aged childrenReference Vermiglio, Sidoti, Finocchiaro and Battiato23–Reference Vitti, Aghini-Lombardi, Antonangeli and Rago25. Even when an iodine deficiency is borderline, as observed in many European countries, impaired school achievements may occur in apparently normal children. Iodine deficiency is one of the most prevalent causes of mental retardation in children that could easily, at least in theory, be prevented and eliminated by adequate iodine supplementation. Any thyroid deficit that occurs during a crucial stage in the neurological development of the foetus may have a harmful effect on the final intelligence and scholastic ability of the progeny. This is presumably related to an insufficient trans-placental transfer of maternal thyroid hormones to the foetus and combined with the inability of the foetal thyroid gland to produce adequate amounts of thyroid hormones itselfReference Vulsma, Gons and De Vijlder26, Reference Zoeller27.
Conclusions
The main changes in thyroid function associated with the pregnant state are due to increased hormone requirements, which begin in the first trimester of gestation. Increased hormone requirements can only be met by proportionally increased hormone production that, in otherwise healthy women, depends directly upon the availability of iodine in the diet. When dietary iodine is lacking, adequate physiological adaptation is difficult to achieve and is progressively replaced by pathological alterations occurring in parallel with the degree and duration of iodine deprivation which leads to maternal hypothyroxinaemia and enhanced glandular stimulation. Therefore, pregnancy typically acts to reveal an underlying iodine deficiency even in conditions with only a marginally poor intake, a state that is observed in many European countries. Iodine deficiency during pregnancy has important repercussions for both the mother and the foetus, particularly thyroid under-function and goitrogenesis. Furthermore, iodine deficiency may be associated with alterations of the neurological, psychological and intellectual development of the child during both gestation and the postnatal period. Therefore, iodine prophylaxis should be given systematically to women during pregnancy. In localities where the deficiency is severe, the administration of iodine has proved highly beneficial to prevent mental deficiency disorders: the many actions undertaken to eradicate iodine deficiency have prevented the occurrence of mental retardation in millions of people throughout the world. In most public health programmes dealing with the correction of iodine deficiency disorders, iodised salt has been the preferred means of delivering iodine to households. Iodised salt, however, is not the ideal means of delivering iodine in the specific instances of pregnancy, breast-feeding and complementary feeding, because of the need to limit salt intake during these periods. Finally, the results of the recent nutritional survey in the United States has disclosed, with some concern, that iodine deficiency thought to have been eradicated for many years may actually be resurgent, particularly in young women. This issue will need to be considered seriously by the medical community and public health authorities, since similar situations may occur in other countries as well.




