Pregnancy increases the demand for iron in women due to the expanding blood volume of the mother, the increasing maternal supply of iron to the foetus and the considerable iron loss due to blood loss at deliveryReference Bothwell1. It has been reported that approximately 40% of Danish women of reproductive age have small or absent iron storesReference Milman, Rosdahl, Lyhne, Jorgensen and Graudal2–Reference Milman, Byg, Ovesen, Kirchhoff and Jurgensen4, i.e. they do not have sufficient iron stores to go through pregnancy without becoming iron-deficient or developing iron-deficiency anaemia. Iron-deficiency anaemia may cause impaired work performance and tiredness, and reduction of immune functionReference Baynes, Brock, Halliday, Pippard and Powell5. Intervention studies with iron supplementation in pregnancy have shown increased iron stores in the supplemented women compared with non-supplemented womenReference Milman, Agger and Nielsen6–Reference Makrides, Crowther, Gibson, Gibson and Skeaff11, and in two randomised controlled trials the risk of adverse pregnancy outcomes was reduced among the women allocated to iron supplementsReference Cogswell, Parvanta, Ickes, Yip and Brittenham12, Reference Siega-Riz, Hartzema, Turnbull, Thorp, McDonald and Cogswell13. The Danish National Board of Health's guidelines on pregnancy for health professionals, published in 1998, state that all Danish pregnant women should be advised to take a daily supplement of 50–70 mg iron from week 20 of gestation until delivery14. Before 1998, no official recommendation on iron supplement use in relation to pregnancy existed. Until now the adherence to this recommendation has not been examined.
A number of sociodemographic and lifestyle factors have been associated with the use of dietary supplements in general. Socio-economic class is one such factor, with individuals from higher socio-economic groups being more likely to consume dietary supplements than individuals from low socio-economic groupsReference Cogswell, Kettel-Khan and Ramakrishnan15–Reference Knudsen, Rasmussen, Haraldsdottir, Ovesen, Bulow and Knudsen21. Smoking has also been associated with the use of dietary supplementsReference Subar and Block19, Reference Knudsen, Rasmussen, Haraldsdottir, Ovesen, Bulow and Knudsen21, Reference Haslam and Lawrence22: non-smokers and former smokers being most likely to take supplements. Self-perceived health was associated with the use of dietary supplements in one surveyReference Knudsen, Rasmussen, Haraldsdottir, Ovesen, Bulow and Knudsen21, but not in anotherReference Lyle, Mares-Perlman, Klein, Klein and Greger18, and a wish to improve health has been reported as a reason for taking supplementsReference Overvad, Diamant, Holm, Holmer, Mortensen and Stender23. Users of supplements are often found to have healthier, more nutrient-dense diets than non-usersReference Lyle, Mares-Perlman, Klein, Klein and Greger18, Reference Beitz, Mensink, Hintzpeter, Fischer and Erbersdobler24, Reference Elmstahl, Wallstrom, Berglund, Janzon, Johansson and Larson25.
The aims of the present study, conducted within the Danish National Birth Cohort (DNBC), were: (1) to examine how many of the participants took iron supplements during pregnancy; (2) to estimate the amount of iron from supplements; and (3) to describe differences between compliers and non-compliers with the recommendation with respect to gestational, lifestyle and dietary factors.
Materials and methods
Danish National Birth Cohort
Data derive from the DNBC, which is a nationwide birth cohort including more than 100 000 pregnant women recruited in early pregnancy in the period from 1997 to 2002. Data were collected through four telephone interviews: in week 12 and week 30 of gestation, and when the child was 6 and 18 months old. Further, all participants received a food-frequency questionnaire (FFQ) in week 25 of gestation, covering diet and use of dietary supplements in mid-pregnancyReference Olsen, Melbye, Olsen, Sorensen, Aaby and Andersen26.
Study population
Approximately 70% of the participants returned the FFQ. During the study period, the FFQ was improved several times and not all versions recorded the use of dietary supplements in a reliable way, which reduced the sample size to 58 078 observations. Further reductions due to incomplete or erroneous completion of the FFQ left 54 371 observations for analyses. Of these, data from telephone interviews were available for 50 902 women. All of the women included in the analyses were recruited after the recommendation on iron supplements was implemented.
Iron supplements
The proportion of women taking iron supplements during pregnancy was assessed using information from the FFQ and the second telephone interview. In the FFQ the women were asked to write the brand name of all supplements, the nutrient content (if single-nutrient supplements) and the number of daily doses taken in the previous four weeks. In the second telephone interview, the women were asked if they had been taking iron supplements during pregnancy (‘Have you been taking iron pills during pregnancy?’), and if yes, in which weeks of gestation they had been taking it, ranging from the beginning of pregnancy until the time of interview, which took place around week 30.
To examine the characteristics of a regular user of iron supplements, a user of supplemental iron was defined as a woman who in the FFQ had reported taking at least one of the above-mentioned iron supplements for at least three out of four days.
Diet
The FFQ employed in the DNBC was a semi-quantitative 360-item questionnaire, which recorded intake of foods and drinks in the previous four weeks. Intakes of foods and nutrients were calculated by means of the program Foodcalc (www.foodcalc.dk), the Danish food composition tables27 and assumptions on standard portion sizesReference Andersen, Jensen and Haraldsdottir28. The FFQ was validated using a 7-day weighed food diary as gold standard, which showed statistically significant correlations between intakes for fruit and vegetables (r = 0.55), protein (0.39), folate (0.53) and retinol (0.37)Reference Mikkelsen, Osler and Olsen29. The differences in dietary habits between users and non-users of iron supplements were examined by comparison of intake of total energy, macronutrients and overall food groups.
Other factors
Information on gestational, anthropometric, lifestyle and sociodemographic factors derived from the second telephone interview, conducted in week 30 of gestation. The following factors were regarded as potential determinants of use of iron supplements and included in the analyses: parity (0 vs. ≥ 1); whether the pregnancy was planned or not; the woman's age; pre-pregnancy body mass index (BMI); smoking habit during pregnancy; physical activity (yes vs. no) during pregnancy; socio-economic group (based upon the job description of the woman or her partner, the higher was chosen); housing; whether the woman had a partner or not; whether the woman had worries about her unborn child; the woman's self-perceived health; and alcohol intake in pregnancy (0 vs. ≥ 0.5 drinks per week).
Statistical analysis
Differences between women who returned and women who did not return the FFQ were analysed by means of the χ2 test when comparing proportions or the t-test for continuous variables. Users of iron supplements were compared with non-users with respect to total energy intake and intake of macronutrients by the t-test, and with respect to median dietary intake of selected food groups by use of the Wilcoxon signed rank test as the outcome data were not normally distributed. Possible determinants for use of iron supplements were analysed by means of logistic regression analysis, and odds ratios and 95% confidence intervals were estimated. All analyses were conducted using SAS version 9.1.3 for Windows (SAS Institute Inc.).
Results
Women who had completed the FFQ and women who had not completed it, or who had completed it erroneously, were compared with respect to background variables. There were significantly higher proportions of primiparous, normal-weight, non-smoking and physically active women among those who completed the FFQ (and thus were included in the analysis) than among those who did not or completed it erroneously (and thus were excluded from the analysis) (Table 1), and more of the included women had planned their pregnancy. The women included in the analyses were slightly older than the women who were excluded.
Table 1 Comparison of women who were included vs. not included in the analyses (N=101 068)
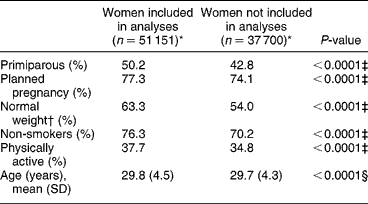
SD – standard deviation.
* Not all women participated in the telephone interviews, thus the sum is not equal to 101 068.
† Body mass index = 25–29 kg m− 2.
‡ χ2 test.
§ t-test.
From information on brand names recorded in the FFQ, iron supplements were defined as pure iron supplements, liquid iron supplements and supplements with haem iron. Seventy-seven per cent of the women reported use of iron supplements in the FFQ, irrespective of dose ingested (Table 2). This is in accordance with the results from the second interview, as approximately three-quarters of the participants reported taking iron supplements from week 20 of gestation as recommended, see Fig. 1 (the small decrease in the proportion of women taking supplements from week 28 onwards is due to missing information, as a small proportion of the participants were interviewed before week 30 of gestation).
Table 2 Intake of iron supplements in the Danish National Birth Cohort (N=54 371)*
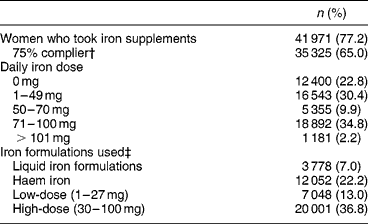
* Data are based on information from the food-frequency questionnaire, covering intake in weeks 21–25 of gestation.
† 75% complier – women who took iron supplements for a minimum of 75% of the recording time (four weeks).
‡ The sum of all formulations used is higher than the number of women who reported taking iron supplements, as a small number of women reported use of more than one iron supplement.

Fig. 1 Proportion of women (%) reporting intake of iron supplements on a weekly basis in each week of gestation from week 1 to week 30
The dietary patterns among users and non-users of iron supplements are shown in Table 3. No statistically significant difference was seen in daily energy intake or intake of macronutrients. With respect to food groups, supplement users had significantly higher intakes of fruit and poultry, while non-users had slightly higher intake of cereals than users. However, although statistically significant, the differences were small.
Table 3 Intakes of energy, macronutrients and food groups in users (at least 75% of daily doses) and non-users of supplemental iron (N=54 371)

Data are presented as mean (standard deviation) or median (25th percentile; 75th percentile).
* t-test.
† Wilcoxon signed rank test.
The gestational and lifestyle factors that were regarded as potential determinants for use of iron supplements were analysed in univariate models. Furthermore, all explanatory variables were included in a multiple logistic regression model, with use and non-use of supplemental iron as the outcome variable. The results are shown in Table 4. The woman's age, BMI, parity, planning of pregnancy, smoking habits, alcohol habits and physical activity in pregnancy were associated with use of supplemental iron, with primiparous, normal-weight, non-smoking, physically active women above the age of 20 years with planned pregnancies having the highest probability of taking iron supplements. The estimates were slightly weakened after adjusting for the other factors. Compared with the unemployed, all other socio-economic groups had a higher probability of taking iron supplements in the univariate analyses. The association was weakened in the adjusted analysis. In the univariate analysis women who perceived their health as good had a higher probability of taking iron supplements than the women who reported having less good health; the association was weakened in the adjusted analysis. No clear association could be established between use of supplements and the woman's worry about the unborn child. No associations were seen between civil status or housing and use of iron supplements. Women who drank alcohol ( ≥ 0.5 drinks per week) during their pregnancy had a lower probability of taking iron compared with women who abstained from alcohol during pregnancy.
Table 4 Determinants of use (at least 75% of daily doses, n=33 145) vs. non-use (n=17 757) of iron supplements (N=50 902)

OR – odds ratio; CI – confidence interval; BMI – body mass index.
Discussion
More than two-thirds of the participants reported use of iron supplements after week 20 of gestation, which shows that the recommendation on use of iron supplements has to a great extent reached the target group within this population.
We found a lower likelihood of compliance with the recommendation for iron supplementation among young, overweight, physically inactive, smoking women, who had not planned their pregnancy. Young age has been associated with low compliance to health campaigns in earlier studiesReference Erkkola, Karppinen, Jarvinen, Knip and Virtanen30, Reference Knudsen, Orozova-Bekkevold, Rasmussen, Mikkelsen, Michaelsen and Olsen31, indicating that younger individuals are less conscious about health matters or simply do not receive the message. Smoking in pregnancy was previously found to be associated with a low rate of iron supplementationReference Nordeng, Eskild, Nesheim, Aursnes and Jacobsen16, Reference Haslam and Lawrence22, which is supported by results from two surveys where pregnant smokers were found to have a less healthy diet than non-smokersReference Trygg, Lund Larsen, Sandstad, Hoffman, Jacobsen and Bakketeig32, Reference Mathews, Yudkin, Smith and Neil33. In two more surveys in non-pregnant populations, the former smokers were most likely to take dietary supplementsReference Subar and Block19, Reference Knudsen, Rasmussen, Haraldsdottir, Ovesen, Bulow and Knudsen21, while another survey found no association between supplement use and smoking habitReference Block, Cox, Madans, Schreiber, Licitra and Melia17. Smokers may to a high degree belong to a group who care less about their health or they are not reached by public health campaigns. Overweight women also represented a group of individuals who to a lesser degree followed the official guidelines according to iron supplement use, which is also supported by earlier findingsReference Nordeng, Eskild, Nesheim, Aursnes and Jacobsen16, Reference Erkkola, Karppinen, Jarvinen, Knip and Virtanen30. Not surprisingly, the women who had planned their pregnancy were more likely to follow the recommendations, and may be regarded as more conscious about their pregnancy. Multiparous women were less likely to follow the recommendations and the difference remained after adjusting for age. This was also seen in a previous study of compliance with a recommendation of periconceptional folic acid supplementationReference Knudsen, Orozova-Bekkevold, Rasmussen, Mikkelsen, Michaelsen and Olsen31 and indicates that multiparous women may have a more casual attitude towards health matters in relation to pregnancy, possibly because the majority have experienced a previously uncomplicated pregnancy.
We found a clear association between socio-economic class and use of iron supplements, with a higher proportion of iron users among the well-educated women. This is confirmed in several studies, where populations with longer education were more likely to take dietary supplements than groups of less-privileged individualsReference Cogswell, Kettel-Khan and Ramakrishnan15–Reference Knudsen, Rasmussen, Haraldsdottir, Ovesen, Bulow and Knudsen21, Reference Erkkola, Karppinen, Jarvinen, Knip and Virtanen30, Reference Mathews, Yudkin, Smith and Neil33. This is also somewhat supported in two surveys where low educational level was found to be associated with low haemoglobin concentration in pregnancyReference Bergmann, Gravens-Muller, Hertwig, Hinkel, Andres and Bergmann34, Reference Bodnar, Siega-Riz, Arab, Chantala and McDonald35.
The differences in dietary intake between users and non-users were small and reached statistical significance in only two cases, and thus were probably not of nutritional importance. However, the difference may give a picture of the dietary patterns of users and non-users of iron supplements pointing towards the fact that users of supplements to a higher extent eat a diet closer to current recommendations. This is supported by earlier findings in non-pregnant individuals, showing that users of dietary supplements in general have a healthier diet than non-users, and that individuals who take dietary supplements are the most unlikely to need themReference Lyle, Mares-Perlman, Klein, Klein and Greger18, Reference Beitz, Mensink, Hintzpeter, Fischer and Erbersdobler24.
A large proportion of the women took iron supplements in low doses and consequently did not meet the recommended dose of 50–70 mg iron per day. In contrast, many of the women took twice the recommended daily dosage or more per day. Many of the iron preparations available on the Danish market contain 100 mg iron per tablet, and only a few of the iron preparations recorded in this cohort contained iron in the recommended dose, 50–70 mg. This makes it difficult for women to follow the recommendation. As a consequence, many of the pregnant women were exposed to high doses of iron; this is considered unfavourable as excess iron intake may lead to increased oxidative stress, which has been associated with gestational hypertensionReference Casanueva and Viteri36 and increased risk of cancerReference Fisher and Naughton37. Furthermore, high intakes of iron impair absorption of zinc and other metal ionsReference Sandstrom38 which are also of increased need during pregnancyReference Scholl, Hediger, Schall, Fischer and Khoo39. The prevalence of hereditary haemochromatosis, which is characterised by high intestinal iron absorption, is 0.25% for the homozygotic mutation and 9.2% for the heterozygotic mutationReference Ellervik, Mandrup-Poulsen, Nordestgaard, Larsen, Appleyard and Frandsen40, and pregnant women having this condition should be cautious about taking the high-dose iron supplements.
In a Swedish survey of adherence to recommendations on iron supplements in pregnancy, side-effects of the supplements (gastrointestinal problems) were mentioned as a reason for poor compliance, as the recommended daily dose was high (100 mg) and side-effects of the supplements were reported to be dose-dependentReference Wulff and Ekstrom41. On the other hand, in a randomised controlled trial with iron supplements in varying doses, no difference in occurrence of side-effects was reported in the groups who received 20, 40, 60 or 80 mg iron per dayReference Milman, Bergholt, Eriksen, Byg, Graudal and Pedersen42. However, this may be a reason for not taking iron supplements, as many of the available products contain 100 mg iron per daily dosage.
The strengths of this study are the large number of participants and the wide variety of information on lifestyle and sociodemographic factors collected routinely in DNBC. A limitation of the cohort is that the participants cannot be regarded as representative for the whole population of Danish pregnant womenReference Olsen, Melbye, Olsen, Sorensen, Aaby and Andersen26. The latter is assumed to cause a selection of women in the cohort who are more concerned about health matters in relation to pregnancy compared with the background populationReference Hennekens and Buring43. The proportions of iron supplement users found in this study must therefore be regarded as being overestimated, if not representative. However, the results from this study indicate that pregnant women belong to a group who in general pays attention to health recommendations.
Responders and non-responders to FFQs are often found to differ with respect to lifestyle variables such as smoking and BMIReference Margetts and Nelson44. This was also the case in this study, as seen in Table 1. However, even though the women included in these analyses may not be representative, we do believe that the patterns in differences between users and non-users of iron supplements may reflect the patterns in the background population.
Conclusion
The recommendation for pregnant women to take supplemental iron from week 20 of gestation has been met with success although the doses taken are not optimal. Many pregnant women take iron in excess of recommended levels due to inappropriate product formulations on the Danish market. Not surprisingly, well-educated, non-smoking women above 20 years of age comply with the recommendations to a higher degree than young smoking women with short or no education. In the future, campaigns should be aimed at more vulnerable groups, and more attention should be paid to ensure availability of supplements with appropriate doses of iron on the Danish market.
Acknowledgements
Sources of funding: The DNBC was funded by the Danish National Research Foundation, March of Dimes Birth Defects Foundation, Pharmacy Foundation, Egmont Foundation, Augustinus Foundation, the Health Foundation and the European Union (QLK1-2000-00 083). The DNBC managerial team consisted of Jørn Olsen (chair), Mads Melbye, Anne Marie Nyboe Andersen, Sjurdur F Olsen, Thorkild IA Sørensen and Peter Aabye.
Conflict of interest declaration: None of the authors had any conflicts of interest.
Authorship responsibilities: V.K.K., H.S.H., L.O. and S.F.O. were involved in generating the purpose of the work. V.K.K. was involved in data collection, analysing and interpreting data, and writing the manuscript. H.S.H., L.O. and S.F.O. were involved in interpreting the data and editing the manuscript. T.B.M. was involved in data collection, interpreting the data and editing the manuscript.







