Fe deficiency is the most common nutritional disorder in the world and is often the cause of anaemia, a frequently encountered haematological problem in pregnancy care. Currently, the national policy in India is to provide universal supplementation equivalent to 100 mg elemental Fe/d to all pregnant women, regardless of their initial Fe/Hb status( 1 ).
A number of recent studies suggest that Fe provided in excess may induce oxidative stress through the production of reactive oxygen species. Supplementation of 100 mg Fe/d to mildly anaemic women resulted in an increase in oxidative stress( Reference Tiwari, Mahdi and Chandyan 2 ), while a dose of 50 mg/d given in the second trimester to pregnant women with high Hb (≥135 g/l) resulted in a higher number of babies born small for gestational age and a higher incidence of hypertensive disorders( Reference Ziaei, Norrozi and Faghihzadeh 3 ). Intra-uterine oxidative stress is associated with adverse birth outcomes such as intra-uterine growth restriction, pre-eclampsia and an elevated risk of premature rupture of membranes( Reference Burton and Jauniaux 4 ), as well as an elevated risk of metabolic syndrome in later life( Reference Weinberg 5 ).
An earlier Indian study showed that women with high Hb values in trimester 1 (T1), who consumed the recommended dose of supplemental Fe during pregnancy, had a higher risk of delivering a term low-birth-weight (LBW) baby( Reference Shastri, Mishra and Dwarkanath 6 ).
The standard marker of oxidative stress is the level of lipid peroxides (LPO) formed from unsaturated lipids, mainly from oxidized LDL. One of the key enzymes that protects LDL from oxidation is paraoxonase-1 (PON-1). Studies have shown that decreased serum PON-1 and increased LPO may be indices of oxidative stress leading to early pathogenesis of atherosclerotic heart disease in pregnant women( Reference Mackness, Durrington and Ayub 7 ). Another enzyme that is involved in the oxidative modification of lipoproteins, as well as in inflammation, is myeloperoxidase (MPO). Increased MPO activity has been reported in pregnant women with pre-eclampsia and intra-uterine growth restriction( Reference Hung, Chen and Lo 8 ).
The assessment of oxidative stress in the current study was therefore based on measurement of the enzymatic activities of PON-1, LPO, MPO and total antioxidants (ToAx). The purpose of the current study was to assess if Hb status in T1 (Hb1) was associated with oxidative stress and if this could be related to birth outcomes.
Participants and methods
The current study was part of a prospective observational cohort of pregnant women conducted at St. John’s Research Institute and St. John’s Medical College Hospital in Bangalore, India. The details of the cohort have been described previously( Reference Shastri, Mishra and Dwarkanath 6 , Reference Dwarkanath, Barzilay and Thomas 9 ). Briefly, the cohort consisted of 1838 pregnant women in the age group 17–40 years, who had no known morbidities and who delivered live babies at St. John’s Medical College Hospital. All women of this cohort received routine Fe supplementation of 45 mg/d, although only 642 women could be considered anaemic in T1 (Hb1<110 g/l). From this cohort, 100 participants were chosen based on their Hb1 values: forty with Hb1<110 g/l and sixty with Hb1≥120 g/l. All participants were supplemented with 45 mg elemental Fe/d in trimesters 2 and 3( Reference Shastri, Mishra and Dwarkanath 6 ). The study by Genc et al.( Reference Genc, Uzun and Benian 10 ) reported a difference of 40 U/ml in PON-1 between pre-eclampsia and normal pregnancy. To observe this difference between the low/normal and high Hb1 groups, with 5 % level of significance and 80 % power, the sample size required was forty-five per group. We could get valid data in only forty low/normal Hb1 women. To improve the power, we increased the number in the high Hb1 group to sixty.
Blood sample collection and analysis
Venous whole blood samples were collected into EDTA-coated anticoagulant tubes (Becton Dickenson, NJ, USA) by trained laboratory assistants at T1 (11·9 (sd 2·3) weeks of gestation) and cord blood (CB) samples were collected at birth. Hb concentration was analysed using an automated cyanmethaemoglobin technique (ABX Pentra 60 C+ haematology analyser; Horiba ABX Diagnostics, Germany). The measuring range was 80–180 g/l with a within-run precision of >99 %.
Analysis of oxidative stress parameters
The arylesterase activity of PON-1 was assayed using p-nitrophenyl acetate as the substrate as described by Burlina et al.( Reference Burlina, Michielin and Galzigna 11 ). Briefly, the rate of formation of p-nitrophenol was measured at 405 nm and arylesterase activity was expressed as pmol p-nitrophenol generated/min per ml plasma. MPO was measured as the rate of oxidation of o-dianisidine in the presence of H2O2 at 450 nm( Reference Gan, Smolen and Eckerson 12 ). Enzyme activity was expressed as U/min per ml plasma (1 U=1 OD change at 450 nm). LPO were measured as mmol thiobarbituric acid-reactive species formed on reaction of plasma with malondialdehyde. Briefly, plasma proteins were first precipitated with 15 % (w/v) TCA and the supernatant was reacted with 20-mm thiobarbituric acid in the presence of 10 % (w/v) SDS. The thiobarbituric acid-reactive species formed were measured in a fluorescence spectrophotometer with λ ex=520 nm and λ em=570 nm, and data were expressed as nmol malondialdehyde/ml plasma. ToAx were measured using the TEAC method (Trolox equivalent antioxidant capacity) based on the suppression of absorbance of the radical cation of 2,2′-azinobis(3-ethyl benzothiazoline sulfonate) by plasma antioxidants( Reference Miller, Rice-Evans and Davies 13 ) and were expressed as nmol Trolox equivalent/ml plasma.
Delivery and birth information
Infants were weighed to the nearest 10 g on an electronic weighing scale (Salter Housewares 914 Electronic Baby and Toddler Scale, NY, USA) immediately after birth. LBW was defined as birth weight less than 2500 g irrespective of gestational age.
Statistical analysis
The comparison of the oxidative stress markers among the different groups of Hb1 was performed using the independent-sample t test or the Mann–Whitney U test if not normally distributed. The data were also analysed based on dividing the participants into two groups based on birth outcome (LBW and others). Normally distributed data are presented as mean and standard deviation and the rest as median (quartile 1–quartile 3). For all analyses, two-sided P values <0·05 were considered statistically significant. The oxidative stress markers were compared between four combined groups of low/normal and high Hb1 and low and normal birth weight using one-way ANOVA. All statistical analyses were performed using the statistical software package IBM SPSS Statistics for Windows, Version 18.0.
Results
The general characteristics and birth details of the women participating in the study (total, low/normal Hb1 and high Hb1) are summarized in Table 1. There were no differences in characteristics between the two groups except their Hb1 values. Mean compliance to Fe supplementation was about 70 % among all women. Among the women who had low/normal Hb1, 25 % of babies were born LBW, whereas 38 % of women with high Hb1 gave birth to LBW babies.
Table 1 General characteristics of the study population (n 100): pregnant women attending a tertiary hospital in urban South India
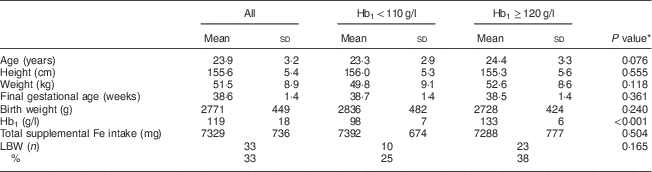
Hb1, Hb in trimester 1; LBW, low birth weight.
* Calculated by the independent-sample t test for continuous data and by the χ 2 test for categorical data. P<0·05 was considered to be statistically significant.
Table 2 describes the results of oxidative stress parameters in women with low/normal v. high Hb1. PON-1 showed a significantly lower activity in T1 in the high Hb1 group (424·7 (sd 163·7) pmol p-nitrophenol formed/min per ml plasma) compared with the low/normal Hb1 group (532·9 (sd 144·7) pmol p-nitrophenol formed/min per ml plasma, P=0·002). The same trend held for PON-1 in CB samples (P=0·020). The high Hb1 group also had a higher mean LPO level in T1 (P=0·046) but not in CB. Although not statistically significant, women with high Hb1 had babies with a mean birth weight that was 108 g less than women with low/normal Hb1 (P=0·240). No differences were seen in MPO activity or ToAx levels between the groups.
Table 2 Comparison of oxidative stress markers within groups according to low/normal and high Hb in the first trimester, among pregnant women attending a tertiary hospital in urban South India
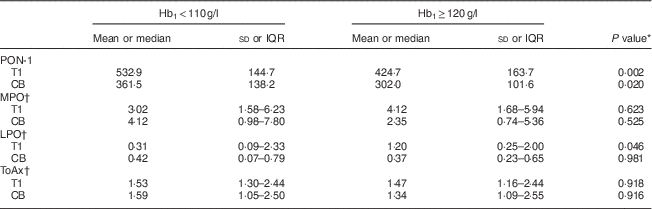
Hb1, Hb in T1; IQR, interquartile range (quartile 1–quartile 3); PON-1, paraoxonase-1; T1, trimester 1; CB, cord blood; MPO, myeloperoxidase; LPO, lipid peroxides; ToAx, total antioxidants.
PON-1 expressed as pmol p-nitrophenol formed/min per ml plasma; MPO expressed as U/min per ml plasma; LPO expressed as nmol malondialdehyde/ml plasma; ToAx expressed as nmol Trolox equivalent/ml plasma.
* P value calculated from the independent-samples t test. P<0·05 was considered to be statistically significant.
† Median and IQR with P value calculated from the Mann–Whitney U test. P<0·05 was considered to be statistically significant.
The participants were further sorted into two additional groups based on birth weight: Group 1, low/normal Hb1 and normal birth weight (NBW); Group 2, low/normal Hb1 and LBW; Group 3, high Hb1 and NBW; and Group 4, high Hb1 and LBW. Among these groups, both T1 and CB PON-1 were lower in Group 4 compared with the control Group 1 (Fig. 1), although only the T1 values were statistically significant.

Fig. 1 Mean paraoxonase-1 (PON-1) in the trimester 1 (T1) blood of mothers (●) and the cord blood of babies (○) expressed as pmol p-nitrophenol formed/min per ml plasma. Values are means with their 95 % confidence limits represented by vertical lines. *P=0·004 when Group 4 of T1 PON-1 is compared with Group 1. All groups were given the routine iron supplementation (Group 1, low/normal Hb1 and normal birth weight; Group 2, low/normal Hb1 and low birth weight; Group 3, high Hb1 and normal birth weight; Group 4, high Hb1 and low birth weight; Hb1, Hb in T1)
Subsequently, these parameters were also analysed based on a birth weight grouping (Table 3). Women who delivered LBW babies had a significantly lower T1 PON-1 activity compared with those who delivered babies with NBW. The LBW group also had a higher mean LPO level in T1, although this was not significant. No differences were seen in any of the parameters in the CB samples.
Table 3 Comparison of oxidative stress markers within groups according to birth weight, among pregnant women attending a tertiary hospital in urban South India

NBW, normal birth weight; LBW, low birth weight; IQR, interquartile range (quartile 1–quartile 3); Hb1, Hb in T1; PON-1, paraoxonase-1; T1, trimester 1; CB, cord blood; MPO, myeloperoxidase; LPO, lipid peroxides; ToAx, total antioxidants.
PON-1 expressed as pmol p-nitrophenol formed/min per ml plasma; MPO expressed as U/min per ml plasma; LPO expressed as nmol malondialdehyde/ml plasma; ToAx expressed as nmol Trolox equivalent/ml plasma.
* P value calculated from the independent-sample t test. P<0·05 was considered to be statistically significant.
† Median and IQR with P value calculated from the Mann–Whitney U test. P<0·05 was considered to be statistically significant.
Discussion
Fe-deficiency anaemia is among the commonest problems encountered during pregnancy, especially in developing countries such as India. A routine supplementation of Fe and folic acid during the second and third trimesters of pregnancy is believed to be essential for the benefit of both the fetus and the mother. However, an increasing number of studies especially from developing countries suggest that high levels of Fe can cause undesirable secondary effects, including gestational diabetes mellitus and pre-eclampsia( Reference Weinberg 5 ). Epidemiological studies also show negative associations between birth outcomes and both low and high Hb levels during pregnancy( Reference Scanlon, Yip and Schieve 14 , Reference Zhou, Yang and Hua 15 ). However, in India, the current national guideline prescribes a universal dosage of 100 mg elemental Fe/d during pregnancy( 1 ), despite the possibility of high Fe supplementation leading to adverse outcomes( Reference Ribot, Aranda and Giralt 16 , Reference Beard 17 ), especially in women who start out the pregnancy with high Hb( Reference Shastri, Mishra and Dwarkanath 6 ).
In the current study, all women were supplemented with 45 mg elemental Fe/d during their second and third trimesters of pregnancy, irrespective of their initial Hb status( Reference Shastri, Mishra and Dwarkanath 6 ). We have earlier reported that even this dose of Fe supplementation in pregnant women with a high Hb1 has an adverse impact on birth outcome( Reference Shastri, Mishra and Dwarkanath 6 ). Similar results have been reported with 50 mg elemental Fe provided daily for 8 weeks during pregnancy( Reference Kamp and Donangelo 18 ). Supplementing Fe to healthy, non-anaemic pregnant women has been reported to increase oxidative stress, resulting in deleterious effects on lipid peroxidation( Reference Lachili, Hininger and Faure 19 , Reference Bhatla, Kaul and Lal 20 ) as well as protein oxidation( Reference Kamp and Donangelo 18 ). Some of these effects have been shown to be ameliorated by the concomitant consumption of antioxidants such as vitamin C( Reference Lachili, Hininger and Faure 19 ). In the current study, effects on both lipid peroxidation as well as protein oxidation (using PON-1 activity as a surrogate marker( Reference Gelisgen, Genc and Kayali 21 )) were investigated. It was seen that women who started their pregnancy with a high Hb1 actually had a higher level of oxidative stress as evidenced by higher levels of LPO and lower PON-1 activity in T1 (Table 2).
Furthermore, Fig. 1 suggests that women with high Hb1, who eventually delivered babies that were LBW, had further lower PON-1 activity in T1 compared with those who had low/normal Hb1. Considering the fact that all women had similar Fe supplementation during their pregnancy, irrespective of Hb1 values, these data suggest that women with high Hb1 associated with low PON-1 activity may be more susceptible to the possible deleterious effects of this routine Fe supplement. On the other hand, it is important to emphasize that anaemic women appear to have benefited by the Fe supplementation, as evidenced by the higher PON-1 activity in CB as well as NBW.
PON-1 is an important anti-oxidative enzyme associated with HDL which prevents the oxidation of LDL and reduces the formation of atheromatous plaques in the blood vessels. It therefore has a protective role against cellular damage from oxidative stress( Reference La Du, Aviram and Billecke 22 ) and low serum PON-1 activity is associated with increased risk of atherosclerosis( Reference Mackness, Durrington and Ayub 7 , Reference Mogarekar and Rojekar 23 ) as well as adverse birth outcomes( Reference Genc, Uzun and Benian 10 , Reference Gelisgen, Genc and Kayali 21 ).
The activity of PON-1 in T1 was significantly lower among women who delivered LBW babies (Table 3). One explanation for this is that PON-1 is itself very susceptible to oxidative stress and can be inactivated by free-radical-induced oxidation( Reference Vlachos, Bartzeliotou and Schulpis 24 ). PON-1 activity has been reported to decrease as pregnancy progresses, as it is consumed when stabilizing the oxidative stress levels in the blood( Reference Vlachos, Bartzeliotou and Schulpis 24 ). In addition to the effects of haemodilution, it has been postulated that the lower levels could be a result of decreased protein synthesis due to cellular injury, which in turn decreases the enzymatic turnover, and thus contributes to the decrease in serum levels of the enzyme( Reference Mogarekar and Rojekar 23 ). Recent studies have shown that LBW babies have low PON-1 levels in CB( Reference Mogarekar and Rojekar 23 ); however, we did not observe such an effect. This might be due to the fact that the levels of LPO, which are the key influencers of PON-1 activity, were unaffected in the CB of the LBW group.
Studies have shown that increased protein oxidation markers and lowered PON-1 activity in early pregnancy could predict clinical complications like pre-eclampsia and gestational diabetes mellitus( Reference Genc, Uzun and Benian 10 , Reference Gelisgen, Genc and Kayali 21 ). In combination with the current finding of higher oxidative stress and lower PON-1 activity in T1 also being associated with high Hb1 as well as LBW, the potential of PON-1 being used as an early marker of gestational morbidities is worth investigating.
MPO is an enzyme that is produced and released by activated neutrophils in response to an inflammatory stress( Reference Hung, Chen and Lo 8 ) and plays a key role in the pathogenesis of CVD. Many of the morbidities of pregnancy such as pre-eclampsia and intra-uterine growth restriction exhibit increased systemic inflammatory responses, and it has been hypothesized that MPO plays an important role here as well( Reference Gandley, Rohland and Zhou 25 ). Hung et al.( Reference Hung, Chen and Lo 8 ) recently reported that higher MPO levels in maternal blood before delivery were associated with pre-eclampsia and intra-uterine growth restriction. However, in the current study no differences were seen in MPO activity. This is not surprising since the maternal blood sampling in the current study was in the first trimester, where there probably was no inflammatory stress, even though there appears to be oxidative stress.
The limitations of the present study are mainly the small sample sizes, the focus on only T1 and CB rather than all trimesters, and the lack of heterogeneity of the population studied. Although many more such studies on larger populations are required, the present study reprises the question regarding the prudency of a universal high-dose Fe supplementation in pregnant women with high Hb1. In conclusion, the study suggests that low PON-1 activity in the first trimester of pregnancy, particularly in women with high Hb1, could be an early indicator of oxidative stress, which could potentially have adverse effects on the fetus.
Acknowledgements
Acknowledgements: The authors would like to acknowledge Priyanka Bannikoppa for her help in sample retrieval and analysis and Dr Pratibha Dwarkanath for providing essential details regarding the cohort. Financial support: This research was funded by the Department of Biotechnology, Government of India (reference 102/IFD/SAN/3325/2013–2014). The funder had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: R.S.P., L.S., I.M. and A.V.K. were responsible for formulating the research question and designing the study. R.S.P., L.S. and I.M. were responsible for doing the sample analysis. T.T., L.S., I.M. and R.S.P. analysed the data and all authors contributed to the writing of the manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Institutional Ethical Review Board of St. John’s Medical College Hospital, Bangalore, India. Written informed consent was obtained from all participants at enrolment.






