Introduction
Acetolactate synthase (ALS; EC 4.1.3.18), also known as acetohydroxyacid synthase (EC 2.2.1.6), catalyzes the first step in the biosynthesis of the branched-chain amino acids Valine, Leucine, and Isoleucine (Schloss Reference Schloss1990). ALS isoforms are common targets of five chemical families of herbicides, including sulfonylurea (SU), imidazolinone (IMI), triazolopyrimidine (TP), pyrimidinyl-thiobenzoate (PTB), and sulfonyl-aminocarbonyl-triazolinone (SCT) (Garcia et al. Reference Garcia, Nouwens, Lonhienne and Guddat2017; Lonhienne et al. Reference Lonhienne, Garcia, Pierens, Mobli, Nouwens and Guddat2018; McCourt et al. Reference McCourt, Pang, King-Scott, Guddat and Duggleby2006). These herbicides have been widely used across the globe to control both grass and broadleaf weed species in a range of crops. However, their excessive use has led to the rapid evolution of ALS-resistant weed biotypes. To date, 171 weed species have been reported to be resistant to ALS inhibitors worldwide (Heap Reference Heap2023).
Herbicide resistance can be categorized into target-site resistance (TSR) and non-TSR (NTSR) (Gaines et al. Reference Gaines, Duke, Morran, Rigon, Tranel, Küpper and Dayan2020). TSR is mostly caused by amino acid substitution (AAS) in the target enzyme that reduces herbicide action on the enzyme’s active pocket or by target gene duplication (Yu and Powles Reference Yu and Powles2014). To date, at least 32 AASs at eight codon positions of ALS (Ala-122, Pro-197, Ala-205, Asp-376, Arg-377, Trp-574, Ser-653, and Gly-654) have been identified that confer resistance to different ALS inhibitors (Murphy and Tranel Reference Murphy and Tranel2019; Yu and Powles Reference Yu and Powles2014), with most field-evolved AASs occurring at Pro-197 and Trp-574. Also, most of the AASs can confer cross-resistance to herbicides from two or more ALS families (Beckie and Tardif Reference Beckie and Tardif2012). In addition to AASs, ALS overexpression has also been reported as a potential mechanism of resistance to mesosulfuron-methyl and pyroxsulam in shortawn foxtail (Alopecurus aequalis Sobol.) and barren brome (Bromus sterilis L.), respectively (Sen et al. Reference Sen, Hamouzová, Mikulka, Bharati, Košnarová, Hamouz, Roy and Soukup2021; Zhao et al. Reference Zhao, Yan, Wang, Bai, Wang, Liu and Wang2018). In contrast, NTSR is caused by a variety of mechanisms that reduce the amount of active herbicide reaching the target site. Among these, enhanced rate of herbicide metabolism (i.e., metabolic resistance) has been identified as the predominant NTSR mechanism for ALS resistance (Jugulam and Shyam Reference Jugulam and Shyam2019). It often involves the increased activity of multiple herbicide-detoxifying enzymes such as cytochrome P450 monooxygenases (P450s), glutathione S-transferases (GSTs), UDP-glycosyl transferases, and ATP-binding cassette transporters (Yuan et al. Reference Yuan, Tranel and Stewart2007). Metabolic resistance can often confer an unpredictable resistance pattern to herbicides with different modes of action (MOAs) and has become a research hot spot in recent years (Nandula et al. Reference Nandula, Riechers, Ferhatoglu, Barrett, Duke, Dayan, Goldberg-Cavalleri, Tétard-Jones, Wortley, Onkokesung, Brazier-Hicks, Edwards, Gaines, Iwakami, Jugulam and Ma2019).
Alopecurus aequalis is a partly cross-pollinated (ca. 40%) winter annual weed of the Poaceae family (Morishima and Oka Reference Morishima and Oka1980). It can germinate under a wide range of temperatures (15/5 to 35/25 C) and pH levels (4 to 10) and even under high NaCl concentrations (up to 200 mM) and has become a dominant weed throughout North America, Europe, and temperate Asia (Cope Reference Cope, Nasir and Ali1982; Hashim et al. Reference Hashim, Hachinohe and Matsumoto2010). In China, A. aequalis has been found to infest some overwintering crop regions such as canola (Brassica napus L.) and wheat (Triticum aestivum L.) fields, causing great reductions in grain yields (Guo et al. Reference Guo, Yuan, Liu, Bi, Du, Zhang, Li and Wang2015). In recent decades, Chinese growers have relied heavily on herbicides for weed control in wheat fields, particularly acetyl-CoA carboxylase (ACCase; EC 6.4.1.2)- and ALS-inhibiting herbicides. Of these, the ALS inhibitor mesosulfuron-methyl has been regularly used for A. aequalis control during the past 20 yr, which also led to its subsequent evolution of resistance. Today, both TSR and NTSR have been identified as the main mechanisms of resistance to ALS inhibitors in A. aequalis (Zhao et al. Reference Zhao, Yan, Du, Zhang, Liu and Wang2020, Reference Zhao, Yan, Liu and Wang2022), severely threatening our ability to control this species. Investigating the unknown resistance mutations and potential resistance-conferring genes and characterizing their resistance patterns are critical for designing integrated weed management (IWM) and resistance management methods (Matzrafi et al. Reference Matzrafi, Peleg and Lati2021).
In the summer of 2021, an A. aequalis population, AHFT-4, with suspected herbicide resistance was collected from a wheat field with a rice (Oryza sativa L.) rotation in Fengtai County, Anhui Province, China. The farmer noticed that the A. aequalis population could not be effectively controlled with mesosulfuron-methyl applied at the field-recommended rate. The main objectives of this study were to (1) determine the resistance level of AHFT-4 to mesosulfuron-methyl, (2) investigate the potential TSR and NTSR mechanisms to mesosulfuron-methyl, and (3) characterize its cross-resistance to different ALS-inhibiting herbicides.
Materials and Methods
Plant Materials and Growth Conditions
The susceptible (S) population AHFY-3 and the suspected herbicide-resistant (R) population AHFT-4 of A. aequalis originated from Fengyang County (32.75°N,117.64°E) and Fengtai County (32.78°N, 116.65°E), Anhui Province, China, respectively. In May 2021, the seeds of the S population were collected from uncultivated land with no history of herbicide application (Wang et al. Reference Wang, Tang, Liao, Cao and Zhao2023). The seeds of the R population were collected from a wheat field that had undergone repeated rice–wheat rotations for several decades and had received mesosulfuron-methyl application for approximately 17 yr. Additionally, the wheat field had been treated with mesosulfuron-methyl at its field-recommended rate during the year of seed collection. For each population, mature seeds were randomly harvested from more than 200 individual plants and thoroughly mixed. After being air-dried separately, they were stored in paper bags at 4 C for at least 5 mo until further use.
Seeds were randomly selected from each population and pre-germinated in petri dishes containing 6 g L−1 agar, as described by Wang et al. (Reference Wang, Tang, Liao, Cao and Zhao2023). Eight germinated seeds were then sown into separate square plastic pots (10 cm by 10 cm by 8.5 cm) filled with loam soil and grown in a controlled glasshouse under natural light (∼75% relative humidity, 25 C/15 C). The pots were regularly watered and rearranged. Once the plants reached the 2- to 3-leaf stage, they were thinned to five similar-sized plants per pot and allowed to grow to the 3- to 4-leaf stage.
Single-Dose Herbicide-Resistance Testing
Single-dose herbicide-resistance testing was carried out following a previously described method with minor modifications (Zhao et al. Reference Zhao, Yan, Ge, Zhu, Liu and Wang2019). Briefly, 30 seeds were randomly selected from each population and grown to the 3- to 4-leaf stage under the conditions described earlier. For each population, 15 plants were treated with mesosulfuron-methyl (30 g L−1 oil-miscible flowable, Bayer, Hangzhou, China) at the field-recommended rate (9 g ai ha−1), while the remaining 15 plants were treated with water and served as the control. The herbicide and water were applied using a cabinet sprayer (3WP-2000, Nanjing Mechanization Research Institute of the Ministry of Agriculture, Nanjing, China) with a spray volume of 450 L ha−1 at a pressure of 275 kPa. After treatment, the weed seedlings were returned to the glasshouse and allowed to grow for an additional 3 wk. At 21 d after treatment (DAT), the survival of weed seedlings was assessed. A plant was considered “dead” if it had necrotic leaf tissue that was easily breakable and showed no signs of new tiller formation (Kumar and Jha Reference Kumar and Jha2017).
PCR Amplification and Gene Sequencing
Seeds from S and R populations were randomly selected, and seedlings were grown to the 3- to 4-leaf stage under the conditions described earlier. At this stage, fresh leaf tissues were harvested from each individual seedling. At least 10 individual plants were randomly selected from each population for ACCase gene sequencing. Genomic DNA was extracted using the classical cetyltrimethylammonium bromide method (Porebski et al. Reference Porebski, Bailey and Baum1997). Two pairs of primers (ALS1-F1: 5′-CAATAAAAATCTCATGCCCGT-3′, and ALS1-R1: 5′-CATGGTTCACAGTTGACCACA-3′; ALS2-F1: 5′-ACGCTCGCATAAAAAGCCA-3′, and ALS2-R1: 5′-GTCCTCTAGGTCGAGCTCTTGATT-3′) previously reported by Iwakami et al. (Reference Iwakami, Shimono, Manabe, Endo, Shibaike, Uchino and Tominaga2017) were used to amplify the different copies (ALS1 and ALS2) of the full-length ALS genes. The polymerase chain reaction (PCR) systems and cycling profiles were prepared using 2× Super Pfx MasterMix (CWBIO, Beijing, China) according to the manufacturer’s instructions. The PCR products with expected sizes were purified using an EasyPure Quick Gel Extraction Kit (TransGen Biotech, Beijing, China) and directly Sanger sequenced on both strands by Tsingke Biotech (Nanjing, China). Subsequently, the purified gene fragments were cloned into pEASY-T1 simple vectors (TransGen Biotech), and the inserts were sequenced on both strands again.
The resulting nucleotide and deduced amino acid sequences from the S and R plants were compared and aligned with the ALS genes of A. aequalis (GenBank accession nos. LC200800 to LC200801) using DNAMAN v. 6.0.3.99 (Lynnon Biosoft, Quebec, Canada) and BioEdit v. 7.1.3.026 (Hall Reference Hall1999), respectively.
Development of the Derived Cleaved Amplified Polymorphic Sequence (dCAPS) Assay for ALS Genotyping
A dCAPS assay was developed to detect the ALS mutation in A. aequalis at the molecular level. Based on the wild and mutant ALS sequences obtained in the previous experiment, a pair of primers (d197F: 5′-CTCCATCCCCATGGTCGCCATCACGGGGCAGGTG-3′, and d197R: 5′- GGAGGAGGCGAGGAAGAA-3′) were designed using the dCAPS Finder v. 2.0 software (Neff et al. Reference Neff, Turk and Kalishman2002). The d197F primer was modified by introducing a “C” to “G” mismatch to create a restriction site “GG^CC” for the HaeIII enzyme. The PCR systems and cycling profiles were prepared using 2× Super Pfx MasterMix (CWBIO), and the resulting products were directly digested with HaeIII (TaKaRa, Dalian, China). The pair of primers amplified 178-bp DNA fragments, but only the mutant sequences could be digested by the enzyme. The resulting digestion products were analyzed on 2.0% agarose gels.
ALS Expression Assays
Real-time quantitative PCR (RT-qPCR) analysis of ALS expression was conducted as described in our prior study (Zhao et al. Reference Zhao, Yan, Wang, Bai, Wang, Liu and Wang2018). The S and R seedlings were grown to the 3- to 4-leaf stage and then treated with mesosulfuron-methyl at the field-recommended rate (9 g ai ha−1). Fresh shoots were collected from individuals of each biotype at 0 (CK), 12, 24, and 48 h after treatment (HAT). RNA was then extracted from each sample using the TRIzol Reagent Kit (Invitrogen, Carlsbad, CA, USA), and complementary DNA (cDNA) was obtained using HiScript III 1st Strand cDNA Synthesis Kit (+gDNA wiper) (Vazyme, Nanjing, China). The PCR system was prepared using a 2× TransStart Top Green qPCR SuperMix (TransGen Biotech), and RT-qPCR was performed using the CFX96 Real-Time PCR system (Bio-Rad Laboratories, Hercules, CA, USA).
In this study, three genes, including capsine phosphatase (CAP), eukaryotic initiation factor 4a (EIF4A), and tubulin (TUB), were conjunctively used as internal controls. The total expression of different ALS copies relative to that of the internal controls was analyzed by the 2−ΔCt method (Livak and Schmittgen Reference Livak and Schmittgen2001), with all the primers referred to Zhao et al. (Reference Zhao, Yan, Wang, Bai, Wang, Liu and Wang2018) and were summarized in Supplementary Table S1. Two threshold values, fold change ≥ 2 and Student’s t-test of P < 0.05, were considered when determining the up- or downregulation of ALS in the R compared with the S plants. The experiment had six biological replicates, each consisting of an individual plant, and each biological replicate contained three technical replicates.
Cross-Resistance to Different Herbicides
To determine the resistance level of the R population AHFT-4 to different herbicides, greenhouse pot experiments at the whole-plant level were performed. A total of five ALS-inhibiting herbicides and one photosystem II (PSII)-inhibiting herbicide were selected for the testing, and a series of doses for each herbicide was determined (summarized in Table 1) via a preliminary experiment (data not shown). At 21 DAT, the aboveground biomass of the plants per pot was determined and expressed as a percentage of the untreated control. The experiment was conducted twice in the same place under similar conditions, first in the autumn of 2021 and then again in the spring of 2022, with each treatment consisting of at least three biological replicates.
Table 1. Herbicides used and rates applied in the Alopecurus aequalis whole-plant dose–response experiments. a
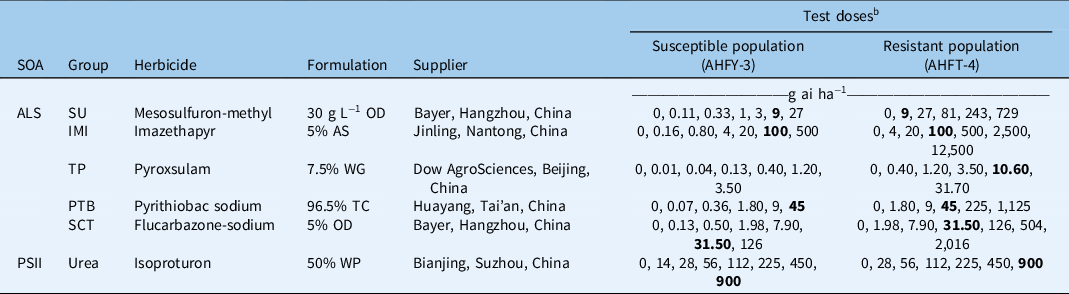
a Abbreviations: ALS, acetolactate synthase; AS, aqueous solution; IMI, imidazolinone; OD, oil dispersion; PSII, photosystem II; PTB, pyrimidinyl-thiobenzoate; SCT, sulfonyl-aminocarbonyl-triazolinone; SOA, site of action; SU, sulfonylurea; TC, technical concentrate; TP, triazolopyrimidine; WG, water-dispersible granule; WP, wettable powder.
b The field-recommended rate is shown in bold.
Susceptibility to Mesosulfuron-Methyl following P450 or GST Inhibition
Alongside the whole-plant dose–response assays, the susceptibility of both S and R populations to mesosulfuron-methyl following P450 or GST inhibition was also determined. In this study, malathion (95%; Sunjoy AgroScience, Ningbo, China) and 4-chloro-7-nitrobenzoxadiazole (NBD-Cl) (98%; Aladdin, Shanghai, China) were separately used as P450 and GST inhibitors (Christopher et al. Reference Christopher, Preston and Powles1994; Cummins et al. Reference Cummins, Wortley, Sabbadin, He, Coxon, Straker, Sellars, Knight, Edwards and Hughes2013), respectively, and the experiment was conducted as described in Zhao et al. (Reference Zhao, Yan, Ge, Zhu, Liu and Wang2019). Briefly, the seedlings of S and R populations were grown to the 3- to 4-leaf stage under the conditions described earlier and were treated with malathion, malathion plus mesosulfuron-methyl, NBD-Cl, or NBD-Cl plus mesosulfuron-methyl. Of these, malathion at 1,000 g ai ha−1 and NBD-Cl at 270 g ai ha−1 were applied 1 h and 48 h before the mesosulfuron-methyl application, respectively, and mesosulfuron-methyl was applied at the same doses used in the cross-resistance testing (Table 1). At 21 DAT, the aboveground biomass of the plants per pot was recorded and expressed as a percentage of the untreated control. As stated previously, the experiment was also conducted twice, with each treatment consisting of at least three biological replicates.
Statistical Analyses
One-way ANOVA of the data sets from the repeated runs of each experiment was conducted in SPSS v. 19.0 software (IBM, Armonk, NY, USA). As no significant difference (P > 0.05) was detected between the repeated runs, the data from the same trial were pooled across runs and fit to a four-parameter nonlinear logistic model in SigmaPlot v. 12.0 software (Systat Software, San Jose, CA, USA):
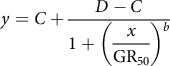 $$y = C + {{D - C} \over {1 + {{\left( \displaystyle{{x \over {{\rm{G}}{{\rm{R}}_{50}}}}} \right)}^b}}}$$
$$y = C + {{D - C} \over {1 + {{\left( \displaystyle{{x \over {{\rm{G}}{{\rm{R}}_{50}}}}} \right)}^b}}}$$
where the dependent variable y is the growth response at the independent variable x (i.e., herbicide dose), C is the lower limit of response, D is the upper limit of response, and b is the slope at the herbicide dose resulting in a 50% growth reduction (GR50).
The resistance index (RI) was calculated based on the GR50 values of the R versus S population, with the population susceptibility to each herbicide categorized according to Beckie and Tardif (Reference Beckie and Tardif2012).
Results and Discussion
Susceptibility and Resistance Level of Alopecurus aequalis to Mesosulfuron-Methyl
The herbicide susceptibility of the S (AHFY-3) and R (AHFT-4) populations of A. aequalis was assessed by applying mesosulfuron-methyl at a label rate of 9 g ai ha−1 postemergence. Based on a visual check at 21 DAT, all the mesosulfuron-methyl–treated plants in the AHFY-3 population had died, while all those in the AHFT-4 population survived (Figure 1), confirming susceptibility in S and resistance in suspected resistant R to mesosulfuron-methyl.
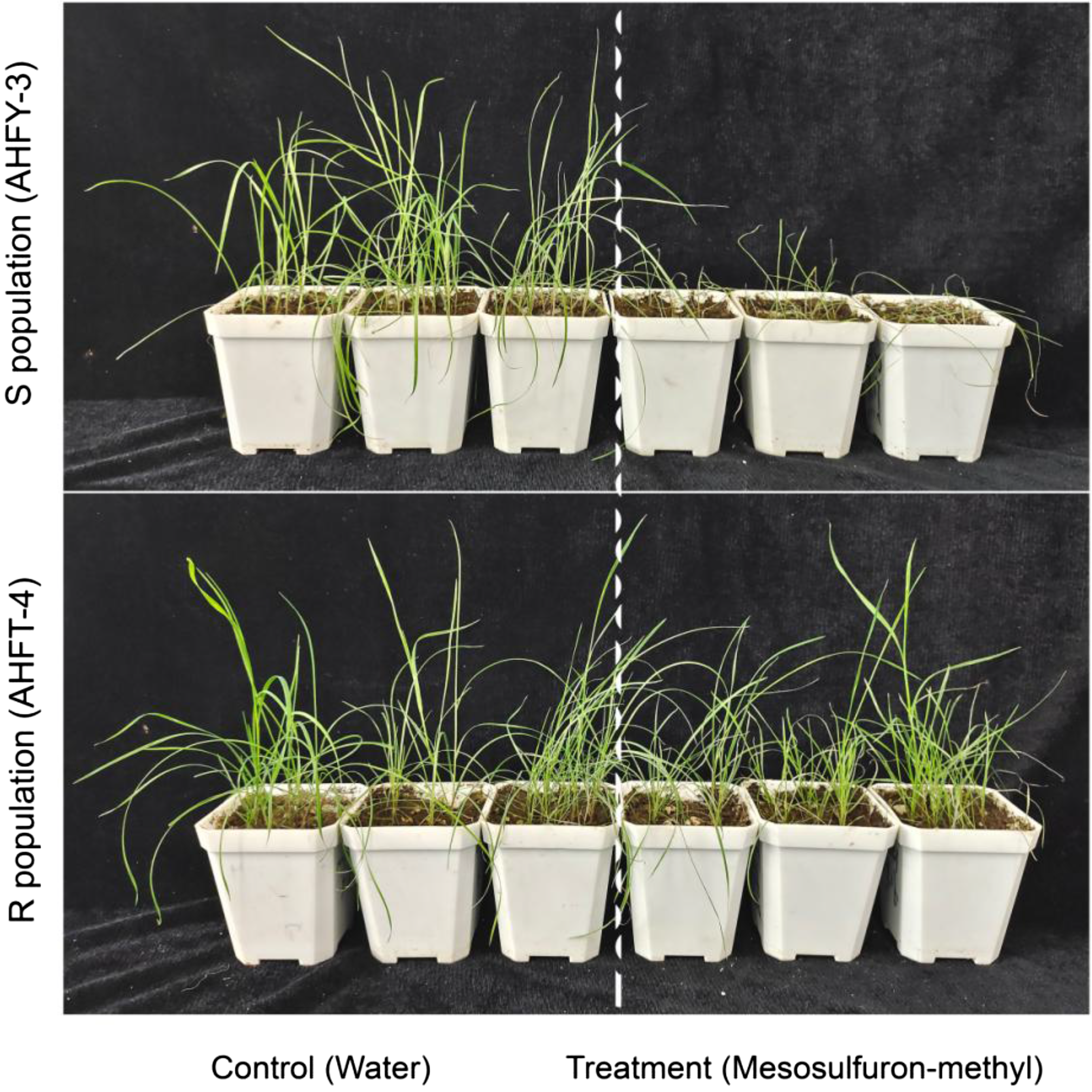
Figure 1. Herbicide susceptibility of the susceptible (S, AHFY-3) and putative resistant (R, AHFT-4) Alopecurus aequalis populations to mesosulfuron-methyl. Weed seedlings of both populations were treated with mesosulfuron-methyl at the field-recommended rate (9 g ai ha−1) when they reached the 3- to 4-leaf stage, followed by photography at 21 days after treatment.
The GR50 value of AHFT-4 treated with mesosulfuron-methyl was 40.99 g ai ha−1, which was much higher than the field-recommended rate (9 g ai ha−1) and that of the S population AHFY-3 (0.48 g ai ha−1) (Table 2). Based on the RI value, AHFT-4 was confirmed to be about 85-fold more resistant to mesosulfuron-methyl than AHFY-3.
Table 2. Parameter values of the four-parameter log-logistic equation (Equation 1) used to fit the plant growth response (as a percentage of the untreated control) resulting from the different herbicide doses. a
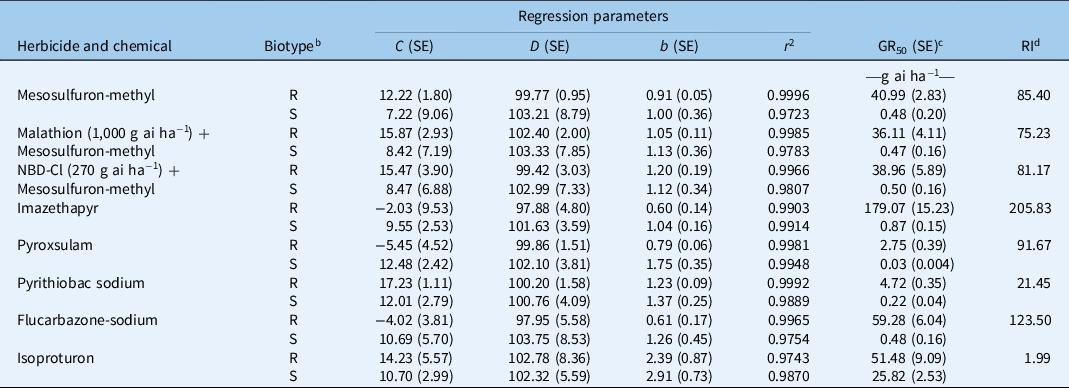
a Equation 1: y = C + (D – C)/[1 + (x/GR50) b ], where C is the lower limit, D is the upper limit, b is the slope of the curve at GR50 (the herbicide dose resulting in a 50% growth reduction), and y is the response at the herbicide dose x.
b R, resistant Alopecurus aequalis population AHFT-4; S, susceptible A. aequalis population AHFY-3.
c No significant difference (P > 0.05) was observed in the susceptibilities of R or S plants to mesosulfuron-methyl without or with cytochrome P450 monooxygenases (P450s)/glutathione S-transferases (GSTs) inhibition.
d RI, resistance index. RI was calculated by dividing the obtained GR50 value of the R population by that of the S population.
Due to its high efficiency and selectivity, the ALS inhibitor mesosulfuron-methyl has been widely used for controlling both grass and broadleaf weeds in wheat fields worldwide. However, it has been ascertained that the long-term use of the same MOA herbicides is one of the greatest risk factors for rapid resistance evolution (Norsworthy et al. Reference Norsworthy, Ward, Shaw, Llewellyn, Nichols, Webster, Bradley, Frisvold, Powles and Burgos2012). Unsurprisingly, resistance to mesosulfuron-methyl has occurred in diverse arable weeds in recent years, such as jointed goatgrass (Aegilops cylindrica Host) (Rodriguez et al. Reference Rodriguez, Hauvermale, Carter, Zuger and Burke2021), B. sterilis (Sen et al. Reference Sen, Hamouzová, Mikulka, Bharati, Košnarová, Hamouz, Roy and Soukup2021), and Italian ryegrass [Lolium perenne L. ssp. multiflorum (Lam.) Husnot] (Bobadilla et al. Reference Bobadilla, Hulting, Berry, Moretti and Mallory-Smith2021; Tehranchian et al. Reference Tehranchian, Nandula, Matzrafi and Jasieniuk2019). In the present study, the putative resistant A. aequalis population AHFT-4 collected from a wheat field with a rice rotation had been treated with mesosulfuron-methyl for approximately 17 yr. Single-dose testing and whole-plant dose–response bioassay confirmed that AHFT-4 had evolved high-level resistance to mesosulfuron-methyl. ALS resistance in A. aequalis was first documented in 2015 in Shou County, Anhui Province, China (Guo et al. Reference Guo, Yuan, Liu, Bi, Du, Zhang, Li and Wang2015), and this study indicates that resistance is continuing to evolve along with the large-scale use of ALS herbicides.
ALS Sequencing, dCAPS Genotyping, and ALS Expression
The full-length sequences of ALS1 and ALS2 spanning all eight known mutation sites were amplified from each individual of both populations. Sequence alignment showed that the two populations had approximately 99.97% homology with the same regions of the documented ALS genes of A. aequalis (GenBank accession nos. LC200800 and LC200801), indicating the targeted ALS genes were successfully amplified. Comparison between the ALS genes of the two populations revealed a single nucleotide change, of CCC to GCC, resulting in a homozygous or heterozygous Pro-197-Ala substitution in the ALS1 of each R individual (Figure 2). No known resistance AAS occurred in the ALS2 of the R plants (data not shown).
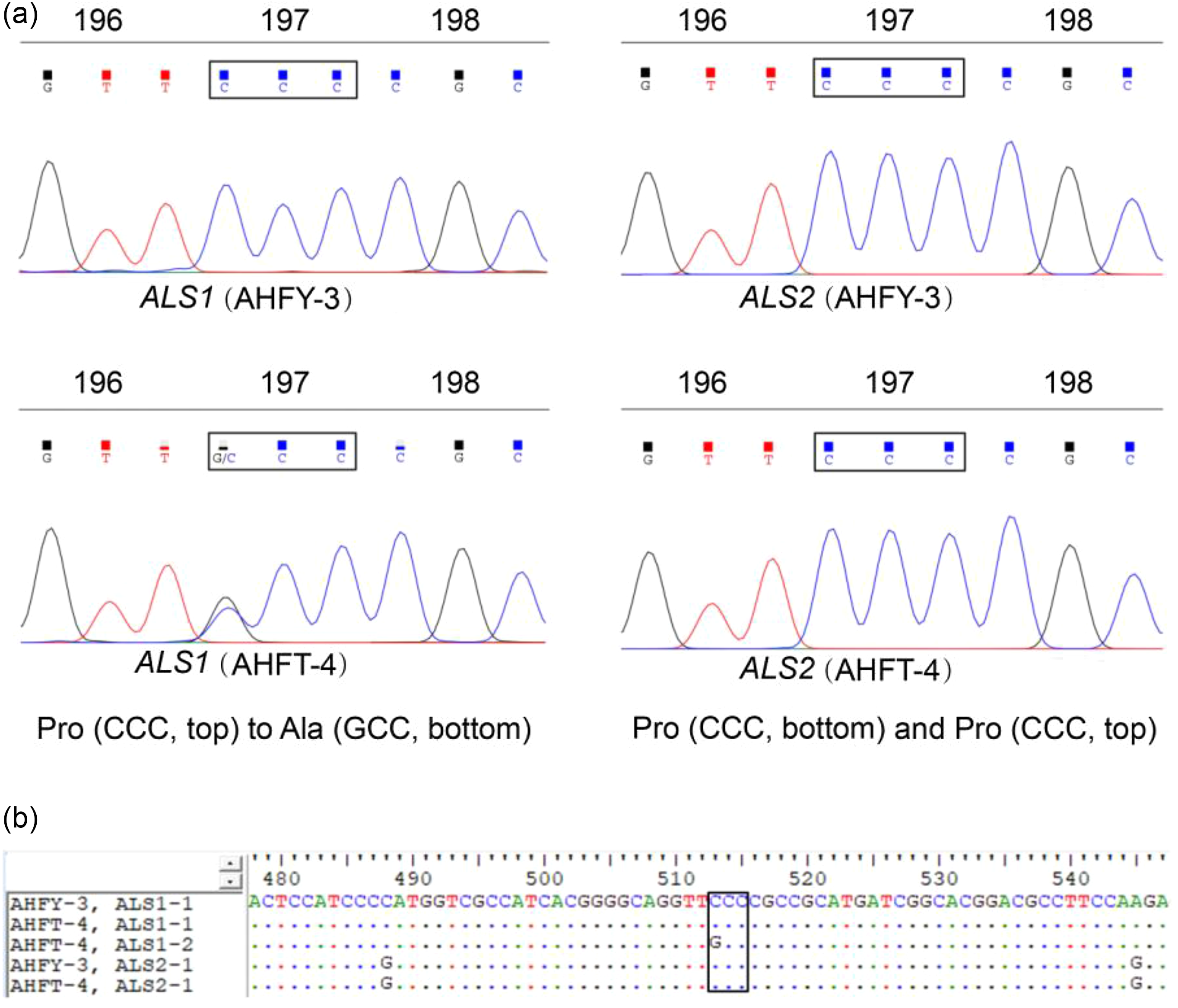
Figure 2. (A) Direct sequencing of different copies of acetolactate synthase (ALS) showing the Pro-197-Ala substitution in ALS1 of the resistant (R, AHFT-4) Alopecurus aequalis plants compared with the susceptible (S, AHFY-3) plants. (B) Partial sequences of ALS1 and ALS2 derived from both S and R plants, with the boxed region indicating the Pro-197-Ala substitution at codon position 197 of ALS1.
All the S and R individuals used for ALS sequencing were selected for the dCAPS assays. In this study, the dCAPS primers designed could not distinguish the different copies of ALS. Therefore, the same regions of both ALS1 and ALS2 would have been amplified from A. aequalis. After restriction digestion, the wild ALS sequences of each S plant showed only an undigested band of 178 bp (SS band in Figure 3), indicating that they were all homozygous susceptible at the Pro-197 codon for both ALS1 and ALS2. In contrast, the ALS sequence of each R plant showed both an undigested band of 178 bp and a digested band of 144 bp (and a digested band of 34 bp invisible on the agarose gel) (RS bands in Figure 3), indicating that they each carried the wild Pro-197 and mutant Pro-197-Ala sequences. Because no RR bands (i.e., two digested bands of 144 bp and 34 bp) were detected, no plant was expected to be homozygous resistant at the Pro-197 codon for both ALS copies. However, based on the sequencing data obtained in identifying the ALS mutation, the R plants could be homozygous resistant at the Pro-197 codon for ALS1.
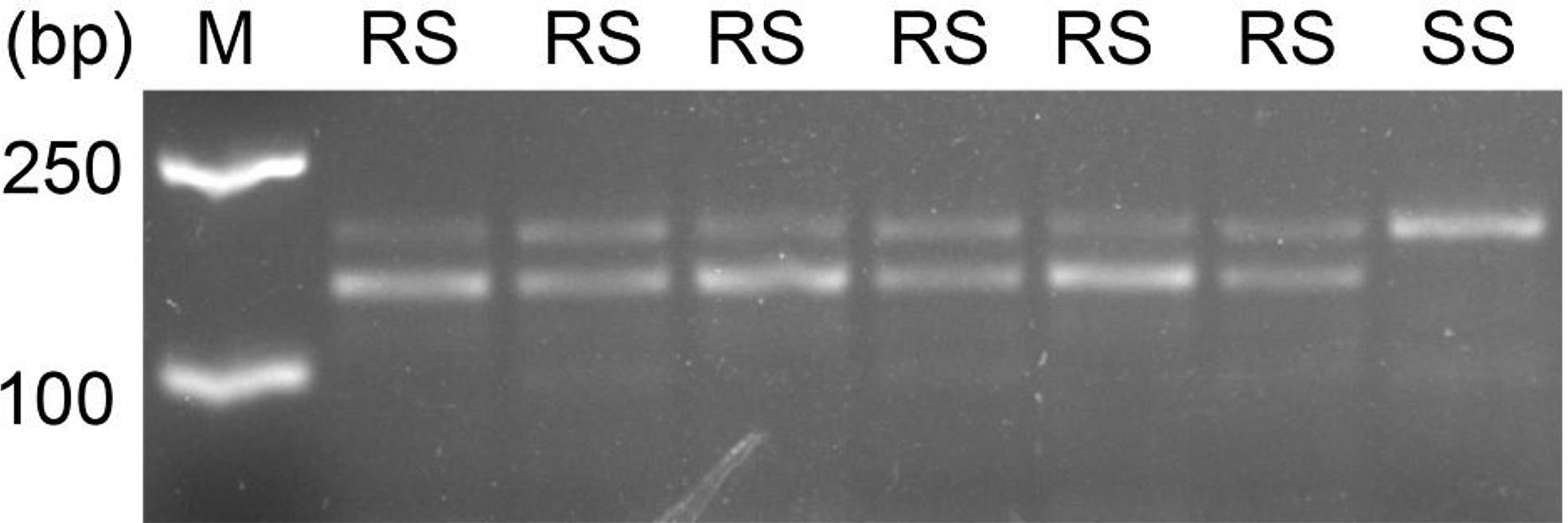
Figure 3. The derived cleaved amplified polymorphic sequence (dCAPS) marker developed for detection of Pro-197-Ala mutation in the acetolactate synthase (ALS) of resistant Alopecurus aequalis plants. The HaeIII digestion pattern indicates that the unrestricted fragment (178 bp) corresponds to the wild-type Pro-197 allele (S, susceptible), and the two restricted fragments (144 and 34 bp) correspond to resistant Pro-197-Ala allele (R, resistant). M, 2k DNA marker; RS, heterozygous resistant; SS, homozygous susceptible.
The total expression of different ALS copies was normalized relative to that of CAP, EIF4A, and TUB and compared between the S and R populations of A. aequalis at 0 (CK), 12, 24, and 48 HAT. Based on the data obtained (Figure 4), there was no significant difference (fold change <2) in total ALS expression between the two populations before and after mesosulfuron-methyl treatment. This suggested that mesosulfuron-methyl resistance in the R plants could not involve ALS overexpression.
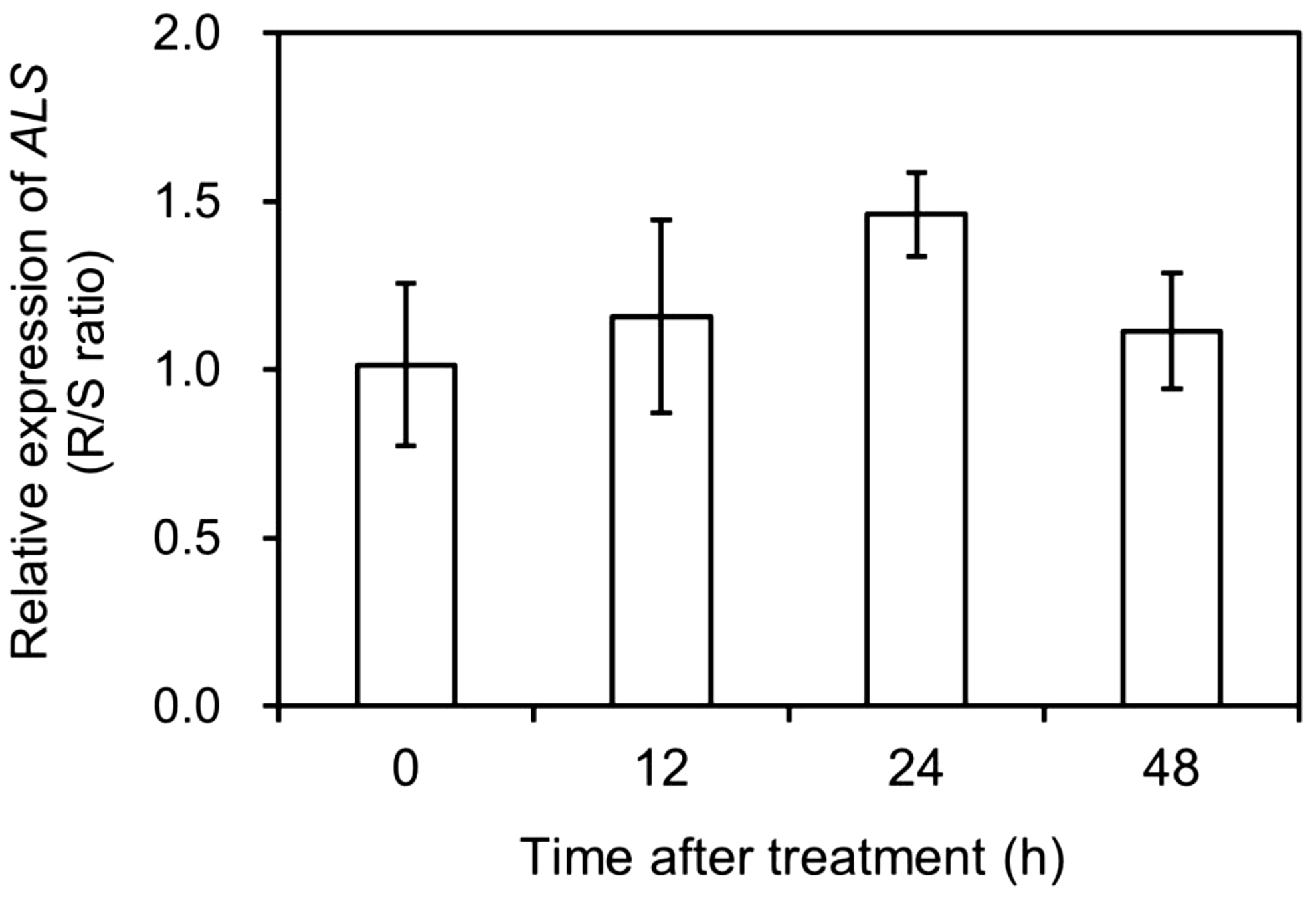
Figure 4. Relative expression levels of acetolactate synthase (ALS) in Alopecurus aequalis plants at 0 (CK), 12, 24, and 48 h after mesosulfuron-methyl treatment. No significant difference (Fold change < 2) was detected in total ALS expression between the susceptible (S) and resistant (R) plants at each time point. Vertical bars represent the standard errors of the means.
To date, the ALS gene mutation is one of the most commonly reported mechanisms responsible for ALS resistance, and diverse mutations have been detected in different resistant weed species (Murphy and Tranel Reference Murphy and Tranel2019; Yu and Powles Reference Yu and Powles2014). For A. aequalis specifically, five mutations (Pro-197-Arg/Ser/Thr/Tyr and Trp-574-Leu) at codon positions 197 and 574 of ALS are now known to impact the efficacies of ALS-inhibiting herbicides (Guo et al. Reference Guo, Chi, Feng, Tian, Liu and Wang2018; Xia et al. Reference Xia, Pan, Li, Wang, Feng and Dong2015; Zhao et al. Reference Zhao, Yan, Du, Zhang, Liu and Wang2020). In the current study, sequence analysis of the S and R populations of A. aequalis revealed a Pro-to-Ala substitution at codon position 197 of ALS1 in the R plants, which has been reported to confer ALS resistance in many other weed species (Kaloumenos et al. Reference Kaloumenos, Tsioni, Daliani, Papavassileiou, Vassileiou, Laoutidou and Eleftherohorinos2012; Mora et al. Reference Mora, Cheimona, Palma-Bautista, Rojano-Delgado, Osuna-Ruiz, de la Cruz and De Prado2019; Ntoanidou et al. Reference Ntoanidou, Kaloumenos, Diamantidis, Madesis and Eleftherohorinos2016; Sada and Uchino Reference Sada and Uchino2017; Yu et al. Reference Yu, Han and Powles2008). The follow-up RT-qPCR assays showed no significant difference in total ALS expression between the S and R plants, implying the molecular basis of TSR in the resistant population AHFT-4 was the Pro-197-Ala mutation of ALS1. A dCAPS assay was then developed to specifically detect the ALS mutation, because it was identified for the first time in the ALS-resistant A. aequalis. Similar results have also been reported in both grass and broadleaf weeds, such as the ALS-resistant L. multiflorum (Tehranchian et al. Reference Tehranchian, Nandula, Matzrafi and Jasieniuk2019) and redroot pigweed (Amaranthus retroflexus L.) (Sibony et al. Reference Sibony, Michel, Haas, Rubin and Hurle2001).
Cross-Resistance of Alopecurus aequalis to Other Herbicides
Whole-plant dose–response experiments were conducted to determine the cross-resistance pattern of the R population AHFT-4 to different classes of ALS inhibitors (Figure 5B–E). As expected, the GR50 values of the S population AHFY-3 treated with each herbicide ranged from 0.03 to 0.87 g ai ha−1 (Table 2), much lower than their respective field-recommended rates (Table 1). This indicated that AHFY-3 was susceptible to all the ALS inhibitors tested. In comparison, AHFT-4 was highly resistant to the IMI herbicide imazethapyr (Figure 5B), the TP herbicide pyroxsulam (Figure 5C), the PTB herbicide pyrithiobac sodium (Figure 5D), and the SCT herbicide flucarbazone-sodium (Figure 5E), with RI values ranging from 21 to 206 (Table 2).
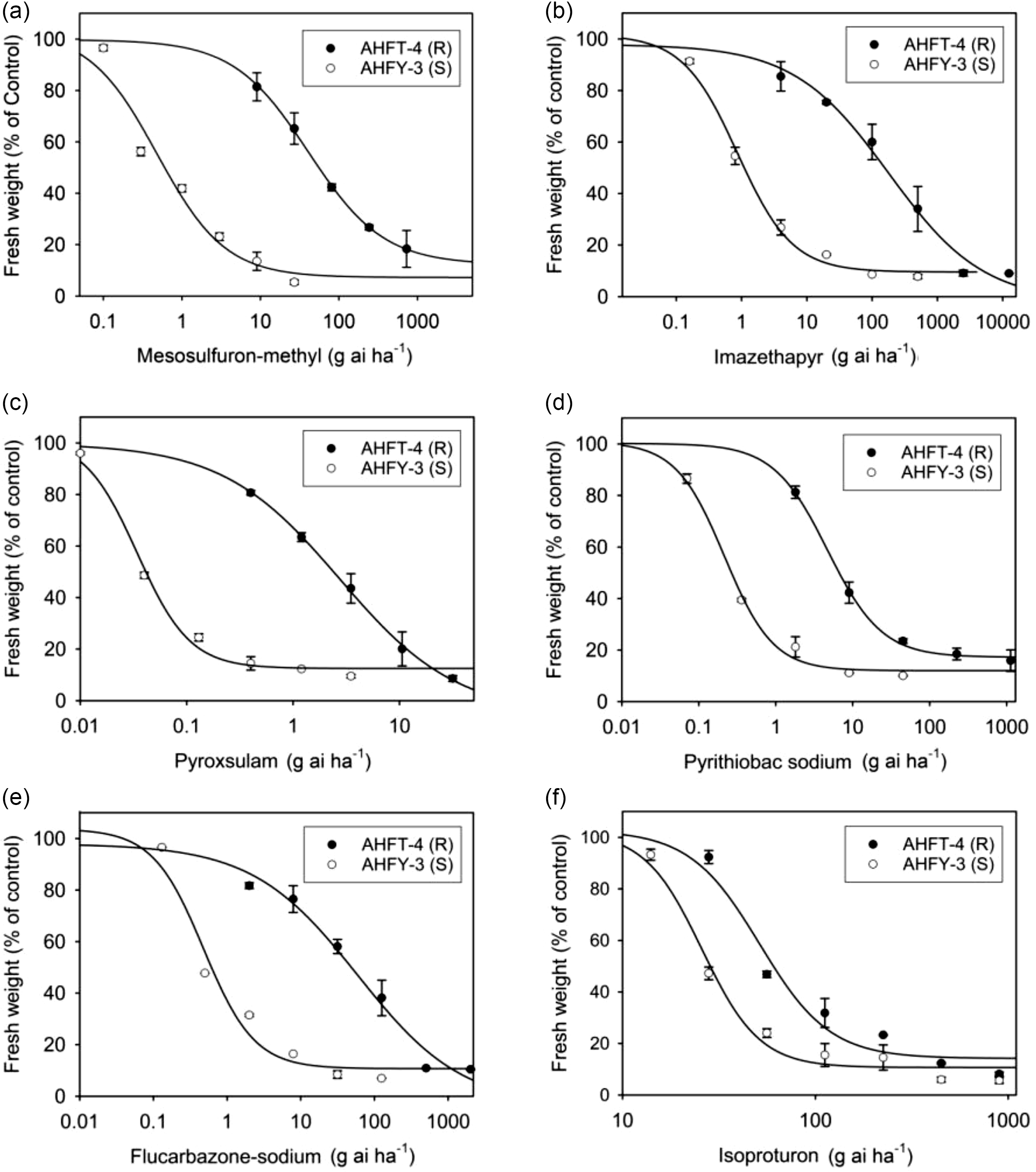
Figure 5. Whole-plant dose–response experiments showing the susceptibility levels of susceptible (S) and resistant (R) Alopecurus aequalis populations to different herbicides, including (A) mesosulfuron-methyl, (B) imazethapyr, (C) pyroxsulam, (D) pyrithiobac sodium, (E) flucarbazone-sodium, and (F) isoproturon. Vertical bars represent the standard errors of the means.
As documented, different ALS mutations can confer distinct cross-resistance patterns, depending on the position and type of substitution, and sometimes, the weed species (Yu and Powles Reference Yu and Powles2014). Generally, AASs at codon 197 tend to confer SU and TP resistance; AASs at codons 122, 653, and 654 tend to confer IMI resistance; and AASs at codons 376 and 574 can confer broad-spectrum ALS resistance (Tranel et al. Reference Tranel, Wright and Heap2022). In this study, the ALS1 gene Pro-197-Ala mutant plants of A. aequalis showed high-level resistance to ALS-inhibiting herbicides of all five chemical families tested, with RI values ranging from 21 to 206. It is worth noting that the resistance pattern observed in this study differed from that reported in other weed species with the same mutation, wherein the mutant plants only showed confined cross-resistance (Mora et al. Reference Mora, Cheimona, Palma-Bautista, Rojano-Delgado, Osuna-Ruiz, de la Cruz and De Prado2019; Ntoanidou et al. Reference Ntoanidou, Kaloumenos, Diamantidis, Madesis and Eleftherohorinos2016; Sada and Uchino Reference Sada and Uchino2017; Yu et al. Reference Yu, Han and Powles2008). This disparity may be partially attributed to variations in weed species and herbicide application histories, as well as the potential presence of other unidentified mechanisms of resistance, such as enhanced herbicide metabolism and the participation of endophytes (Liu et al. Reference Liu, Luo, Mao, Pan, Yan, Wu, Hu, Liu, Liu and Bai2020). Further analysis of the effects of the Pro-197-Ala mutation on ALS binding affinity for different ALS inhibitors could help elucidate the structural basis for the broad-spectrum cross-resistance observed in herbicide-resistant A. aequalis (Liu et al. Reference Liu, Fang, He, Li and Dong2019).
This study also determined the susceptibility of both populations to the PSII inhibitor isoproturon (Figure 5F). The GR50 values of AHFY-3 and AHFT-4 treated with isoproturon were 25.82 and 51.49 g ai ha−1, respectively (Table 2), much lower than the field-recommended rate (900 g ai ha−1). Moreover, the application of isoproturon at 450 g ai ha−1 resulted in approximately 90% growth inhibition of both S and R plants (Figure 5F). Therefore, although the susceptibility of the R population to isoproturon seemed to have decreased compared with the S population (RI < 2), both populations could still be effectively controlled by its application at the label rates. Isoproturon applied in combination or rotation with herbicides with different MOAs, such as the novel 4-hydroxyphenylpyruvate dioxygenase inhibitor cypyrafluone (Zhang et al. Reference Zhang, Bai, Wang, Liu and Wang2019), may be an ideal alternative to delay the evolution of resistance in A. aequalis.
P450 and GST Inhibitory Effects on the Mesosulfuron-Methyl Resistance
The application of the P450 inhibitor malathion or the GST inhibitor NBD-Cl alone did not have a significant effect on the growth of both S and R plants (data not shown). Moreover, pretreatment with malathion or NBD-Cl did not influence the herbicide susceptibility in either population (Figure 6), as evidenced by the relatively stable GR50 values (P < 0.05) (Table 2). These results suggest that P450s and GSTs may not be involved in mesosulfuron-methyl resistance in the R plants.
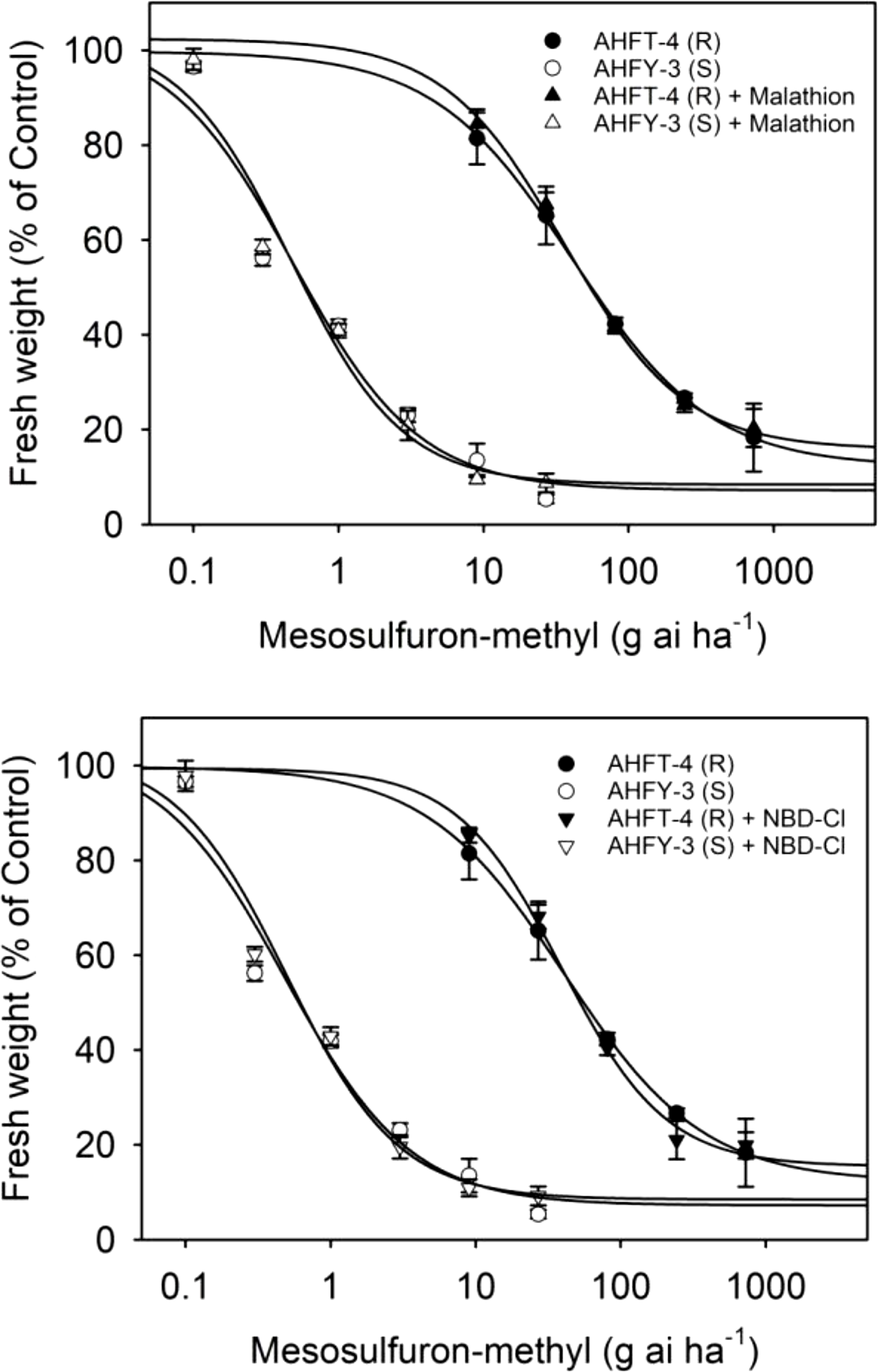
Figure 6. Dose–response curves for the fresh weights of susceptible (S) and resistant (R) Alopecurus aequalis populations treated with a range of mesosulfuron-methyl doses minus or plus 1,000 g ai ha−1 malathion or 270 g ai ha−1 4-chloro-7-nitrobenzoxadiazole (NBD-Cl). Vertical bars represent the standard errors of the means.
Enhanced rates of herbicide metabolism are now recognized as a major NTSR mechanism in many herbicide-resistant weeds (Rigon et al. Reference Rigon, Gaines, Küpper and Dayan2020), including A. aequalis (Zhao et al. Reference Zhao, Yan, Ge, Zhu, Liu and Wang2019). However, this study has found that enhanced herbicide metabolism through P450s and GSTs may not be responsible for the observed resistance in the R plants (Gaines et al. Reference Gaines, Duke, Morran, Rigon, Tranel, Küpper and Dayan2020). Despite this, the R population showed high-level resistance to ALS inhibitors of all five chemical families tested. Further investigation is thus necessary to determine whether enhanced activity of other relevant herbicide-detoxifying enzymes contributes to resistance to other ALS inhibitors in the R plants. Determining the difference in metabolism rates for different ALS herbicides between S and R plants may help explain the distinct resistance pattern observed in the R population.
In summary, to our knowledge, this study has identified the first case of an A. aequalis population that developed high-level resistance to mesosulfuron-methyl due to a Pro-197-Ala mutation in the ALS1 gene. The study also characterized the comprehensive cross-resistance pattern of TSR resulting from this specific ALS1 mutation in A. aequalis. However, it remains to be determined whether other mechanisms, such as enhanced herbicide metabolism, contributed to the resistance to different ALS inhibitors. The ongoing evolution of herbicide resistance in A. aequalis highlights the necessity for implementing an IWM program in wheat production.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/wsc.2023.23
Acknowledgments
This research was funded by the National Natural Science Foundation of China (no. 32102237), the Anhui Provincial Natural Science Foundation (no. 2108085QC115), and the Talent Research Project of Anhui Agricultural University (no. rc342004). No conflicts of interest have been declared.










