Introduction
Benzobicyclon is a 4-hydroxyphenylpyruvate dioxygenase (HPPD)-inhibiting herbicide that has activity on a wide range of aquatic, grass, sedge, and broadleaf weeds in flooded rice (Oryza sativa L.) fields (Sekino et al. Reference Sekino, Koyanagi, Ikuta and Yamada2008). Tolerance in rice can vary depending on the genetic background of each cultivar, but in general, japonica cultivars exhibit tolerance to benzobicyclon, while most but not all indica cultivars are sensitive (Kwon et al. Reference Kwon, Shin, An, Lee, Min, Park, Shin, Jung and Kuk2012; Lv et al. Reference Lv, Zhang, Yuan, Huang, Peng, Peng, Li, Tang, Liu, Zhou, Wang, Pan, Shao, Xin and Zhu2021; Maeda et al. Reference Maeda, Murata, Sakuma, Takei, Yamazaki, Karim, Kawata, Hirose, Kawagishi-Kobayashi, Taniguchi, Suzuki, Sekino, Ohshima, Kato, Yoshida and Tozawa2019; Young et al. Reference Young, Norsworthy, Scott, Bond and Heiser2018). The HPPD Inhibitor Sensitive 1 (HIS1) gene is responsible for tolerance in rice, which is conferred through hydroxylation of benzobicyclon hydrolysate (active parent) into a less phytotoxic compound and not benzobicyclon (proform) (Maeda et al. Reference Maeda, Murata, Sakuma, Takei, Yamazaki, Karim, Kawata, Hirose, Kawagishi-Kobayashi, Taniguchi, Suzuki, Sekino, Ohshima, Kato, Yoshida and Tozawa2019). Sensitivity in indica cultivars is either the result of a loss-of-function frameshift mutation (28-bp deletion) resulting in truncated protein (Maeda et al. Reference Maeda, Murata, Sakuma, Takei, Yamazaki, Karim, Kawata, Hirose, Kawagishi-Kobayashi, Taniguchi, Suzuki, Sekino, Ohshima, Kato, Yoshida and Tozawa2019) or a missense mutation located in the region of the putative substrate pocket that encodes for a glycine instead of a valine at the 286th (Val-286-Gly) amino acid position (Lv et al. Reference Lv, Zhang, Yuan, Huang, Peng, Peng, Li, Tang, Liu, Zhou, Wang, Pan, Shao, Xin and Zhu2021). Hereafter, a less than fully functional HIS1 allele with either mutation will be referred to as his1.
Although the presence or absence of HIS1 in rice is the major contributing factor in determining sensitivity, the spatial–temporal expression profile of HIS1 and its role in tolerance is less understood. Based on the Rice Expression Profile Database, RiceXPro (Sato et al. Reference Sato, Takehisa, Kamatsuki, Minami, Namiki, Ikawa, Ohyanagi, Sugimoto, Antonio and Nagamura2013), HIS1 is not a constitutively expressed gene; however, it was preferentially expressed in leaf blades and sheaths with little to no expression detected in roots (Maeda et al. Reference Maeda, Murata, Sakuma, Takei, Yamazaki, Karim, Kawata, Hirose, Kawagishi-Kobayashi, Taniguchi, Suzuki, Sekino, Ohshima, Kato, Yoshida and Tozawa2019; Xia et al. Reference Xia, Zou, Sang, Xu, Yin, Li, Wu, Hu, Hao and Zhang2017). Ancillary data from Maeda and coauthors (Reference Maeda, Murata, Sakuma, Takei, Yamazaki, Karim, Kawata, Hirose, Kawagishi-Kobayashi, Taniguchi, Suzuki, Sekino, Ohshima, Kato, Yoshida and Tozawa2019) implicated HIS1 as a limiting factor in tolerance, as ‘Nipponbare’ (japonica) was tolerant to benzobicyclon at 0.3 μM but severely bleached at 0.5 μM; however, when HIS1 was constitutively expressed by the 35S promoter in transgenic lines, no bleaching symptomology appeared to be prevalent at 0.5 μM. On this point, research has shown crop response to benzobicyclon can vary among japonica cultivars, albeit minimally. For example, Kwon et al. (Reference Kwon, Shin, An, Lee, Min, Park, Shin, Jung and Kuk2012) observed the phytotoxic ratings for 18 transplanted japonica cultivars (∼25-d old) ranged from 0 to 2 out of a 9 scale after applying benzobicyclon at 400 g ha−1. These differences could potentially be explained by differential gene expression, but this needs to be explored further.
In Arkansas, weedy rice (unwanted rice; Oryza sativa L.) is a problematic weed, and growers are currently heavily reliant on herbicide-resistant rice technology (e.g., Clearfield® and Max-Ace®) for its control (FIFRA 2021; Hardke Reference Hardke2021; Young et al. Reference Young, Norsworthy, Scott, Bond and Heiser2018). In this paper, weedy rice is considered unwanted rice, including red rice or off-types from previous plantings of hybrid rice. Benzobicyclon at the label rate of 371 g ha−1 has shown some potential to be a complementary piece for a grower’s weedy rice control program in both conventional and herbicide-resistant rice. Unfortunately, numerous weedy rice accessions in Arkansas are already segregating for HIS1, and this is mostly likely the consequence of gene flow with the cultivated japonica rice grown in Arkansas (Brabham et al. Reference Brabham, Norsworthy and González-Torralva2020; Chen et al. Reference Chen, Lee, Song, Suh and Lu2004; Shivrain et al. Reference Shivrain, Burgos, Gealy, Sales and Smith2009; Young et al. Reference Young, Norsworthy, Scott, Bond and Heiser2018). Thus, the success of benzobicyclon in a particular field will be highly dependent on the accession’s HIS1 allele frequency; however, preliminary research suggests plant growth stage at the time of benzobicyclon application may have a role in determining weedy rice sensitivity and even crop tolerance regardless of HIS1 genotype (Castner et al. Reference Castner, Norsworthy, Brabham and Sandoski2020; Patterson et al. Reference Patterson, Norsworthy, Lancaster, Beesinger and France2020). We hypothesize that HIS1 expression is growth stage dependent, but more research is needed to understand the genetic contribution of HIS1 to benzobicyclon sensitivity. In this paper, we used different rice cultivars, either indica (‘Purple Marker’ and ‘Rondo’) or japonica (‘CLXL745’, ‘Diamond’, ‘Jupiter’, ‘LaKast’, ‘Roy J’, and ‘XL753’) to explore three major objectives: 1) to confirm the inheritance of HIS1 and the tolerance of heterozygotes to benzobicyclon, 2) to characterize the spatial–temporal gene expression profile of HIS1 and its role in tolerance, and 3) to use these data to build a model toward optimizing the utility of benzobicyclon as a weedy rice control tool.
Material and Methods
Response of HIS1 Heterozygous Plants to Benzobicyclon
Plant Material and Growth Conditions
F 1s of reciprocal crossed Roy J (HIS1/HIS1) × Rondo (his1/his1) and Jupiter (HIS1/HIS1) × Rondo (his1/his1) rice varieties were created at the Rice Research and Extension Center near Stuttgart, AR. The his1 allele in Rondo harbors the 28-bp deletion.
Rice seeds from these crosses were germinated in plastic trays containing potting mix (Sunshine premix No. 1®, Sun Gro Horticulture, Agawam, MA, USA) at the Altheimer Laboratory in Fayetteville, AR. Seedlings at the 1-leaf stage were then transplanted into plastic buckets (7.5 L, 24 cm in diameter) filled to three-quarter capacity with sieved Pembroke silt loam (fine-silty, mixed, active, mesic Mollic Paleudalfs) soil (pH of 6.6 and 2.4% organic matter). Plants were maintained under greenhouse conditions at a 32/22 C day/night temperature regime and a 16-h photoperiod consisting of natural lighting supplemented with halide lights. Plants were fertilized weekly following the manufacturer’s instructions (Miracle-Gro® Water All Purpose Plant Food, Scotts Miracle-Gro, Marysville, OH, USA). When plants reached the 5-leaf growth stage, a 5-cm flood was established 24 h before benzobicyclon treatment and maintained until the end of the experiment.
Dose–Response Experiments
To assess the susceptibility of F 1 progeny to benzobicyclon, dose–response assays were carried out. Experiments were conducted as a completely randomized experimental design. Each bucket contained four plants, with one plant from each reciprocal crossed F 1 and its respective parents. The experiment was repeated in time; however, the number of replicates for each genotype at each rate varied from 6 to 12 because of limited availability of F 1 plants. Benzobicyclon was applied at 0, 23, 46, 93, 185, 371 (1×), 742, and 1,484 g ha−1. Methylated seed oil (MSO; Leci-Tech, Loveland Products, Loveland, CO, USA) was added to all treatments at 1% v/v to optimize performance of the herbicide (Brabham et al. Reference Brabham, Norsworthy, Sandoski, Varanasi and Schwartz-Lazaro2019). Treatments were applied using a research track sprayer equipped with two 1100067 flat-fan nozzles and calibrated to delivered 187 L ha−1. At 35 d after treatment (DAT), aboveground biomass was collected and oven-dried for 3 d at 66 C to obtain dry weight. Dose–response curves for dry weights were generated in R (R Core Team 2020) using the drc package (Ritz et al. Reference Ritz, Baty, Streibig and Gerhard2015). Based upon the Akaike information criterion (AIC) and lack of fit, dry weight data for all genotypes were simultaneously fit using the following four-parameter log-logistic model:
where Y represents dry weight, e is the ED50 (estimated dose required to reduce growth by 50%), c and d represent the lower and upper asymptotes, b represents the relative slope around e, and x represents the applied rate. The EDcomp function was used to compare the benzobicyclon response of F 1 plants to parental lines based on ED50 values.
Spatial–Temporal HIS1 Expression and Growth Stage Sensitivity to Benzobicyclon
A completely randomized mixed level-four factorial experiment was initiated to determine the spatial–temporal expression of HIS1 and its role in benzobicyclon tolerance. The four main factors were: cultivar, growth stage, tissue type, and timing after application. The five japonica varieties included in this study were the inbred cultivars Diamond, LaKast, and Roy J, plus two hybrid cultivars CLXL745 and XL753 (Supplemental Figure S1). Seeds of each hybrid (∼20 seeds) and inbred (∼30 seeds) cultivar were seeded directly onto soil in plastic buckets as described earlier, so the final plant stand, after the six plants were removed for RNA extraction, better mimicked field-like densities recommended by the University of Arkansas (Hardke Reference Hardke2021). To establish a difference in growth stages (second factor), a second planting was started (separate buckets) once plants from the first planting had 2 leaves. At the time of tissue collection and benzobicyclon application, cultivars from the first planting had 5 to 6 leaves (height range over runs was 33 to 45 cm for hybrids and 30 to 38 cm for inbreds), while plants in the second planting had 2 to 3 leaves (height range over runs was 22 to 28 cm for hybrids and 18 to 24 cm for inbreds). Plants were started in the greenhouse, and once the seedlings reached the spiking growth stage, were moved outdoors for the remainder of the experimental run. Plants were fertilized weekly with Miracle-Gro® following the manufacturer’s instructions. All experiments were conducted outside at the Altheimer Laboratory (36.098782°N, 94.179015°W) in 2018 between early May and September.
Once plants reached the desired leaf growth stage, a 5-cm-depth flood was established 96 to 120 h before tissue collection and benzobicyclon treatment and maintained until the end of the experiment (28 DAT). One day before benzobicyclon applications, three plants from each cultivar by growth stage were harvested (cut at the soil line) and dissected into leaf blades, sheaths, and whorls (third factor). The top 2 leaves with a visible leaf collar were dissected into blade and sheath. Afterward, any remaining tissue was removed from the plant to reveal the “whorl.” The whorl contained the growing point and any unfurled tissue with no visible collar, with particular attention paid to selecting plants with a relatively new emerging unfurled leaf. Approximately 24 h later, benzobicyclon at 371 g ha−1 plus MSO (1% v/v) was sprayed broadcast as described earlier. At 6 h after herbicide treatment (timing after application, fourth factor), three plants from each cultivar by growth stage were collected and dissected as described earlier. Tissue from three plants in the nontreated controls were also collected to account for any time of day difference in expression. For all collection time points and factor levels, tissue from the three subsamples was pooled and immediately frozen in liquid nitrogen and maintained at −80 C until use. At 28 DAT, plant height and subsequent dry weight from three plants per replication were recorded. The experiment was repeated three times, and each run had three replicates; however, gene expression data were only determined for the first two runs.
Quantitative Real-Time PCR (qPCR)
Tissue was homogenized in liquid nitrogen using a mortar and pestle, and RNA was extracted using the phenol and guanidine isothiocyanate extraction method (TRIzol Reagent, Invitrogen, Carlsbad, CA, USA). Before use, RNA quality and quantity were checked with a spectrophotometer (NanoDrop 2000c, Thermo Scientific, Waltham, MA, USA) and agarose gel (1%) electrophoresis. Isolated RNA was treated with DNase 1 to remove any DNA contamination, then first-strand complementary DNA (cDNA) was synthesized from 1 μg total RNA according to the iScript cDNA kit (Bio-Rad, Hercules, CA, USA). Primers used for HIS1 amplification were forward 5′- ACTCAACGAAGCTCCTGCATTT-3′ and reverse 5′-GAAGAATCGTCAAGAGGGTGCC-3′. Elongation factor 1 alpha (EF1) was utilized as the reference gene, and primer sequences were forward 5′-GTCATTGGCCACGTCGACTC-3′ and reverse 5′-TGTTCATCTCAGCGGCTTCC-3′ (Caldana et al. Reference Caldana, Scheible, Mueller-Roeber and Ruzicic2007). Primer efficiencies for HIS1 and EF1 across cultivars and tissue type ranged from 92% to 96%. A qPCR reaction mix consisted of 7.5 μl of SYBR Green Master Mix (Bio-Rad), 0.9 μl of a forward and reverse primer mix (final concentration per primer 0.3 μM), 1.5 μl of cDNA, and 5.1 μl water to make the total reaction 15 μl. PCR conditions were 50 C for 2 min; 95 C for 2 min; and 40 cycles of 95 C for 15 s, 57 C for 15 s, and 72 C for 1 min. A melt curve was added for quality analysis. Three biological and technical replicates were used per factor per run.
Before analysis, HIS1 expression was normalized to EF1 (ΔCt), and these values were used for ANOVA. Data met the basic assumptions of ANOVA, and no interactions with run as a factor were significant. Thus, data were pooled over runs. For any significant interactions, data were transformed into 2−ΔΔCt fold change values (Livak and Schmittgen Reference Livak and Schmittgen2001) and were standardized to HIS1 expression in Roy J (baseline cultivar used in most of our benzobicyclon experiments) or blade tissue (expression believed to be highest based on data from Kato et al. [Reference Kato, Maeda, Sunohara, Ando, Oshima, Kawata, Yoshida, Hirose, Kawagishi, Taniguchi, Murata, Maeda, Yamada, Sekino and Yamazaki2015]). Plant height and dry weight data for both treated and nontreated plants were standardized to the average value of the nontreated within a growth stage. Data met the basic assumptions of ANOVA, and no run interactions were detected, allowing data to be pooled across runs. Nontreated controls were included in this analysis. All analyses were performed in JMP (JMP v. 16, Cary, NC, USA), and Tukey’s HSD test was utilized to separate means at α = 0.05.
Weedy Rice Control Model Proof of Concept Study
To test the functionality of our weedy rice control model, proof of concept field experiments were initiated in 2019 at the Pine Tree Research Station near Colt, AR (hereafter referred to as the Pine Tree location; 35.126042°N, 90.9637°W) and at the Rice Research and Extension Center near Stuttgart, AR (hereafter referred to as the Stuttgart location; 34.466036°N, 91.402936°W). Field experiments were conducted on a Dewitt silt-loam soil (fine, smectitic, thermic Typic Albaqualfs) at Stuttgart and on a Calloway silt loam (fine-silty, mixed, active, thermic Aquic Fraglossudalfs) at Pine Tree. The soil at Stuttgart had a pH of 6.0 and organic matter content of 1.8% with 8.4, 71.4, and 20.2% sand, silt, and clay, respectively. The soil at Pine Tree had a pH of 7.5 and organic matter content of 1.3% with 10.6, 68.6, and 20.8% sand, silt, and clay, respectively. Experiments were setup as a quasi–two factor randomized complete block design with three replications but without nontreated controls. Factor A was weedy rice accession or cultivated rice line, and factor B was growth stage at application. A total of 16 weedy rice accessions were screened in this study, with 15 accessions collected in Arkansas (Brabham et al. Reference Brabham, Norsworthy and González-Torralva2020; Young et al. Reference Young, Norsworthy, Scott, Bond and Heiser2018) and XL745 F 2 plants (considered HIS1/HIS1 weedy rice). Roy J × Purple Marker F 1 plants (HIS1/his1), the susceptible cultivars Purple Marker and Rondo (his1/his1), and the tolerant cultivar LaKast (HIS1/HIS1) were included as genotypic internal standards. The HIS1 allele frequency for the 15 accessions was previously characterized by Brabham et al. (Reference Brabham, Norsworthy and González-Torralva2020). Therein, accessions 9, 10, 12, 24, and 25 had contrasting data between observed field sensitivity and predicted HIS1 genotype. Plants from these accessions were re-genotyped for the presence or absence of a 28-bp deletion in the HIS1 gene using the assay provided in Maeda et al. (Reference Maeda, Murata, Sakuma, Takei, Yamazaki, Karim, Kawata, Hirose, Kawagishi-Kobayashi, Taniguchi, Suzuki, Sekino, Ohshima, Kato, Yoshida and Tozawa2019).
Each experiment and subsequent blocks were contained within a single bay (21 by 18 m wide). A 10-row drill was used to create furrows and plot widths. An individual plot was 1.5 by 1.5 m wide with a 1-m alley between plots. A block was four plots long and two wide (two tractor passes), and there were three total blocks. Plots in the right column of a block contained accessions/cultivars at the first planting date and the parallel plot in the left column contained the second planting date. Five different accessions/cultivars were hand planted into every other row within a plot (50 seeds row−1), and the grouping of accessions/cultivars changed across blocks. To maintain experiments weed free, the Pine Tree location received an application of florpyrauxifen-benzyl (30 g ha−1) plus cyhalofop-butyl (313 g ha−1) and Stuttgart location received an application of quinclorac (420 g ha−1). A day before benzobicyclon application, an 8- to10-cm flood was established, with the Stuttgart trial flooded on May 29, and Pine Tree on June 12. At the time of treatment, accessions/cultivars at Pine Tree were at the 4- to 5-leaf growth stage in the first planting and 1- to 2-leaf stage in the second planting. At Stuttgart, plants in the first planting had 1 to 2 tillers and were at the 3- to 4-leaf growth stage in the second planting. Benzobicyclon at 371 g ha−1 plus MSO (1% v/v) was applied to the entire area of the bay using a CO2-pressurized backpack sprayer equipped with six AIXR110015 nozzles and calibrated to deliver 140 L ha−1. Percent control (injury) ratings were taken at 28 DAT using a 0% to 100% scale (where 0% and 100% corresponded to no control and complete control, respectively). Control of weedy rice or injury to cultivated rice was mainly a result of chlorosis or bleaching of plant tissue that eventually led to plant death when severe.
Results and Discussion
Response of HIS1 Heterozygous Plants to Benzobicyclon
Benzobicyclon tolerance in rice is conferred by a major semidominant to dominant gene, HIS1 (Kato et al. Reference Kato, Maeda, Sunohara, Ando, Oshima, Kawata, Yoshida, Hirose, Kawagishi, Taniguchi, Murata, Maeda, Yamada, Sekino and Yamazaki2015; Maeda et al. Reference Maeda, Murata, Sakuma, Takei, Yamazaki, Karim, Kawata, Hirose, Kawagishi-Kobayashi, Taniguchi, Suzuki, Sekino, Ohshima, Kato, Yoshida and Tozawa2019); however, the sensitivity of heterozygous HIS1 plants in comparison to parental lines has not been characterized in a controlled manner. Here, two different tropical japonica cultivars, Roy J and Jupiter (HIS1/HIS1) were reciprocally crossed to a known sensitive indica cultivar Rondo (his1/his1) to create F 1 heterozygous populations. There was no significant difference between reciprocally crossed F 1 families from the same parentage, as expected from a nuclear-encoded gene, and data were pooled across reciprocally crossed F 1 families (data not shown).
Rondo was highly sensitivity to benzobicyclon, with an ED50 value of 54 g ha−1, while Jupiter and Roy J were 3.2 and 3.5 times more tolerant, respectively, based on their ED50 values (Table 1; Supplemental Figure S2). Both F 1 families were significantly different from their respective maternal and paternal cultivars. The intermediate response confirms HIS1 is a semidominant gene. For example, the ED50 value for reciprocally crossed Roy J × Rondo F 1 plants, was 111 g ha−1 and corresponded to 2 and 0.6 times more tolerance in comparison to the parental lines of Rondo and Roy J, respectively. Overall, the ED50 values for Rondo, Jupiter, and Roy J and the average of F 1 families corresponded to roughly 1/6, 1/2, and 3/10 of the benzobicyclon label rate of 371 g ha−1 (FIFRA 2021). In the field, both Jupiter and Roy J (HIS1/HIS1) exhibited excellent crop tolerance to benzobicyclon at 494 g ha−1 (Young et al. Reference Young, Norsworthy, Scott, Bond and Heiser2018), and thus the lack of tolerance in the greenhouse to even a label rate in our study was perplexing. We cannot sufficiently explain this observation, but our overall object for this experiment was still met, as benzobicyclon sensitivity was confirmed to be controlled by HIS1, which is a single semidominant gene.
Table 1. Parameter estimates for benzobicyclon dose–response curves and estimated dose required for 50% growth reduction (ED50) of parental and F 1 families at 35 d after treatment.

a Dose–response curves for dry weight data were generated using a four-parameter log-logistic model. There was no significant difference between reciprocally crossed F 1 families from the same parentage and pooled. SEM, standard error of the mean.
b SI, Selectivity index calculated as ED50 (Rondo) divided by the ED50 of the different rice genotypes, respectively.
An asterisk (*) indicates values are significantly different from both parental ED50 values at α = 0.05.
Spatial–Temporal HIS1 Expression and Growth Stage Sensitivity to Benzobicyclon
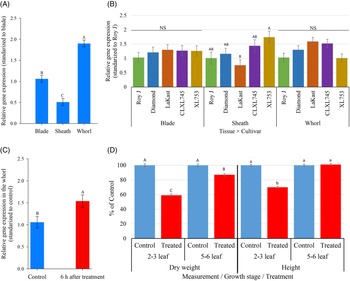
HIS1 is not a constitutively expressed gene (Maeda et al. Reference Maeda, Murata, Sakuma, Takei, Yamazaki, Karim, Kawata, Hirose, Kawagishi-Kobayashi, Taniguchi, Suzuki, Sekino, Ohshima, Kato, Yoshida and Tozawa2019), but expression of the gene in the whorl and how overall expression contributes to benzobicyclon tolerance has yet to be thoroughly explored. Here, we captured and compared the expression profile of HIS1 among five japonica inbred and hybrid cultivars, growth stages (2- to 3-leaf compared with 5- to 6-leaf plants), tissue types (leaf blade, sheath, and whorl), and time after benzobicyclon application. Based on ΔCt values, HIS1 expression within a tissue type for each cultivar at the 2- to 3-leaf compared with 5- to 6-leaf plants was not significantly different; therefore, data were pooled (P = 0.11).
Before benzobicyclon application and averaged over cultivars, there was a clear difference in HIS1 expression among the tested tissue types (P > 0.0001). When standardized to expression in leaf blades, relative expression of HIS1 in whorls was nearly 2-fold higher (1.9 ΔΔCt) and 2-fold less in the sheaths (0.5 ΔΔCt) (Figure 1A). Expression in roots was not investigated, because previous research (Maeda et al. Reference Maeda, Murata, Sakuma, Takei, Yamazaki, Karim, Kawata, Hirose, Kawagishi-Kobayashi, Taniguchi, Suzuki, Sekino, Ohshima, Kato, Yoshida and Tozawa2019) as well as findings in our pilot study (data not shown) indicated HIS1 expression in roots is minimal. Higher expression in the whorl is favorable, because benzobicyclon hydrolysate has ideal physicochemical properties for phloem mobility (Brabham et al. Reference Brabham, Norsworthy, Sandoski, Varanasi and Schwartz-Lazaro2019).

Figure 1. Relative gene expression of HIS1 in different tissue types averaged over cultivars and growth stages and standardized to expression in blade tissue (A); comparison of relative HIS1 expression within a tissue type across rice cultivars. Results are averaged over growth stages and standards to HIS1 expression in Roy J within a tissue type (B). Change in relative HIS1 expression in whorl tissue averaged over cultivars at the 5- to 6-leaf stage at 6 h after treatment with benzobicyclon (371 g ha−1) (C). Comparison of dry weight and height accumulation of rice cultivars at the 2- to 3-leaf and 5- to 6-leaf growth stages at 28 d after treatment with benzobicyclon at 371 g ha−1. No significant interaction between cultivars and growth stage was detected, and data were pooled accordingly (D). A and B show HIS1 gene expression before benzobicyclon application, while C shows HIS1 gene expression after application. Different letters on bars within a group indicate a significant difference (α = 0.05). Bars represent means ± SEM.
In general, relative expression patterns across cultivars within a tissue type were similar, even though a significant interaction was detected (P = 0.01). Relative expression values among cultivars within a tissue type ranged from 0.76- to 1.74-fold when standardized to the respective expression in Roy J (Figure 1B). The only significant differences between cultivars was detected in sheath tissue, where the relative expression of HIS1 in the inbred cultivar LaKast was nearly 2-fold less than in the hybrid XL753. The relative expression of HIS1 at 6 h after application was measured only in the whorl tissue of the five cultivars at the 5- to 6-leaf stage. Again, there was no cultivar by treatment interaction (P = 0.90), but the main effect of treatment was significant (P = 0.027). HIS1 expression in treated plants was 1.5-fold higher in comparison to the nontreated at 6 h after benzobicyclon application; however, this is probably biologically insignificant (<2-fold) (Figure 1C).
Unlike gene expression patterns, the response of a cultivar to benzobicyclon was dependent on the growth stage at application. However, there was no significant difference between the five cultivars within a growth stage based on percent height reduction (P = 0.60) and dry reduction data (P = 0.76). At 28 DAT, plants at the 5- to 6-leaf stage at application were not significantly different from the nontreated based on height data, but a slight significant reduction in dry weight (13%) was detected. In contrast, the height and dry weight of plants at the 2- to 3-leaf stage at application were reduced by 30% and 41%, respectively (Figure 1D). Based on these findings, difference in benzobicyclon tolerance among HIS1 homozygous cultivars is probably not a function of expression, but a bigger cultivar sample size is needed to truly make this conclusion.
Weedy Rice Control Model and Proof of Concept Experiments
Prior research indicates benzobicyclon has the potential to provide rice growers in the southern United States with the added benefit of weedy rice suppression, but the degree to which this occurs can vary considerably (Brabham et al. Reference Brabham, Norsworthy and González-Torralva2020; Young et al. Reference Young, Norsworthy, Scott, Bond and Heiser2018). Therefore, a model was developed to predict the efficacy of benzobicyclon on weedy rice based on data from cultivated rice tolerance studies herein and in the literature. Our findings from greenhouse studies indicate benzobicyclon tolerance in cultivated rice is first and foremost dependent on HIS1 zygosity followed by growth stage; thus, our model predicts weedy rice sensitivity to benzobicyclon is a function of HIS1 zygosity by plant growth stage at application (Table 2). The model predicts benzobicyclon will provide moderate suppression to complete control of weedy rice plants at the ≤2-leaf growth stage regardless of genotype, while activity on older plants is completely dependent on HIS1 zygosity.
Table 2. A model for control of weedy rice in cultivated rice that is derived from lessons learned from crop tolerance research associated with HIS1. Weedy rice, considered unwanted rice, including red rice or off-types from previous plantings of hybrid rice, control is proposed to be a function of genotype by growth stage. a

a Applicable only to U.S. Midsouth rice production practices where benzobicyclon is applied post-flood in a soluble concentrate formulation at 371 g ha−1 plus methylated seed oil (1% v/v).
To test our model, a proof of concept experiment was conducted in 2019 at two field locations (Pine Tree and Stuttgart) looking at weedy rice sensitivity to benzobicyclon at different growth stages. A total of 15 weedy rice accessions plus XL745 F 2 plants (considered HIS1/HIS1 weedy rice) were tested. Roy J × Purple Marker F 1 plants (HIS1/his1), the susceptible cultivars Purple Marker and Rondo (his1/his1), and the tolerant cultivar LaKast (HIS1/HIS1) were included as internal standards. The HIS1 allele frequency in a subsample of plants from each accession was previously determined (Brabham et al. Reference Brabham, Norsworthy and González-Torralva2020) or accessions were re-genotyped. In all but two accessions, HIS1 was the dominant allele (Supplemental Table T1).
Data were grouped according to HIS1 (HIS1/HIS1; HIS1/his1; his1/his1) genotyping, and average percent control or injury was calculated among groups. At Pine Tree, benzobicyclon efficacy (371 g ha−1) on the genotyped HIS1/HIS1 accessions at 28 DAT averaged from 69% to 8% control when plants were at the 1- to 2-leaf stage and 4- to 5-leaf stage at application, respectively. Roy J × Purple Marker F 1 plants together with weedy rice accession 7 were heterogeneously segregating for HIS1/his1 alleles and displayed 77% and 9% control when plants were at the 1- to 2-leaf stage and 4- to 5-leaf stage at application, respectively. Accessions 18 and 20 were predominantly segregating for his1/his1 (HIS1 allele frequency ≤30%) and responded similarly to the susceptible standards Purple Marker and Rondo at both growth stages (98% and 93% of control, respectively) (Table 3).
Table 3. Results from a proof of concept experiment testing our benzobicyclon weedy rice control model in which weedy rice sensitivity is proposed to be a function of genotype by growth stage. a

a Trials were conducted in 2019 at the Pine Tree Research Station near Colt, AR, and at the Rice Research and Extension Center near Stuttgart, AR. The table contains the HIS1 genotype and average percent control or injury of weedy rice accessions and rice cultivars treated with benzobicyclon (371 g ha−1) at 28 d after treatment.
b The HIS1 genotype for each weedy rice accession was previously determined in Brabham et al. (Reference Brabham, Norsworthy and González-Torralva2020). Accessions 9, 10, 12, 24, and 25 were re-genotyped. An accession was categorized as HIS1/HIS1, HIS1/his1, or his1/his1 if the HIS1 allele frequency was ≥70%, 31%–69%, or ≤30%, respectively. XL745 F 2 was considered weedy rice. Purple Marker and Rondo were included as his1 susceptible standards, while LaKast and Roy J × Purple Marker F 1 were the HIS1 homozygous and heterozygous standards, respectively.
c Values correspond to the mean (n = 3), and numbers in parentheses corresponds to SD.
Plants were at an advanced growth stage when applications were made at Stuttgart, and control was less than that observed at Pine Tree. Overall, benzobicyclon efficacy (371 g ha−1) at 28 DAT on weedy rice accessions ranged from 24% to 93% and 4% to 82% when plants were at the 3- to 4-leaf stage and 1- to 2-tiller stage at application, respectively. At the 3- to 4-leaf stage, the homozygous (HIS1/HIS1) LaKast group exhibited 24% control, while the heterozygous (HIS1/his1) standard Roy J × Purple Marker F 1 plants had an intermediate response (37% injury). At the 1- to 2-tiller stage, control of accessions or injury to cultivars genotyped as HIS1/HIS1 or HIS1/his1 did not exceed 12%. Again, the sensitivity of accessions 18 and 20 (HIS1 allele frequency ≤30%) and the susceptible standard cultivars Purple Marker and Rondo was independent of growth stage, and control averaged 82% (Table 3).
Data from the proof of concept field experiments appear to provide supporting evidence for our model. As predicted, his1/his1 accessions/cultivars were highly sensitive to benzobicyclon at 371 g ha−1 regardless of growth stage, and predominantly HIS1 accessions or HIS1/HIS1 cultivars (e.g., LaKast) at the ≥5-leaf growth stage were tolerant. These statements are supported by evidence in the literature (Akasaka et al. Reference Akasaka, Watanabe and Kawana2011; Kwon et al. Reference Kwon, Shin, An, Lee, Min, Park, Shin, Jung and Kuk2012; Lv et al. Reference Lv, Zhang, Yuan, Huang, Peng, Peng, Li, Tang, Liu, Zhou, Wang, Pan, Shao, Xin and Zhu2021; Maeda et al. Reference Maeda, Murata, Sakuma, Takei, Yamazaki, Karim, Kawata, Hirose, Kawagishi-Kobayashi, Taniguchi, Suzuki, Sekino, Ohshima, Kato, Yoshida and Tozawa2019; Young et al. Reference Young, Norsworthy, Scott and Barber2017). For HIS1/his1 accessions/cultivars, the model predicted severe suppression to complete control at ≤2-leaf growth stage, which was observed at the Pine Tree location, but the model would indicate greater control at the 5-leaf growth stage. For instance, benzobicyclon activity on Roy J × Purple Marker F 1 plants and accession 7 (mixed HIS1/his1 population) dropped to less than 15% once plants had ≥ 4 leaves at application in both trial locations. This result contrasts with work from Akasaka et al. (Reference Akasaka, Watanabe and Kawana2011) and Kwon et al. (Reference Kwon, Shin, An, Lee, Min, Park, Shin, Jung and Kuk2012), who observed moderate to significant benzobicyclon injury on transplanted indica × japonica cultivated rice.
Moderate suppression of homozygous HIS1 plants at the ≤2-leaf growth stage was predicted and observed at the Pine Tree location, where weedy rice accessions and cultivars at the 1- to 2-leaf stage were moderately to severely suppressed (69% control). We believe the increased sensitivity is related to overall HIS1 expression but not expression pattern, and we therefore theorize that it is possible to “overload” the HIS1 enzyme in smaller plants. Anecdotal evidence from our gene expression experiment indicated HIS1 is probably not a highly expressed gene, as Ct values for HIS1 ranged from 29 to 31 (EF1 averaged 20). This statement can be strengthened substantially by RNA-seq data at RiceXPro (Sato et al. Reference Sato, Takehisa, Kamatsuki, Minami, Namiki, Ikawa, Ohyanagi, Sugimoto, Antonio and Nagamura2013) and the Rice Expression Database (Xia et al. Reference Xia, Zou, Sang, Xu, Yin, Li, Wu, Hu, Hao and Zhang2017) indicating HIS1 (Loc_Os02g17940) is a low to at most moderately expressed gene (even in comparison to HPPD gene Loc_Os02g07160). Thus, we hypothesize HIS1/HIS1 weedy rice plants will be at least suppressed at ≤2-leaf growth stage; this would be exacerbated in the field by the flood and competition from the 5-leaf rice crop.
In closing, the model we present currently only applies to drill-seeded rice production practices in the U.S. Midsouth, where benzobicyclon is applied post-flood in a soluble concentrate formulation at 371 g ha−1 to the rice crop at the 5-leaf growth stage or beyond. It should also be assumed that all weedy rice accessions in the U.S. Midsouth are segregating for HIS1, and it is probably the dominant allele, as previous screening efforts have indicated (Brabham et al. Reference Brabham, Norsworthy and González-Torralva2020; Young et al. Reference Young, Norsworthy, Scott, Bond and Heiser2018). Therefore, in general, benzobicyclon should not be expected to provide acceptable weedy rice control, but based on our model, benzobicyclon can provide some degree of suppression on plants with ≤2 leaves at the time of application regardless of the HIS1 genotype. Further research is needed to validate our benzobicyclon weedy rice control model.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/wsc.2022.17
Acknowledgments
The authors are grateful to the Arkansas Rice Promotion Board and Gowan Company for their support of this research.
No conflicts of interest have been declared.