Introduction
Invasive winter annual grasses present the largest threat to the arid and semiarid ecosystems of western North America (DiTomaso Reference DiTomaso2000). Winter annual grasses typically germinate in the late summer or fall and overwinter in a semidormant state, resuming growth in early spring and taking advantage of early-season moisture and nutrients before native plant communities break dormancy (Beck Reference Beck2009). Plants then produce seed and senesce by early summer (Beck Reference Beck2009). Although fall germination is typical, invasive winter annual grasses are opportunistic and can germinate whenever the growing conditions are favorable (Beck Reference Beck2009). This opportunistic life cycle and prolific seed production have allowed introduced winter annual grasses to spread through perennial-dominated rangelands (Mack and Pyke Reference Mack and Pyke1983). The ecological impacts of winter annual grass invasions include increased fire frequency, altered nutrient cycling, decreased species diversity, and degraded wildlife and pollinator habitat (Corbin and D’Antonio Reference Corbin and D’Antonio2004; Knapp Reference Knapp1996; Thill et al. Reference Thill, Beck and Callihan1984).
Downy brome (Bromus tectorum L.) is the most widespread winter annual grass, invading over 22 million ha of rangeland in the United States with an estimated annual spread rate of 14% (Duncan et al. Reference Duncan, Jachetta, Brown, Carrithers, Clark, Ditomaso, Lym, McDaniel, Renz and Rice2004). Since its introduction into North America in the mid-1800s, B. tectorum has undergone rapid range expansion, becoming the most dominant and impactful weed in the Intermountain West region (Johnson and Davies Reference Johnson and Davies2012; Mack Reference Mack1981). It has been projected that an additional 31 million ha in the western United States are susceptible to invasion by exotic winter annual grasses (Pellant and Hall Reference Pellant and Hall1994).
While B. tectorum impacts the most acreage in the western United States (DiTomaso Reference DiTomaso2000), two other winter annual grasses, medusahead [Taeniatherum caput-medusae (L.) Nevski] and ventenata [Ventenata dubia (Leers) Coss.], have emerged as major threats to western rangelands (Wallace et al. Reference Wallace, Pavek and Prather2015; Young Reference Young1992). Taeniatherum caput-medusae was first discovered in the United States in 1887, although populations appear to have remained relatively static until around the 1950s, when it became a major concern for livestock producers (Bovey et al. Reference Bovey, Le Tourneau and Erickson1961). It infests more than 2 million ha of rangeland, has low palatability, and can reduce grazing capacity by 80% (Dahl and Tisdale Reference Dahl and Tisdale1975; Hironaka Reference Hironaka1961). The invasive winter annual grass V. dubia was first reported in Washington in 1952 and spread throughout the Intermountain Pacific Northwest, becoming a major invader in Conservation Reserve Program lands and Palouse grasslands (Wallace et al. Reference Wallace, Pavek and Prather2015). To demonstrate the spread potential of these two relatively new invaders, populations were recently identified for the first time in the Great Plains ecoregion (Sheridan, WY) (USDA-NRCS 2019). Before this discovery it was uncertain whether these species were adapted to the climatic conditions of the Great Plains, but with these recent findings it appears as though, if left unchecked, T. caput-medusae and V. dubia could potentially become as widespread as B. tectorum.
Winter annual grass infestations accumulate large quantities of litter, or thatch, on the soil surface as plants senesce yearly and decompose slowly (Evans and Young Reference Evans and Young1970). Litter facilitates invasions by promoting winter annual grass germination and suppressing native plants (Evans and Young Reference Evans and Young1970). Litter accumulation also contributes to increased fire frequency in the western United States by providing a continuous layer of fine fuels, especially in the Great Basin Desert (a region covering nearly all of Nevada, the western half of Utah, and portions of Oregon, Wyoming, Idaho, and California), where historically there was a significant amount of bare soil between plants (Abatzoglou and Kolden Reference Abatzoglou and Kolden2011; DiTomaso Reference DiTomaso2000; Knapp Reference Knapp1996). With this continuous fine fuel layer, fire return intervals have decreased from between 60 and 110 yr to less than 5 yr, resulting in displacement of desirable grasses, forbs, and shrubs that are not fire-adapted species (Whisenant Reference Whisenant1990).
It has been widely speculated that winter annual grass litter, which can exceed 15 cm in thickness, can adsorb soil-active herbicides and reduce overall performance (DiTomaso et al. Reference DiTomaso, Brooks, Allen, Minnich, Rice and Kyser2006; Evans and Young Reference Evans and Young1970; Kessler et al. Reference Kessler, Nissen, Meiman and Beck2015; Mangold et al. Reference Mangold, Parkinson, Duncan, Rice, Davis and Menalled2013; Monaco et al. Reference Monaco, Osmond and Dewey2005). Past studies have reported improved performance with herbicides when litter has been eliminated, although many of these studies have been confounded by the fact that fire was used to remove the litter layer (Davies and Sheley Reference Davies and Sheley2011; Kessler et al. Reference Kessler, Nissen, Meiman and Beck2015; Kyser et al. Reference Kyser, DiTomaso, Doran, Orloff, Wilson, Lancaster, Lile and Porath2007; Monaco et al. Reference Monaco, Osmond and Dewey2005; Sheley et al. Reference Sheley, Carpinelli and Morghan2007). The impacts of winter annual grass litter on soil-active herbicides have only been empirically evaluated in one published study (Kessler et al. Reference Kessler, Nissen, Meiman and Beck2015). In that study, up to 74.6% of imazapic and tebuthiuron were intercepted when applied over high amounts of B. tectorum litter, and only 69% of the intercepted herbicide could be desorbed from the litter with 15 mm of rainfall 7 d after treatment (DAT) (Kessler et al. Reference Kessler, Nissen, Meiman and Beck2015). That study and several others evaluating crop residues have hypothesized that some irreversible binding to the litter may be occurring. This hypothesis is based on the fact that rainfall is not able to recover 100% of the applied herbicide (Banks and Robinson Reference Banks and Robinson1982; Carbonari et al. Reference Carbonari, Gomes, Trindade, Silva and Velini2016; Cavenaghi et al. Reference Cavenaghi, Rossi, Negrisoli, Costa, Velini and Toledo2007; da Silva Reference da Silva2018; Ghadiri et al. Reference Ghadiri, Shea and Wicks1984; Kessler et al. Reference Kessler, Nissen, Meiman and Beck2015).
Herbicide sorption increases with the lipophilic components of litter such as lignin, which increases as a percent of the dry weight as litter decays (Barak et al. Reference Barak, Dinoor and Jacoby1983; Dao Reference Dao1991; Van Beinum et al. Reference Van Beinum, Beulke and Brown2006). A herbicide’s chemical properties also influence sorption, with adsorption to organic matter dependent on the herbicide’s lipophilic properties (Gennari et al. Reference Gennari, Negre and Vindrola1998; Stevenson Reference Stevenson1972). Therefore, lipophilic herbicides may adsorb more readily to litter; unfortunately, little is known about differences in adsorption and subsequent desorption from litter with rainfall between water-soluble and water-insoluble herbicides (Barak et al. Reference Barak, Dinoor and Jacoby1983; Dao Reference Dao1991).
Imazapic and rimsulfuron, hydrophilic Group 2 herbicides (Shaner Reference Shaner2014), are used for winter annual grass control on rangeland (Kyser et al. Reference Kyser, DiTomaso, Doran, Orloff, Wilson, Lancaster, Lile and Porath2007, Reference Kyser, Wilson, Zhang and DiTomaso2013; Mangold et al. Reference Mangold, Parkinson, Duncan, Rice, Davis and Menalled2013; Morris et al. Reference Morris, Morris and Surface2016). Both herbicides have PRE residual activity and can provide POST winter annual grass control when applied at the seedling stage (Kyser et al. Reference Kyser, Wilson, Zhang and DiTomaso2013; Sebastian et al. Reference Sebastian, Sebastian, Nissen and Beck2016; Wallace and Prather Reference Wallace and Prather2016). Winter annual grass control can be highly variable with imazapic and rimsulfuron, and although selective at low use rates, perennial grass injury can occur (Kyser et al. Reference Kyser, Wilson, Zhang and DiTomaso2013; Mangold et al. Reference Mangold, Parkinson, Duncan, Rice, Davis and Menalled2013; Sebastian et al. Reference Sebastian, Sebastian, Nissen and Beck2016, Reference Sebastian, Fleming, Patterson, Sebastian and Nissen2017a).
Indaziflam, a lipophilic Group 29 herbicide, controls winter annual grasses in rangeland and natural areas by inhibiting seedling establishment (Brabham et al. Reference Brabham, Lei, Gu, Stork, Barrett and DeBolt2014; Sebastian et al. Reference Sebastian, Fleming, Patterson, Sebastian and Nissen2017a; Tompkins, Reference Tompkins2010). Indaziflam has no significant POST activity (Brabham et al. Reference Brabham, Lei, Gu, Stork, Barrett and DeBolt2014) but provides much longer (3+ yr) soil residual control compared with imazapic and rimsulfuron (Sebastian et al. Reference Sebastian, Sebastian, Nissen and Beck2016, Reference Sebastian, Fleming, Patterson, Sebastian and Nissen2017a). This long-term residual control provides an opportunity to start the process of winter annual grass seedbank depletion (Sebastian et al. Reference Sebastian, Nissen, Sebastian and Beck2017b). Indaziflam’s low water solubility (3.6 mg L−1 at pH 7) suggests that its interaction with winter annual grass litter will differ compared with imazapic and rimsulfuron. There are also indications that V. dubia and T. caput-medusae litter have different physical and chemical properties compared with B. tectorum litter (Bovey et al. Reference Bovey, Le Tourneau and Erickson1961; Wallace et al. Reference Wallace, Pavek and Prather2015); therefore, the ability to remove herbicide from litter with rainfall could vary between litter types. The objectives of this research were to (1) quantify imazapic, indaziflam, and rimsulfuron, intercepted at two levels of B. tectorum, T. caput-medusae, and V. dubia litter; (2) determine the efficiency of various simulated rainfall events to remove the intercepted herbicide from litter, and (3) determine whether time-dependent binding decreases the amount of herbicide that can be removed from litter by rainfall.
Materials and Methods
Litter Collection and Description of Experimental Units
Three separate experiments were conducted to evaluate interception and subsequent desorption with rainfall of herbicides applied to winter annual grass litter. Three winter annual grass litter types were collected in August 2016. Bromus tectorum litter was collected from Colorado Parks and Wildlife’s Wellington State Wildlife Area in Wellington, CO (40.71°N, 104.95°W); V. dubia litter was collected from a Paradise Ridge in Latah County, ID (46.67°N, 116.97°W); and T. caput-medusae litter was collected from a natural area in Cache County, UT (41.66°N, 112.08°W). Litter was allowed to dry at room temperature (25 to 28 C) for a minimum of 1 wk before the experiments were conducted. Samples were taken from each litter type and oven-dried at 60 C for 48 h to determine moisture content. After 1 wk of drying at room temperature, moisture content averaged 7% in B. tectorum and V. dubia, while moisture content averaged 6% in T. caput-medusae. Litter was sieved to remove soil, and shoot segments were then cut to 140 mm in length. Each experimental unit consisted of a 150 by 150 by 50 mm glass dish (Pyrex®, Corelle Brands, Rosemont, IL 60018) below a 150 by 150 by 50 mm stainless-steel mesh basket (TWP, Berkeley, CA 94710). The baskets consisted of 0.5-mm stainless-steel mesh with 6.35-mm openings. All experiments were conducted in a complete randomized design with three replicates, and each of the three experiments described in the following sections were repeated.
Herbicide Interception
At the B. tectorum field site, 1-m2 frames were used to collect three litter samples. Litter was allowed to dry under the same conditions described previously and was then weighed. The average biomass of the three litter samples was equivalent to 2,600 kg ha−1, which is consistent with biomass production in high litter sites for all three winter annual grass types (Evans and Young Reference Evans and Young1970; Kessler et al. Reference Kessler, Nissen, Meiman and Beck2015; Wallace et al. Reference Wallace, Pavek and Prather2015). Therefore, this biomass was used to simulate high litter conditions, while 50% of the biomass measurement (1,300 kg ha−1) was used to simulate low litter conditions. Bromus tectorum, V. dubia, and T. caput-medusae litter was then weighed out into sample sizes of 2.82 g or 5.64 g, which corresponded to the field rate of 1,300 and 2,600 kg ha−1, respectively,when adjusted for the size of the mesh basket. Litter was then spread evenly in the metal baskets and placed on top of the glass dishes. Consistent with field-applied concentrations, commercial formulations of imazapic (Plateau® herbicide, BASF, Research Triangle Park, NC 27709), rimsulfuron (Matrix® herbicide, Bayer Crop Science, Research Triangle Park, NC 27709), and indaziflam (Esplanade 200 SC® herbicide, Bayer Crop Science, Research Triangle Park, NC 27709) were applied at 122, 70, and 102 g ai ha−1, respectively, over the top of the litter using a Generation III research track sprayer (DeVries Manufacturing, Hollandale, MN 56045) equipped with a TeeJet® 8002 EVS flat-fan spray nozzle (TeeJet Technologies, Springfield, IL 62703) calibrated to deliver 187 L ha−1 at 172 kPa. Immediately after herbicide application, dishes were washed with methanol (Millipore Sigma, St Louis, MO 63103) for imazapic and rimsulfuron applications and acetonitrile (Millipore Sigma) for indaziflam applications. These solvents were chosen based on previously developed methods for analysis of these herbicides with liquid chromatography–tandem mass spectrometry (LC-MS/MS) (Demoliner et al. Reference Demoliner, Caldas, Costa, Gonçalves, Clementin, Milani and Primel2010; Pirard et al. Reference Pirard, Widart, Nguyen, Deleuze, Heudt, Haubruge, De Pauw and Focant2007; Xu Reference Xu2008). The methanol and acetonitrile volumes were recorded, and samples were transferred to 15-ml glass tubes (Pyrex® 9826 Culture Tubes, Corning, Corning, NY 14830) and stored at 0 C for analysis. A dish with an empty metal basket was included for all experiments as a control to determine the total quantity of herbicide applied.
Leaf Area Quantification
As a follow-up to the herbicide interception experiment, leaf and shoot area differences between B. tectorum and T. caput-medusae litter were investigated. A leaf area meter (LI-3100, Li-Cor, Lincoln, NE 68504) was used to quantify the leaf area index (LAI) of B. tectorum and T. caput-medusae litter. Six samples of each litter type weighing 5.64 g were measured by the leaf area meter, and means were calculated.
Desorption by Litter Types
To determine herbicide desorption from the three litter types, the litter amount equivalent to 2,600 kg ha−1 was spread in the metal baskets and placed over the glass dishes. All three herbicides were applied as previously described to the three litter types. After herbicide application, baskets were removed, and the glass dishes were washed. Baskets were then placed back on the dishes, and 12 mm of simulated cumulative rainfall was immediately applied to half of the experimental units, using the same overhead sprayer previously described equipped with a TeeJet® 8004E nozzle (TeeJet Technologies) at 0.38 m above the experimental unit surface traveling at 0.45 m s−1. The research track sprayer simulates rainfall via an automated system that delivers constant pressure (set to 172 kPa) from a hydraulic pump, allowing for simulated rainfall at desired volumes. The system applied 1 mm of rainfall min−1 with the conditions described above. The artificial raindrops produced had a median volumetric diameter of 300 µm. The other half of the experimental units were removed from the dishes and stored at 25 to 28 C under laboratory inflorescent light conditions at 200 µmol m−2 s−1. After 1 d, the remaining treated litter was placed over clean dishes, and 12 mm of simulated rainfall was applied. After the simulated rainfall, the total water volume was recorded for each dish, and an aliquot was taken and stored in a 15-ml glass tube at 0 C.
Herbicide Desorption of Bromus tectorum
The final experiment was conducted to determine herbicide desorption from B. tectorum litter at different wait periods and rainfall amounts. The three herbicides were applied to B. tectorum litter (2,600 kg ha−1), and rainfall was simulated using the previously described procedures. Rainfall was applied cumulatively in amounts of 3, 6, 12, and 24 mm after periods of 0, 1, and 7 d. The experimental units were stored at 25 to 28 C under laboratory inflorescent light conditions for the 1- and 7-d periods. Aliquots were collected and stored in the same manner as previously described until further analysis. The samples from the dishes without litter were diluted 20 times with methanol for imazapic and rimsulfuron samples and acetonitrile for indaziflam samples before filtration.
Herbicide Quantification
For imazapic and rimsulfuron, the herbicide–methanol and herbicide–water samples were prepared for LC-MS/MS analysis by filtering 1-ml aliquots of each sample through a 0.24-µm membrane syringe filter (VWR International, Radnor, PA 19087) into 1.5-ml autosampler vials (Shimadzu Scientific Instruments, Columbia, MD 21046). For indaziflam, the herbicide–acetonitrile samples from the interception experiment were prepared in the same manner. For the indaziflam–water samples, 80% of the original solution was diluted with 20% acetonitrile, and 1-ml aliquots were filtered through a 0.24-µm membrane syringe filter into 1.5-ml autosampler vials. The samples collected from the dishes without litter were prepared by diluting them 20 times with methanol for imazapic and rimsulfuron applications or acetonitrile for indaziflam applications and using the same filtration process as described earlier. The herbicide concentration in each sample was determined by an LC-MS/MS system (Shimadzu LCMS-8040, Shimadzu Scientific Instruments, Columbia, MD 21046). The LC-MS/MS conditions for each herbicide are listed in Table 1. Five concentrations ranging from 0.01 and 1 µg ml−1 were created from analytical standards and included as the calibration curve (rimsulfuron and imazapic, 99.9% purity, Sigma-Aldrich, St Louis, MO 63146; indaziflam, 99.3% purity, Bayer Crop Science).
Table 1. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) conditions for rimsulfuron, imazapic, and indaziflam.
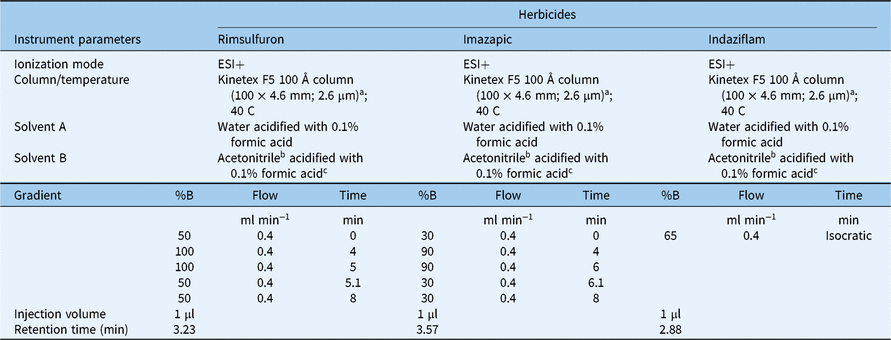
a Material source: Phenomenex, Torrance, CA.
b Material source: Millipore Sigma, St Louis, MO.
c Material source: Thermo Fisher Scientific, Waltham, MA.
The herbicide concentration from the samples based on the LC-MS/MS analysis was transformed into mass (µg) by adjusting for the volume recorded from the simulated rainfall. For the interception experiment, the measured herbicide concentrations that passed through the litter were subtracted from the total herbicide applied to the empty dishes to determine the herbicide amount intercepted by the litter. These data were then transformed to a percentage of the total applied herbicide. For the desorption experiments, the concentrations from the simulated rainfall samples were compared against the total herbicide intercepted by the litter in order to calculate percent desorption.
Statistical Analysis
After failing to reject the null hypothesis of a Levene’s test that experimental variances are equal, repeated studies for all three experiments were combined for analysis. Interception data were analyzed in R v. 3.4.3 (R Core Team 2017) using ANOVA, with herbicide, litter type, and litter amount as the factors. Post hoc analysis was performed using the emmeans package (Lenth Reference Lenth2018) in R v. 3.4.3 (R Core Team 2017) to obtain comparisons between all pairs of least-squares means with a Tukey-Kramer adjustment (P < 0.05). To compare desorption among the three litter types, data were analyzed by herbicide and wait period (0 d and 1 d) using ANOVA, with litter type as the factor in R v. 3.4.3 (R Core Team 2017). For the desorption from B. tectorum litter, a model comparison procedure was conducted to determine the best fit for these data. Data were subjected to an asymptotic regression (AR) model, a rectangular hyperbolic model, and linear regression. For each herbicide, best fit was determined by using the procedure outlined by Kniss et al. (Reference Kniss, Vassios, Nissen and Ritz2011), which chooses models with lowest bias-corrected Akaike information criterion corrected for small sample size (AICc) value while also considering residual standard errors and AICc ratios between models. The analysis was conducted in R v. 3.4.4 (R Core Team 2017) using the drm (Ritz and Streibig Reference Ritz and Streibig2005) and qpcR (Ritz and Spiess Reference Ritz and Spiess2008) packages. For rimsulfuron and imazapic at all three wait periods (0, 1, and 7 d), as well as indaziflam at 1- and 7-d wait periods, the AR model was chosen as the best fit. The AR equation used to regress rain amount (mm) with percent desorption of intercepted herbicide was:
where y is desorption as a percentage of the total intercepted herbicide, A max is the maximum desorption at large values of r, r is rainfall amount, and r 80 is the rainfall amount required for 80% of maximum desorption to occur. The AR procedure was performed, and parameter estimates of A max and r 80 values with standard errors were established. A likelihood ratio test was conducted to compare the parameters statistically among time periods and herbicides. Linear regression was chosen as the best fit for indaziflam data from the 0-d time point, and regression analysis and ANOVA were performed in R v. 3.4.3 (R Core Team 2017).
Results and Discussion
Interception by Litter
For the interception experiment, herbicide and the interaction of herbicide by litter type were not significant (P = 0.807 and P = 0.1631, respectively), although there was a difference in interception among the litter types (P < 0.001). Therefore, an ANOVA was conducted for all herbicides combined, with litter type and litter amount included as main effects. More herbicide was intercepted by 2,600 kg ha−1 of litter compared with 1,300 kg ha−1 of litter (P < 0.001) across all three litter types (Table 2). Bromus tectorum litter also intercepted a higher percent of the herbicide than T. caput-medusae and V. dubia litter at both litter amounts (P < 0.001) (Table 2). At 1,300 kg ha−1, B. tectorum litter intercepted 69.9 ± 1.1% (mean ± SE) of the herbicide, while V. dubia and T. caput-medusae litter intercepted 52.5 to 54.0 ± 1.3%. Bromus tectorum litter at 2,600 kg ha−1 intercepted 84.3 ± 1.0% of the herbicide, while V. dubia and T. caput-medusae litter averaged 75.5 to 76.4 ± 1.0% interception (Table 2).
Table 2. Amount of herbicide intercepted by Bromus tectorum, Taeniatherum caput-medusae, and Ventenata dubia litter, as a percentage of total herbicide applied, at two litter amounts (1,300 kg ha−1 and 2,600 kg ha−1). a

a The data for rimsulfuron, imazapic, and indaziflam were combined for ANOVA.
b Mean value ± SE (n = 18). Means followed by the same letter are not significantly different at the P < 0.05 level as determined by Tukey’s multiple comparison test.
The different interception rates for the three litter types at the same weights indicates there may be a difference in the leaf area to weight ratio among the litter types. The mean LAI for B. tectorum litter was 540.7 ± 32.4, while the mean LAI for T. caput-medusae was 239.0 ± 9.4. The higher LAI potentially explains why B. tectorum litter intercepted significantly more herbicide in the experiment, as there was a higher ratio of leaf area to weight compared with T. caput-medusae.
Several field studies reported better control with annual grass herbicides in sites where litter had been removed compared with sites with litter (Kessler et al. Reference Kessler, Nissen, Meiman and Beck2015; Kyser et al. Reference Kyser, DiTomaso, Doran, Orloff, Wilson, Lancaster, Lile and Porath2007; Monaco et al. Reference Monaco, Osmond and Dewey2005; Sheley et al. Reference Sheley, Carpinelli and Morghan2007). Herbicide interception by the litter layer could account for the inconsistent control observed in the field. In high litter situations, less than 25% of soil-active herbicides may be available immediately after application. The 2,600 kg ha−1 litter amount used in this study is based on the biomass present in the site where the B. tectorum litter was collected, although past studies have reported litter amounts of more than 8,000 kg ha−1 (Evans and Young Reference Evans and Young1970; Ogle et al. Reference Ogle, Reiners and Gerow2003). Kessler et al. (Reference Kessler, Nissen, Meiman and Beck2015) demonstrated herbicide interception increased in a linear relationship as B. tectorum litter increased. In the current study, 14% to 24% more herbicide was intercepted by the litter as the amount increased from 1,300 to 2,600 kg ha−1 (Table 2); therefore, at very high litter sites, herbicide interception could approach 100%.
Desorption Comparison among Litter Types
For all three herbicides, total desorption from the three litter types yielded no differences with 12 mm of simulated rainfall at both 0 and 1 d after application (Supplementary Table 1). These data suggest that herbicide desorption is not dependent on litter type. Because B. tectorum is more widespread than T. caput-medusae and V. dubia, we conducted a more in-depth desorption study using only B. tectorum litter. This comparison study demonstrates that the desorption study with B. tectorum should be applicable to T. caput-medusae and V. dubia litter.
Herbicide Desorption from Bromus tectorum Litter
For herbicide desorption from B. tectorum litter, differences were observed among herbicides and wait periods. Overall, rimsulfuron and imazapic behaved similarly in the amount that could be recovered from the litter for each time point. For rimsulfuron, parameter estimates indicated that all the herbicide could be desorbed with rainfall at 0 d, while only 64.6% to 72.3% could be desorbed at 1 and 7 d (Table 3; Figure 1). Based on the A max parameters for imazapic, rainfall is estimated to desorb a maximum of 101.2%, 69.5%, and 66.2% of the herbicide on the litter at 0, 1, and 7 d, respectively (Table 3; Figure 2). A comparison of the A max parameter indicates the higher desorption at 0 d was significant compared with 1 and 7 d for both rimsulfuron and imazapic (P < 0.001), while there was not a difference between the A max at 1 and 7 d (rimsulfuron, P = 0.2478; imazapic, P = 0.3972). The model estimated that for rimsulfuron only, 8.3, 10.9, and 13.1 mm of rainfall would be required to achieve 80% of the total desorption (r 80) realized at 0, 1, and 7 d, respectively (Table 3). For imazapic, the r 80 parameters estimated that between 6 to 9 mm of rainfall would be needed to achieve 80% of the total desorption at any time period (Table 3).
Table 3. Parameter estimates describing desorption of rimsulfuron, imazapic, and indaziflam from Bromus tectorum, Taeniatherum caput-medusae, and Ventenata dubia litter after 0, 1, and 7 d with a maximum of 24 mm of simulated rainfall by applying an asymptotic regression model.

a
![]() $y = {A_{{\rm{max}}}}X\left\{ {1 - \exp \left[ {\left( {{\rm{log}}0.1} \right)X\left( {{r \over {{r_{80}}}}} \right)} \right]} \right\}$
y, desorption as a percentage of the total intercepted herbicide; A max, maximum desorption at large values of r; r, rainfall amount; r 80, rainfall amount required for 80% maximum desorption.
$y = {A_{{\rm{max}}}}X\left\{ {1 - \exp \left[ {\left( {{\rm{log}}0.1} \right)X\left( {{r \over {{r_{80}}}}} \right)} \right]} \right\}$
y, desorption as a percentage of the total intercepted herbicide; A max, maximum desorption at large values of r; r, rainfall amount; r 80, rainfall amount required for 80% maximum desorption.
b Observed maximum indicates the maximum value obtained by any single observation in the study.
c Indaziflam at 0 d was fit with linear regression, therefore only the observed maximum is reported.
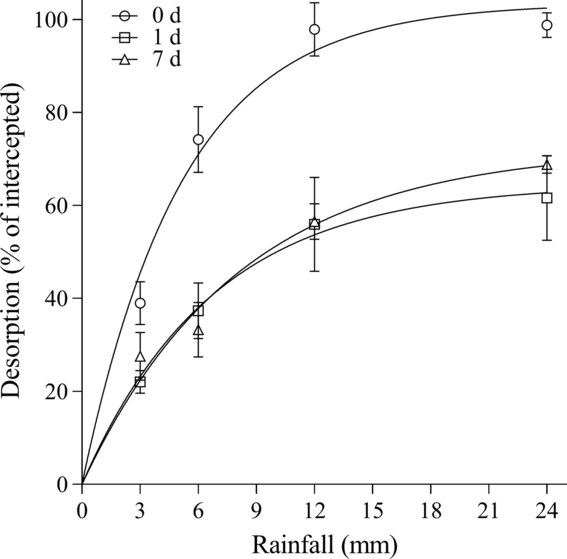
Figure 1. Rimsulfuron desorption from Bromus tectorum litter as a function of the amount of simulated rainfall after 0, 1, and 7 d expressed as a percentage of total herbicide intercepted. Data points are the means of replications with bars indicating the standard error of the mean (n = 6): 0 d:
![]() $y = 103.45X\left\{ {1 - \exp \left[ {\left( {{\rm{log}}0.1} \right)X\left( {{{24} \over {8.35}}} \right)} \right]} \right\}$
; 1 d:
$y = 103.45X\left\{ {1 - \exp \left[ {\left( {{\rm{log}}0.1} \right)X\left( {{{24} \over {8.35}}} \right)} \right]} \right\}$
; 1 d:
![]() $y = 64.58X\left\{ {1 - \exp \left[ {\left( {{\rm{log}}0.1} \right)X\left( {{{24} \over {10.87}}} \right)} \right]} \right\}$
; 7d:
$y = 64.58X\left\{ {1 - \exp \left[ {\left( {{\rm{log}}0.1} \right)X\left( {{{24} \over {10.87}}} \right)} \right]} \right\}$
; 7d:
![]() $y = 72.31X\left\{ {1 - \exp \left[ {\left( {{\rm{log}}0.1} \right)X\left( {{{24} \over {13.06}}} \right)} \right]} \right\}$
.
$y = 72.31X\left\{ {1 - \exp \left[ {\left( {{\rm{log}}0.1} \right)X\left( {{{24} \over {13.06}}} \right)} \right]} \right\}$
.
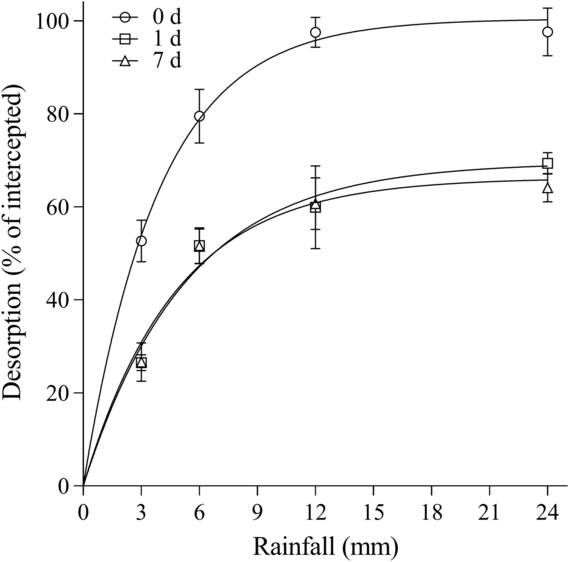
Figure 2. Imazapic desorption from Bromus tectorum litter as a function of the amount of simulated rainfall after 0, 1, and 7 d expressed as a percentage of total herbicide intercepted. Data points are the means of replications with bars indicating the standard error of the mean (n = 6): 0 d:
![]() $y = 101.19X\left\{ {1 - \exp \left[ {\left( {{\rm{log}}0.1} \right)X\left( {{{24} \over {6.39}}} \right)} \right]} \right\}$
; 1 d:
$y = 101.19X\left\{ {1 - \exp \left[ {\left( {{\rm{log}}0.1} \right)X\left( {{{24} \over {6.39}}} \right)} \right]} \right\}$
; 1 d:
![]() $y = 69.53X\left\{ {1 - \exp \left[ {\left( {{\rm{log}}0.1} \right)X\left( {{{24} \over {8.54}}} \right)} \right]} \right\}$
; 7d:
$y = 69.53X\left\{ {1 - \exp \left[ {\left( {{\rm{log}}0.1} \right)X\left( {{{24} \over {8.54}}} \right)} \right]} \right\}$
; 7d:
![]() $y = 66.22X\left\{ {1 - \exp \left[ {\left( {{\rm{log}}0.1} \right)X\left( {{{24} \over {7.69}}} \right)} \right]} \right\}$
.
$y = 66.22X\left\{ {1 - \exp \left[ {\left( {{\rm{log}}0.1} \right)X\left( {{{24} \over {7.69}}} \right)} \right]} \right\}$
.
Indaziflam desorption at 0 d was linear, and there was a positive correlation between rainfall amount and desorption (R2 = 0.96, P < 0.001). At the lowest rainfall amount (3 mm), 9.3 ± 1.1% of the intercepted herbicide was removed from the litter, while 53.7 ± 1.9% was desorbed with the highest rainfall amount (24 mm) (Figure 3). Although this relationship was linear, it would eventually become asymptotic as it reaches the maximum amount that can be desorbed from the litter. The 1- and 7-d wait periods fit an asymptotic curve, and the AR model estimated A max values of 37.7% and 40.9% desorption, respectively. Comparison of the A max indicated no difference between the two time points (P = 0.54) (Table 3; Figure 3). The r 80 parameters indicated that 19 and 24 mm of rainfall are required to achieve 80% of the maximum desorption at 1 and 7 d, respectively. Although 0-d data could not be directly compared with 1 and 7 d using the A max value, the fact that these data presented as linear instead of asymptotic demonstrates there were differences in desorption with rainfall at 0 d compared with the other wait periods.

Figure 3. Indaziflam desorption from Bromus tectorum litter as a function of the amount of simulated rainfall after 0, 1, and 7 d expressed as a percentage of total herbicide intercepted. Data points are the means of replications with bars indicating the standard error of the mean (n = 6): 0 d: y = 2.27x, R2 = 0.94; 1 d:
![]() $y = 37.72X\left\{ {1 - \exp \left[ {\left( {{\rm{log}}0.1} \right)X\left( {{{24} \over {19.29}}} \right)} \right]} \right\}$
; 7d:
$y = 37.72X\left\{ {1 - \exp \left[ {\left( {{\rm{log}}0.1} \right)X\left( {{{24} \over {19.29}}} \right)} \right]} \right\}$
; 7d:
![]() $y = 40.89X\left\{ {1 - \exp \left[ {\left( {{\rm{log}}0.1} \right)X\left( {{{24} \over {23.99}}} \right)} \right]} \right\}$
.
$y = 40.89X\left\{ {1 - \exp \left[ {\left( {{\rm{log}}0.1} \right)X\left( {{{24} \over {23.99}}} \right)} \right]} \right\}$
.
For rimsulfuron and imazapic, 12 mm of rainfall was sufficient to achieve maximum desorption at all time points. Although nearly all the herbicide was recovered when rainfall was received immediately after application (0 d), more than 30% of the intercepted herbicide could not be desorbed from the litter with 24 mm of rainfall at 1 and 7 d after application (Table 3; Figures 1 and 2). For indaziflam, desorption continued to increase between the 12- and 24-mm rainfall amounts, although only 53.7 ± 1.9% could be desorbed from the litter, even with immediate rainfall (0 d). Recovery rates went down to an average of 32.6 ± 1.1% by 1 and 7 d after application (Table 3; Figure 3). Interestingly, for all three herbicides, there were no differences in maximum desorption if rainfall was received 1 or 7 d after application.
The amount of herbicide recovered from the litter decreases as time without rainfall increases, implying irreversible binding to litter may be occurring (Carbonari et al. Reference Carbonari, Gomes, Trindade, Silva and Velini2016; da Silva Reference da Silva2018; Kessler et al. Reference Kessler, Nissen, Meiman and Beck2015; Tofoli et al. Reference Tofoli, Velini, Negrisoli, Cavenaghi and Martins2009). Johnson et al. (Reference Johnson, Shaner, Deane, Mackersie and Tuxhorn2000) determined adsorption of imazethapyr to soil was time dependent, with a rapid, initial adsorption phase occurring in the first 1 to 4 d following application, and adsorption becoming stronger over time. This time-dependent binding may be occurring as herbicides bind to the lipophilic litter components. For example, only 69.5% of imazapic and 59.5% of tebuthiuron could be recovered with 15 mm of rainfall at 7 d after application to B. tectorum litter (Kessler et al. Reference Kessler, Nissen, Meiman and Beck2015). Between 15% and 25% of the imazapic and tebuthiuron could not be desorbed from the litter with methanol extraction (Kessler et al. Reference Kessler, Nissen, Meiman and Beck2015). In our study, there was a 30% to 40% decrease in herbicide recovery when rainfall was delayed for 1 d after application, although there was no additional decrease in recovery from 1 to 7 d. Other studies have demonstrated that herbicide recovery continues to decrease as time without rainfall increases from 1 to 60 DAT (Carbonari et al. Reference Carbonari, Gomes, Trindade, Silva and Velini2016; Cavenaghi et al. Reference Cavenaghi, Rossi, Negrisoli, Costa, Velini and Toledo2007; Tofoli et al. Reference Tofoli, Velini, Negrisoli, Cavenaghi and Martins2009).
The recovery rate for indaziflam was approximately half that for rimsulfuron and imazapic at all three time points, suggesting that the amount of herbicide that can be desorbed from the litter with rainfall is dependent on the physical and chemical characteristics of the herbicide. Rimsulfuron and imazapic are both highly water-soluble herbicides, with log K ow (octanol–water partition coefficient) values of −1.47 and 0.393, respectively (Shaner Reference Shaner2014), while indaziflam is lipophilic, with a log K ow of 2.8 (Tompkins Reference Tompkins2010). Because lipophilicity increases adsorption to organic matter for most herbicides, the lipophilic nature of indaziflam appears to increase its adsorption to litter (Barak et al. Reference Barak, Dinoor and Jacoby1983; Cox et al. Reference Cox, Celis, Hermosin, Cornejo, Zsolnay and Zeller2000; Hance Reference Hance1965).
Inconsistencies in winter annual grass control provided by soil-active herbicides have been reported (Kyser et al. Reference Kyser, Wilson, Zhang and DiTomaso2013; Mangold et al. Reference Mangold, Parkinson, Duncan, Rice, Davis and Menalled2013; Sebastian et al. Reference Sebastian, Sebastian, Nissen and Beck2016, Reference Sebastian, Fleming, Patterson, Sebastian and Nissen2017a; Shinn and Thill Reference Shinn and Thill2004). These inconsistencies may be due to the amount of litter present at a site and how soon rainfall occurs after herbicide application. This information is critical for land managers using soil-active herbicides, especially in high litter sites. Applying herbicides before forecasted rain could potentially improve herbicide performance, as maximum desorption from the litter may be attained with as little as 12 mm of rainfall. Another option for land managers is to combine soil-active herbicides with a POST herbicide, such as glyphosate, and apply herbicides during native species dormancy, while winter annual grasses are in a semidormant state (Sebastian et al. Reference Sebastian, Fleming, Patterson, Sebastian and Nissen2017a). This combination would provide immediate, POST winter annual grass control and allow time for precipitation events to desorb the soil-active herbicide from the residue, providing PRE control of seeds germinating from the soil seedbank.
Additionally, land managers could consider using the higher labeled rates of effective herbicides under high litter situations. This may increase the amount of active ingredient reaching the soil immediately after application and with subsequent rainfall events. Even though a large percentage of the herbicide may be bound to the litter layer, long-term control with indaziflam has been consistently achieved with very low rates (44 to 102 g ai ha−1) in high litter sites, outperforming the hydrophilic herbicides imazapic and rimsulfuron (Sebastian et al. Reference Sebastian, Sebastian, Nissen and Beck2016, Reference Sebastian, Fleming, Patterson, Sebastian and Nissen2017a). This suggests that although interception and adsorption of indaziflam occurs, this herbicide is highly active when incorporated into the soil profile via rainfall. Future research should evaluate the relationship between litter degradation and release of the adsorbed herbicide back into the soil profile. For herbicides like imazapic and rimsulfuron, which photodegrade, this may not be significant; however, indaziflam has limited photodegradation and could be released and activated into the soil profile as litter naturally degrades over time. Research has suggested that herbicides may be released from the litter in active form as it decays, providing a slow release of the herbicide back to the soil and extending control (Dao Reference Dao1991); however, additional research is needed to determine impacts of litter decay for indaziflam.
Acknowledgments
This research was funded by Bayer CropScience. No conflicts of interest have been declared. The authors would also like to express their appreciation for the assistance provided by Rachel Seedorf, KaMele Sanchez, Ana Beatriz Figueiredo, Jessica Scarpin, and Giuliana Piccirilli.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/wsc.2019.45.








