Introduction
Common waterhemp and tall waterhemp [Amaranthus tuberculatus (Moq.) J. D. Sauer] are the most common and problematic weeds in corn (Zea mays L.) and soybean [Glycine max (L.) Merr.] throughout the midwestern United States (Nordby et al. Reference Nordby, Hartzler and Bradley2007). Due to genetic similarity and hybridization between these two species, most botanists now group them into one collective waterhemp species (Pratt and Clark Reference Pratt and Clark2001; Nordby et al. Reference Nordby, Hartzler and Bradley2007), hereby both will be referred to as A. tuberculatus. A. tuberculatus is a prolific seed producer, and a single female plant can produce at least 250,000 seeds in a growing season under a noncompetitive environment, with some plants capable of producing up to 1,000,000 seeds (Hager et al. Reference Hager, Wax, Simmons and Stoller1997; Patzoldt et al. Reference Patzoldt, Tranel and Hager2002; Sellers et al. Reference Sellers, Smeda, Johnson, Kendig and Ellersieck2003). Amaranthus tuberculatus has an aggressive growth habit and can germinate early (as soon as 350 growing degree days) as well as later in the growing season than most other summer annual weed species (Hager et al. Reference Hager, Wax, Stoller and Bollero2002; Sellers et al. Reference Sellers, Smeda, Johnson, Kendig and Ellersieck2003). It is highly competitive and can reduce soybean yield by 56% at a density of 8 plants m−1 of row (Bensch et al. Reference Bensch, Horak and Peterson2003) and corn yield by 10% to 36% with densities ranging from 82 to 445 plants m−2 (Cordes et al. Reference Cordes, Johnson, Scharf and Smeda2004).
Since the introduction of glyphosate-resistant crops, growers changed their weed control practices from using conventional tillage, crop rotation, and herbicides with different mechanisms of action (MOAs) to an almost complete reliance on glyphosate and conservation tillage for weed control (Givens et al. Reference Givens, Shaw, Kruger, Johnson, Weller, Young, Wilson, Owen and Jordan2009; Johnson et al. Reference Johnson, Davis, Kruger and Weller2009; Shaner Reference Shaner2000). Heavy reliance on herbicides with a single active ingredient or MOA over a long period of time can lead to evolution of herbicide resistance in weeds (Jasieniuk et al. Reference Jasieniuk, Brule-Babel and Morrison1996). The first glyphosate-resistant A. tuberculatus population was identified in Platte County, MO, in 2005 (Bradley et al. Reference Bradley, Li and Monnig2006). Glyphosate-resistant A. tuberculatus populations are now found in 19 states in the United States, and three cases have been reported in Ontario, Canada (Heap Reference Heap2017). A. tuberculatus has also been reported with resistance to herbicides from one or more of the following sites of action (SOAs): 5-enylpyruvylshikimate-3-phosphate synthase (EPSPS) inhibitor, acetolactate synthase (ALS) inhibitor, protoporphyrinogen oxidase (PPO) inhibitor, photosystem II (PSII) inhibitor, 4-hydroxphenylpyruate dioxygenase (HPPD) inhibitor, and TIR1 auxin receptor (growth regulator) (Heap Reference Heap2017).
The first confirmed population of 2,4-D–resistant A. tuberculatus was discovered in 2009 in a warm-season native grass seed production field in Nebraska (Bernards et al. Reference Bernards, Crespo, Kruger, Gaussoin and Tranel2012). This population exhibited 10-fold resistance to 2,4-D and reduced sensitivity to dicamba as a result of continual use of low rates of 2,4-D to control broadleaf weeds in this setting (Bernards et al. Reference Bernards, Crespo, Kruger, Gaussoin and Tranel2012). A second population resistant to 2,4-D has since been discovered in Illinois in a row-crop setting and is resistant to herbicides of five different MOAs (Sabate et al. Reference Sabate, Tranel and Hager2016). The mechanism responsible for the resistance to 2,4-D in these A. tuberculatus populations is currently unknown (Bernards et al. Reference Bernards, Crespo, Kruger, Gaussoin and Tranel2012; Sabate et al. Reference Sabate, Tranel and Hager2016). In a controlled greenhouse experiment, Tehranchian et al. (Reference Tehranchian, Norsworthy and Powles2016) reported selection for dicamba resistance after three generations of sublethal doses of dicamba in a Palmer amaranth (Amaranthus palmeri S. Watson) population. Historically, the use of synthetic auxin herbicides was restricted to cereal crops and pastures, but with the introduction of soybean and cotton (Gossypium hirsutum L.) varieties with traits that confer resistance to 2,4-D or dicamba, there will likely be more of these herbicides applied in the near future. These technologies provide growers with new options for the control of glyphosate-resistant broadleaf weeds like A. tuberculatus in cotton and soybean production systems (Craigmyle et al. Reference Craigmyle, Ellis and Bradley2013). However, as previously mentioned, at least two 2,4-D–resistant A. tuberculatus populations have already been discovered.
In 2014, a grower in central Missouri claimed that he was unable to control A. tuberculatus with 2,4-D after routinely applying the herbicide as part of his preplant burndown program for several years consecutively. The field had been in continuous soybean production and had a history of repeated glyphosate, fomesafen, and 2,4-D use as a preplant burndown. Therefore, experiments were conducted to confirm resistance to multiple herbicides, including 2,4-D.
The objective of these experiments were to determine the level of 2,4-D and multiple herbicide resistances in this A. tuberculatus population through field and greenhouse dose–response experiments. Additionally, an experiment was conducted with an objective to study gene stacking for multiple herbicide resistance at the individual plant level in this population. This study documents the first known case of multiple resistance to six SOA groups of herbicides in a single population of A. tuberculatus.
Materials and Methods
Field Experiments
Experiments were conducted during the summers of 2015 and 2016 in the location where the suspected 2,4-D–resistant A. tuberculatus (designated MO-Ren) was reported in Randolph County, MO (39.32°N, 92.39°W). The soil type in this location was a Putnam silt loam (fine, smectitic, mesic Vertic Albaqualfs) with a pH of 5.9 and organic matter content of 1.9%. Individual plots measured 3 by 8 m in size, and treatments were arranged in a randomized complete block design with four replications. Because of the diversity of herbicide treatments evaluated, the experiment was conducted as a bare-ground trial in the absence of any crop. During both years, the field was prepared following conventional tillage practices to secure uniformity of A. tuberculatus before the herbicide treatments. Each year, a different area in the same field was chosen to conduct the experiments. All treatments were applied at a constant speed of 5 km h−1 with a handheld CO2-pressurized research backpack sprayer that delivered 140 L ha−1 and were sprayed on August 8, 2015, and June 6, 2016. All treatments that contained a synthetic auxin herbicide were applied using TTI11002 nozzles, and all other treatments that did not contain a Group 4 herbicide were applied using 8002 flat-fan nozzles (TeeJet®, Spraying Systems, Wheaton, IL). Treatments evaluated in the experiment are shown in Table 1, while the source of materials is presented in Table 2. All treatments were applied when the majority of the A. tuberculatus reached an average height of 10 cm. The average A. tuberculatus density in untreated plots 48 d after herbicide application (DAA) was greater than 120 plants m−2 in 2015 and 250 plants m−2 in 2016. Visual control ratings were taken at 28 and 42 DAA and were assessed on a scale of 0% to 100%, where 0 represented no plant death or injury, and 100 was equivalent to complete plant death. At the 42 DAA timing, surviving A. tuberculatus plants were harvested by clipping them at the soil surface in two 1-m2 quadrats per plot. The relative biomass of all plants for each treatment was determined by drying the harvested A. tuberculatus plants for 5 d at 50 C in a forced-air oven (JPW Industrial Ovens and Furnaces, Trout Run, PA) and expressing biomass as a percentage of the untreated control.
Table 1 Herbicide rates and adjuvants used in field experiments in 2015 and 2016 at Renick, MO, field site.Footnote a
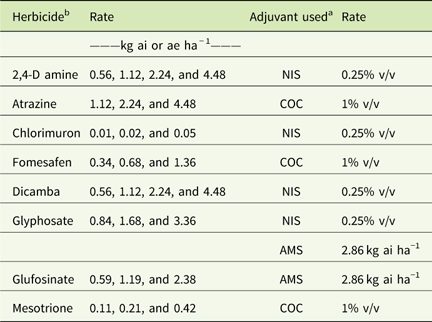
a Abbreviations: % v/v, percent volume to volume; AMS, ammonium sulfate; COC, crop oil concentrate; NIS, nonionic surfactant.
b A drift control agent at 0.29 kg ai ha−1 was used with all the herbicide treatments.
Table 2 Sources of materials used in the experiments.
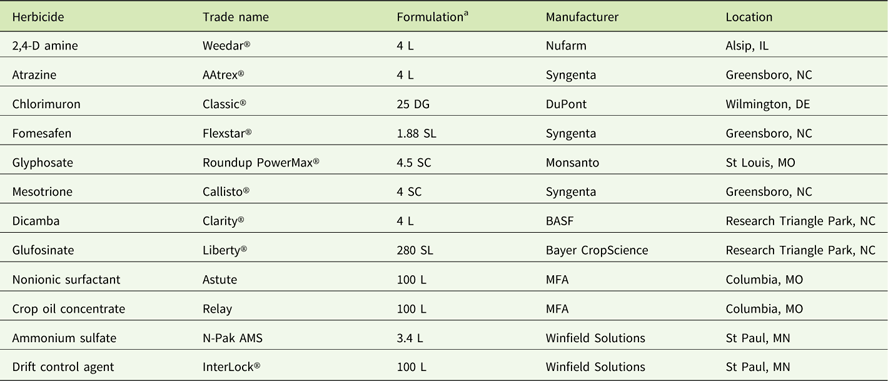
a Abbreviations: DG, dispersible granule; L, liquid; SC, soluble concentrate; SL soluble liquid.
Greenhouse Dose Response
Seed were collected in the fall of 2014 from the suspected resistant MO-Ren population from the field site mentioned earlier. Seeds were treated with a 50:50 water:bleach solution containing 0.1% Tween 20 (Sigma-Aldrich, St Louis, MO) for 5 min, then rinsed with water and suspended in a 0.1% v/v agarose solution and stored at a constant 4 C before the start of each experiment. Before the experiments, an A. tuberculatus population (designated MO-S) was confirmed to be susceptible to 2,4-D, atrazine, fomesafen, dicamba, and mesotrione through a preliminary greenhouse screening and was used as the susceptible standard. Because no chlorimuron-susceptible standard was available due to widespread ALS-inhibitor resistance, the MO-S population was used as a resistant standard. The MO-S population was not susceptible to glyphosate, so a different susceptible population (designated MO-GS) from the preliminary screening was used as a glyphosate-susceptible standard. In each experiment, 0.15 g of seed from the MO-S and MO-GS populations was weighed and broadcast into 25 by 55 cm greenhouse flats filled with a 2:1 mixture of commercial potting medium (Premier Tech Horticulture, Quakertown, PA) and topsoil. Simultaneously, approximately 75 seeds from the MO-Ren population were pipetted from the agarose solution onto flats containing the same potting mixture. After emergence, plants were thinned to 20 to 25 plants flat−1 with four replications per treatment. Experiments were conducted in a randomized complete block design with a factorial arrangement of populations and herbicides rates. Plants were maintained in a greenhouse at 25 to 30 C, watered and fertilized as needed, and provided with artificial lighting from metal-halide lamps (600 µmol photon m−2 s−1) simulating a 16-h photoperiod day during the normal growing season (May to October) in 2015 and 2016.
When the A. tuberculatus plants reached 10 cm in height, they were counted, and herbicide treatments were applied using a compressed air laboratory spray chamber equipped with an 8001EVS nozzle (Teejet®, Spraying Systems) delivering 140 L ha−1 at 234 kPa. The herbicides and rates evaluated are listed in Table 3. The herbicide rates ranged from one-tenth the standard labeled rate (0.1X) up to 20 times the standard labeled rate (20X), depending on the herbicide and likelihood of low- or high-level resistance. An untreated control was included from both populations in each experiment for comparison. Visual control and plant survival ratings were taken at 21 DAA. Visual control ratings were made as described earlier. For plant survival ratings, plants with new green leaf tissue were recorded as resistant, whereas those that displayed severe chlorosis or no new growth were recorded as susceptible. Survival (%) was calculated relative to the number of plants in each flat counted before herbicide application.
Table 3 Herbicide rates and adjuvants used in greenhouse dose–response experiments conducted in 2015 and 2016.
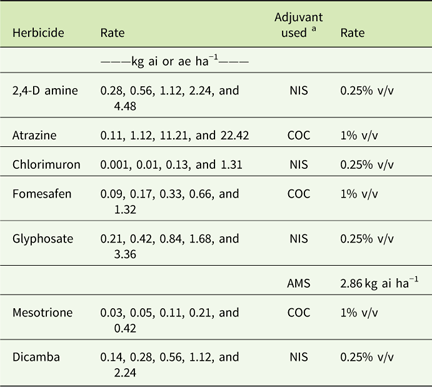
a Abbreviations: % v/v, percent volume to volume; AMS, ammonium sulfate; COC, crop oil concentrate; NIS, non-ionic surfactant.
Gene Stacking by Phenotype
A greenhouse experiment was conducted in 2017 to characterize herbicide-resistant gene stacking within the MO-Ren population. To minimize the influence of genetic variability during the screening, vegetative clones of individual A. tuberculatus were produced. Plants from the MO-Ren population were grown in 25 by 55 cm greenhouse flats as previously described. Once seedlings emerged and reached an average height of 15 cm, the shoot apices were removed to promote elongation and development of the lateral buds into branches. Once axillary branches from each plant reached 4 to 5 cm in length, eight branches were removed from the plant and used to generate clones by dipping the lower end of the excised branch in 0.1% indole-3-butyric acid powder (Bontone® II, Bonide Products, Oriskany, NY). Each treated branch was moved to a 13.5 by 13.5 cm plant propagation tray (four 216-cm3 pots tray−1) containing the commercial potting medium, and 168 MO-Ren plants were propagated using this method. Treated branches were maintained in a greenhouse under conditions similar to those described earlier, except the treated branches were watered for 1 min every consecutive hour during the day for the first 2 wk using an automatic sprinkler irrigation system (Orbit Easy Dial™, Orbit Irrigation, Bountiful, UT) to maintain moist and humid conditions. After the first 2 wk, the plants were watered normally as needed.
When cloned plants reached 10 cm in height, six clones were selected and sprayed with one of the six herbicide treatments at recommended field rates: 2,4-D amine at 1.12 kg ae ha−1, atrazine at 1.12 kg ai ha−1, chlorimuron at 0.01 kg ai ha−1, fomesafen at 0.33 kg ai ha−1, glyphosate at 0.84 kg ae ha−1, and mesotrione at 0.11 kg ai ha−1. A total of 1,008 clones representing 168 MO-Ren plants were screened. Herbicide application and survival assessments were conducted at 21 DAA as described previously.
Statistical Analyses
Visual control ratings data from the field experiment were analyzed using the PROC GLIMMIX in SAS (SAS v. 9.4, SAS Institute, Cary, NC). Herbicide treatments were analyzed as fixed effects, while replication was considered a random effect. Treatment means were separated using Fisher’s Protected LSD at P ≤ 0.05. Data were analyzed separately due to statistically significant differences in response variables between years. The greenhouse dose–response data (visual control and plant survival) were analyzed by fitting nonlinear regression models (Equations 1 and 2) using GraphPad Prism v. 7.03 (GraphPad Software, San Diego, CA). Due to no statistically significant differences in response variables between experimental runs, the data were pooled. The herbicide dose resulting in 50% visual control (I50) and reduction in plant survival by 50% (LD50) with respect to the untreated control were calculated for each population. The level of resistance from the dose response was derived by calculating the ratio between the I50 or LD50 of the MO-Ren population and the I50 or LD50 of the susceptible standard. The model fit to the visual control ratings data was:
where Y is the visual control (%), x (independent variable) is the herbicide dose, I50 is the dose resulting in 50% visual control, and b is the hillslope of the curve. The model fit to the plant survival data was:
where Y is the plant survival (%), x (independent variable) is the herbicide dose, LD50 is the dose of herbicide required to produce a 50% reduction in plant survival, and b is the hillslope of the curve.
The gene stacking by phenotype binary data were visualized according to the “UpSet” technique (Lex et al. Reference Lex, Gehlenborg, Strobelt, Vuillemot and Pfister2014) to show all possible logical relations between different sets using the ‘UpSetR’ package in R (Conway et al. Reference Conway, Lex and Gehlenborg2017; R Development Core Team 2017). UpSet is a novel technique of data visualization for the quantitative analysis of sets and their intersections. UpSet effectively represents association of data by visualizing data set intersections of more than three or four sets in a matrix layout.
Results and Discussion
Field Experiment
Results from the field experiments indicate that this A. tuberculatus population (MO-Ren) is resistant to six of the eight herbicides tested in the field, including 2,4-D. At 21 DAA, application of 2,4-D at 0.56 to 4.48 kg ha−1 provided visual control in the range of 26% to 77% and 15% to 55% of MO-Ren population in 2015 and 2016, respectively (Table 4). By 42 DAA, the visual control was similar or lower than that observed at 28 DAA in both years. The continual use of the same herbicide or herbicides with the same mechanism of action will inevitably select for tolerant species or for resistant biotypes (Shaner Reference Shaner2014). Biomass reduction of the A. tuberculatus plants treated with 2,4-D followed similar trends as the visual control ratings; plant biomass was reduced by 34% to 87% in 2015 and by 48% to 58% in 2016 in response to the range of 2,4-D rates evaluated. The 4.48 kg ha−1 rate of 2,4-D represents four times the labeled rate for 10- to 15-cm A. tuberculatus, and yet this rate did not provide acceptable levels of control (>90% control) or biomass reduction in the field. Bernards et al. (Reference Bernards, Crespo, Kruger, Gaussoin and Tranel2012) discovered the first 2,4-D–resistant A. tuberculatus in a warm-season grass production field, and 3,584 kg ha−1 of 2,4-D only resulted in a 71% control at 7 DAA. Only one other biotype of A. tuberculatus has been identified as 2,4-D–resistant in a row-crop setting in Illinois in 2016 (Sabate et al. Reference Sabate, Tranel and Hager2016).
Table 4 Visual control and biomass reduction in response to different herbicide chemistries and doses at the Renick, MO, field site in 2015 and 2016.

a Means followed by the same letter are not different P≥0.05.
The population tested in this experiment also exhibited resistance to atrazine (PSII inhibitor), chlorimuron (ALS inhibitor), fomesafen (PPO inhibitor), glyphosate (EPSPS inhibitor), and mesotrione (HPPD inhibitor). This population exhibited very high levels of resistance to atrazine, with visual control never exceeding 27% in either year, and the highest level of biomass reduction was 22% with 4.48 kg ha−1 atrazine in 2016 (Table 4). The first A. tuberculatus population discovered with resistance to PSII-inhibiting herbicides was identified in 1994 in Missouri (Heap Reference Heap2017). Since that time, Schultz et al. (Reference Schultz, Chatham, Riggins, Tranel and Bradley2015) determined that approximately 30% of the A. tuberculatus populations across Missouri are atrazine resistant. Matthew et al. (Reference Matthew, Tranel, Loyd and Edward1998) reported that an Illinois A. tuberculatus population required more than 20 kg ha−1 atrazine to achieve 50% control.
Similarly, this A. tuberculatus population was highly resistant to chlorimuron, with visual control never exceeding 7% either year. Biomass was only reduced by as much as 14% either year (Table 4). These results were expected, since the majority of the A. tuberculatus populations across the Midwest are now resistant to ALS-inhibiting herbicides (Heap Reference Heap2017). For example, out of the 187 populations of A. tuberculatus collected by Schultz et al. (Reference Schultz, Chatham, Riggins, Tranel and Bradley2015) in a survey of Missouri A. tuberculatus populations, 186 were resistant to ALS-inhibiting herbicides.
Visual control of this A. tuberculatus population was greater in 2015 (22% to 47%) than 2016 (2% to 9%) in response to fomesafen (Table 4). Biomass reduction followed similar trends as the visual control, with an 11% to 44% and 3% to 26% biomass reduction in response to increasing fomesafen rates in 2015 and 2016, respectively. The seasonal variability in the efficacy of herbicides between the experiments may be due to the presence of natural variation in seedbank or other environmental factors. Schultz et al. (Reference Schultz, Chatham, Riggins, Tranel and Bradley2015) reported that approximately 17% of the A. tuberculatus populations remaining in Missouri soybean fields at harvest are resistant to PPO herbicides; however, the percentage of fields with PPO-resistant A. tuberculatus continues to increase in Missouri due to widespread POST use of these herbicides as “rescue” treatments (K Bradley, personal communication).
Glyphosate provided only 6% to 24% and 2% to 21% visual control of the MO-Ren population in 2015 and 2016, respectively (Table 4). Biomass reduction was greater in 2016 (18% to 33%) than in 2015 (6% to 11%). This may be a result of the field site receiving 30 mm more precipitation in June 2016 than August 2015 (Table 5). Skelton et al. (Reference Skelton, Ma and Riechers2016) reported that the efficacy of herbicides such as glyphosate is reduced when A. tuberculatus is under drought stress and that A. tuberculatus dry matter was reduced by 2% when receiving 10 ml water day−1, but dry matter of plants that received 40 ml water day−1 was reduced by 56%. Nevertheless, these results clearly indicate glyphosate resistance in the MO-Ren population as well. Schultz et al. (Reference Schultz, Chatham, Riggins, Tranel and Bradley2015) reported that 29% of Missouri A. tuberculatus populations were resistant to glyphosate, and glyphosate-resistant A. tuberculatus now also occurs in 19 U.S. states and 1 province in Canada (Heap Reference Heap2017).
Table 5 Monthly rainfall (mm) and average monthly temperatures (C) in comparison to the 30-yr averages from May through October in 2015 and 2016 at the Renick, MO, field site.
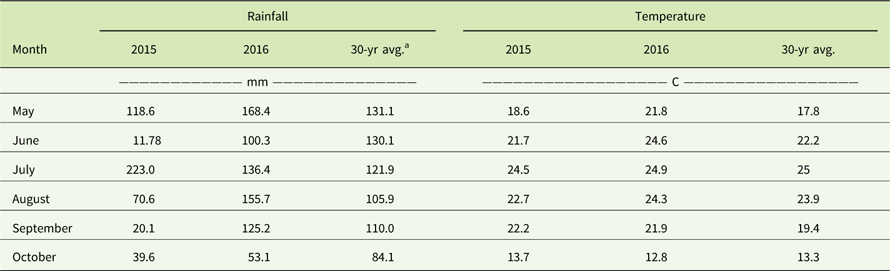
a The 30-yr averages were obtained from the National Climatic Data Center (www.ncdc.noaa.gov).
Mesotrione resulted in 34% to 73% and 38% to 88% visual control of the MO-Ren population by 28 DAA in 2015 and 2016, respectively (Table 4). By 42 DAA in both years, visual control was very similar to that observed 28 DAA. Amaranthus tuberculatus biomass was also reduced by 18% to 78% and 27% to 77% in 2015 and 2016, respectively. These results indicate that this population is also resistant to mesotrione. In other investigations of HPPD resistance in A. tuberculatus, Hausman et al. (Reference Hausman, Singh, Tranel, Riechers, Kaundun, Polge, Thomas and Hager2011) observed from 13% to 27% visual injury in response to the labeled rate of mesotrione in a biotype from Illinois, while McMullan and Green (Reference McMullan and Green2011) reported only 41% injury in response to a 2X rate of mesotrione on an A. tuberculatus biotype from Iowa.
The only two herbicides that provided acceptable control of MO-Ren A. tuberculatus in the field were dicamba and glufosinate (Table 4). By 42 DAA, 2.24 and 4.48 kg ha−1 dicamba provided 93% and 99% control of this A. tuberculatus population and 95% and 100% biomass reduction, respectively. Even though 2,4-D and dicamba are both synthetic auxin, Group 4 herbicides, they belong to different chemical families, which can lead to different plant responses. These results are similar to those reported by Bernards et al. (Reference Bernards, Crespo, Kruger, Gaussoin and Tranel2012), in that both populations exhibit resistance to 2,4-D. Both biotypes are susceptible to dicamba but demonstrate some tendency toward reduced sensitivity at the labeled rates.
Control of the MO-Ren population was also variable in response to glufosinate and was dependent on rate. By 28 DAA in 2015, visual control ranged from 56% to 90% and was very similar to that observed at 42 DAA. In 2016, however, visual control ranged from 47% to 89% at 28 DAA but had fallen to 28% to 71% by 42 DAA. Since glufosinate is primarily a contact herbicide, control was highest after the initial application, but A. tuberculatus plants that were at least partially defoliated put out new growth after all of the initial leaves had abscised. Coetzer et al. (Reference Coetzer, Al-Khatib and Peterson2002) also reported that a single application of glufosinate was not as effective as two applications because of plant regrowth and the contact nature of glufosinate.
Greenhouse Dose–Response Experiments
The I50 and LD50 values for the MO-Ren population were greater than those of the susceptible population for 2,4-D, fomesafen, atrazine, glyphosate, mesotrione, and chlorimuron (Table 6; Figures 1 and 2). Results from the dose–response experiments confirmed that the MO-Ren A. tuberculatus population was 3-fold more resistant to 2,4-D (Table 6; Figures 1 and 2), making this population the third 2,4-D–resistant A. tuberculatus population identified in the United States. Estimates of I50 for 2,4-D for the MO-Ren population were 1.44 kg ha−1, while only 0.47 kg ha−1 was required to achieve the same level of control for the MO-S population. Similarly, Bernards et al. (Reference Bernards, Crespo, Kruger, Gaussoin and Tranel2012) reported a greater level of 2,4-D resistance in a Nebraska A. tuberculatus biotype that required 1.86 kg 2,4-D ha−1 to achieve 50% visual control.

Figure 1 Dose response for visual control of suspected multiple-resistant (MO-Ren) and standard susceptible (MO-S, MO-GS) waterhemp (Amaranthus tuberculatus) populations at 21 DAA in response to 2,4-D, atrazine, fomesafen, glyphosate, mesotrione, and dicamba. Each data point represents the mean percentage visual control; vertical bars represent standard error of mean. Nonlinear regression analysis was performed using Equation 1; b is the hillslope (± SE) of the curve.

Figure 2 Dose response for survival of suspected multiple-resistant (MO-Ren) and standard susceptible (MO-S, MO-GS) waterhemp (Amaranthus tuberculatus) populations at 21 DAA in response to 2,4-D, atrazine, fomesafen, glyphosate, mesotrione, and dicamba. Each data point represents the mean percentage survival; vertical bars represent standard error of mean. Nonlinear regression analysis was performed using Equation 2; b is the hillslope (± SE) of the curve.
Table 6 Estimates of the herbicide dose resulting in 50% visual control (I50) and reduction in plant survival (LD50) for the MO-Ren and susceptible waterhemp (Amaranthus tuberculatus) populations at 21 DAA.

a Values in parentheses are 95% limits of confidence intervals.
b Standard populations used for each herbicide: MO-S, susceptible to 2,4-D, atrazine, fomesafen, mesotrione, and dicamba, and resistant to chlorimuron; MO-GS, susceptible to glyphosate.
c Values could not be estimated by the model, because the dose range was not covering sufficiently large doses.
Similar to the field results described previously, resistance to PSII-, EPSPS-, HPPD-, ALS-, and PPO-inhibiting herbicides was also confirmed in the MO-Ren population. The MO-Ren population exhibited high-level resistance to atrazine and chlorimuron, which is a common response of Amaranthus species to these herbicides in many other locations throughout the United States (Bell et al. Reference Bell, Hager and Tranel2013; Matthew et al. Reference Matthew, Tranel, Loyd and Edward1998; McMullan and Green Reference McMullan and Green2011; Patzoldt et al. Reference Patzoldt, Dixon and Tranel2003, Reference Patzoldt, Tranel and Hager2005; Sarah-Taylor et al. Reference Sarah-Taylor, Loyd, Michael and Peterson1996; Sprague et al. Reference Sprague, Stoller, Wax and Horak1997a, Reference Sprague, Stollfr and Wax1997b). For example, Sprague et al. (Reference Sprague, Stollfr and Wax1997b) reported an A. tuberculatus population with 1,920-fold resistance to chlorimuron that required rates >1,000 gaiha−1 to achieve 50% control. A majority of the A. tuberculatus populations across the Midwest are now resistant to ALS-inhibiting herbicides, and many of them exhibit ultrahigh levels of resistance (Heap Reference Heap2017; Schultz et al. Reference Schultz, Chatham, Riggins, Tranel and Bradley2015). The MO-Ren population in this study had high survival even at the maximum rate of chlorimuron used (20X the labeled rate); therefore, I50 and LD50 values could not be estimated (Table 6). The MO-Ren population exhibited approximately 22-fold resistance to glyphosate based on LD50 values, which is similar to the 19-fold resistance reported in the first glyphosate-resistant A. tuberculatus population discovered (Legleiter and Bradley Reference Legleiter and Bradley2008). The I50 for mesotrione for the MO-Ren population was 0.42 g ha−1, which resulted in an approximate 8-fold level of resistance (Table 6).
Similar to the field study, one of the only herbicides that provided effective control of the MO-Ren population was dicamba (Table 6; Figures 1 and 2). Both the MO-Ren and MO-S populations were effectively controlled with dicamba, and the LD50 values were similar (0.61 and 0.63 kg ha−1, respectively). In contrast, Bernards et al. (Reference Bernards, Crespo, Kruger, Gaussoin and Tranel2012) reported that the Nebraska 2,4-D–resistant population exhibited reduced sensitivity to dicamba, requiring approximately 3-fold more dicamba to achieve 50% visual control than the susceptible populations.
Greenhouse Gene-Stacking Analysis
Field and dose–response experiments confirmed resistance of the MO-Ren population to herbicides with six different SOAs. A screening assay was conducted to determine whether individual plants within the population were resistant to all six SOAs. Vegetative clones derived from 168 individual MO-Ren plants were screened with herbicides from each of the six SOA groups. Of the 168 plants, 27 (16%) survived each herbicide and were classified as six-way resistant (Figure 3). Similarly, 23%, 28%, 26%, 5%, and 1% plants were classified as plants with five-, four-, three-, two-, and one-way resistance, respectively. None of the plants were classified as susceptible to all SOA groups. As expected, the MO-S or MO-GS populations were controlled at the herbicide rates used, except for chlorimuron, because the MO-S population was used as a chlorimuron-resistant standard. These results suggest that 99% of individual plants within the population harbor resistance to more than one of the six different SOAs screened. The presence of stacked genes in the majority of the plants also indicates that a fitness penalty may not be involved, but this needs further investigation. This is similar to previous work in which individuals of an A. tuberculatus population from Illinois were found to harbor resistance to herbicides of four SOA groups (Bell et al. Reference Bell, Hager and Tranel2013). Tranel et al. (Reference Tranel, Riggins, Bell and Hager2011) noted that a population containing individuals with genes stacked for resistance to four groups of herbicides may not be controlled effectively with a tank mix of herbicides representing the four SOAs. Our results imply that mixtures of multiple herbicides from these six SOA groups may not control the MO-Ren population. However, inclusion of these herbicides in the mixtures could still improve A. tuberculatus control due to the presence of plants that contain genes conferring resistance to one herbicide but susceptibility to others.

Figure 3 An UpSet plot showing the gene stacking by phenotype data set (n = 168) for the MO-Ren population. The combination matrix at the bottom identifies the site of action (SOA) intersections, while the bar above it encodes the size of each intersection, that is, the number of plants for each intersection. The three intersection queries are the six-way intersection (red), five-way intersection (mustard), and one-way intersection (olive green) of all six SOA group herbicides. The horizontal bar plot (blue) on the left displays the frequency of resistant plants to each SOA herbicide.
The results of this research indicate that multiple resistances across six herbicide SOAs are present in the MO-Ren A. tuberculatus and that this population is resistant to 2,4-D. This population exhibited 3-fold resistance to 2,4-D, and there are currently only two other known A. tuberculatus populations that have been reported with resistance to 2,4-D: one in Nebraska discovered in a warm-season grass production field, and one in Illinois in a corn and soybean production field (Bernards et al. Reference Bernards, Crespo, Kruger, Gaussoin and Tranel2012; Sabate et al. Reference Sabate, Tranel and Hager2016). Technologies such as 2,4-D–resistant corn and soybean have been developed and are awaiting approval to be commercialized. Dicamba-resistant corn, cotton, and soybean, and 2,4-D–resistant cotton technologies have already been developed and have been recently released for commercial use. The fact that there are already three A. tuberculatus populations with resistance to 2,4-D and that at least one of these populations has demonstrated a reduced sensitivity to dicamba should be emphasized to ensure that proper stewardship of these new technologies is followed (Bernards et al. Reference Bernards, Crespo, Kruger, Gaussoin and Tranel2012; Tehranchian et al. Reference Tehranchian, Norsworthy and Powles2016). There are currently no other reported A. tuberculatus populations with resistance to six different herbicide classes (Heap Reference Heap2017), which also underlines the need to use a diversified approach toward weed management that includes any of the appropriate cultural, mechanical, and biological control tactics available in a given production system rather than relying on any one method alone (Norsworthy et al. Reference Norsworthy, Ward, Shaw, Llewellyn, Nichols, Webster, Bradley, Frisvold, Powles, Burgos, Witt and Barrett2012).











