Introduction
Plants are hosts to an array of fungal symbionts termed “endophytes” that grow symptomless and systemically within plant tissues (Carroll Reference Carroll1988; Faeth and Fagan Reference Faeth and Fagan2002; Rodriguez et al. Reference Rodriguez, White, Arnold and Redman2009). Many fungal endophytes are assumed to be mutualistic, because those most extensively studied to date provide mycotoxins to economically important grasses, which reduces herbivory (Clay and Schardl Reference Clay and Schardl2002), and increase abiotic stress tolerance in the host (Dastogeer et al. Reference Dastogeer, Chakraborty, Sarker and Akter2020; Rodriguez et al. Reference Rodriguez, White, Arnold and Redman2009; Singh et al. Reference Singh, Gill and Narendra2011); these endophytes can confer fitness advantages to the host by altering host plant physiology and oxidative stress tolerance mechanisms (Fu et al. Reference Fu, Schardl, Cook, Cao, Ling, He, Wu, Xue, Li and Shi2024; Shankar Naik Reference Shankar Naik2019). However, most studies on plant–fungal symbioses have been conducted on members of the Poaceae family grown in pots under greenhouse or growth chamber conditions without removal of the endophyte (Liu-Xu et al. Reference Liu-Xu, Vicedo, García-Agustín and Llorens2022) or quantification of mutualism effects (Mathis and Bronstein Reference Mathis and Bronstein2020; May Reference May2016). Our understanding of how fungal endophytes influence other plant genera remains limited, especially for dicotyledonous weedy species.
Species in the genera Astragalus and Oxytropis are perennial legumes that contain fungal endophytes belonging to Alternaria sect. Undifilum. These endophytes produce swainsonine (1, 2, 8-trihydroxyoctahydroindolizine), a toxic alkaloid that causes a wasting disease and symptoms of neurologic disease in wildlife and livestock that graze on the host plants (Cook et al. Reference Cook, Ralphs, Welch and Stegelmeier2009b; Noor et al. Reference Noor, Nava, Cooke, Cook and Creamer2018). Astragalus and Oxytropis species are important rangeland weeds across multiple continents: toxic swainsonine production linked to fungal endophyte presence has been reported in Astragalus and Oxytropis species native to China and North and South America (Cook et al. Reference Cook, Gardner and Pfister2014; He et al. Reference He, Guo, Wang, Zhao, Guo, Cao, Mur and Wei2019; Klypina et al. Reference Klypina, Pinch, Schutte, Maruthavanan and Sterling2017; Martinez et al. Reference Martinez, Lauroua, Borrelli, Gardner and Robles2019) and in several related Swainsona species endemic to Australia (Cook et al. Reference Cook, Gardner and Pfister2014; Grum et al. Reference Grum, Cook, Gardner, Roper, Pfister and Ralphs2012). The erratic behavior and loss of motor control seen in livestock grazing on Astragalus and Oxytropis has led to these plants being collectively referred to as “locoweeds.” Annual economic loss from livestock locoweed poisoning in the United States is estimated to be more than US$300 million (Turner and Allison Reference Turner and Allison2023).
The Alternaria endophyte responsible for this toxicity forms an extensive intercellular network of mycelia between stem parenchyma cells in the host plant, but there is no evidence of pathological effects on cell walls or vascular tissue (Noor et al. Reference Noor, Nava, Cooke, Cook and Creamer2018). The fungus is found throughout the host plant’s aboveground tissues with few mycelia detected in roots (Cook et al. Reference Cook, Gardner, Ralphs, Pfister, Welch and Green2009a). Removal of vegetative tissue does not reduce swainsonine content in subsequent leaf regrowth, because the crown serves as the endophyte source (Cook et al. Reference Cook, Gardner, Roper, Ransom, Pfister and Panter2016). In Astragalus and Oxytropis species, the endophyte, and hence swainsonine synthesis, is vertically transmitted from maternal parent to progeny via seed (Ralphs et al. Reference Ralphs, Cook, Gardner and Grum2011). The fungus resides in the parenchyma and aleurone layers of the seed coat with nearly 100% transmission to the seedling as it germinates, followed by swainsonine synthesis exclusively by the fungus (Oldrup et al. Reference Oldrup, McLain-Romero, Padilla, Moya, Gardner and Creamer2010; Ralphs et al. Reference Ralphs, Cook, Gardner and Grum2011).
Swainsonine concentrations within and among Astragalus and Oxytropis populations are highly variable, ranging from undetectable to 0.4% dry mass; undetectable amounts in some populations are most likely due to the endophyte not establishing (Cook et al. Reference Cook, Gardner, Roper, Ransom, Pfister and Panter2016; Grum et al. Reference Grum, Cook, Gardner, Roper, Pfister and Ralphs2012; Ralphs et al. Reference Ralphs, Gardner, Graham, Greathouse and Knight2002, Reference Ralphs, Creamer, Baucom, Gardner, Welsh, Graham, Hart, Cook and Stegelmeier2008, Reference Ralphs, Cook, Gardner and Grum2011). Swainsonine production varies with the amount of endophyte present (Achata Böttger et al. Reference Achata Böttger, Creamer and Gardner2012; Grum et al. Reference Grum, Cook, Gardner, Roper, Pfister and Ralphs2012) and is strongly influenced by fungal and host plant genotypes (Cook et al. Reference Cook, Grum, Gardner, Welch and Pfister2013; Guo et al. Reference Guo, Zhang, Zhao, Beckmann, Phillip, Meng, Mo, Mur and He2022; He et al. Reference He, Guo, Wang, Zhao, Guo, Cao, Mur and Wei2019). The plant part sampled and host plant phenology also affect detectable swainsonine levels (Achata Böttger et al. Reference Achata Böttger, Creamer and Gardner2012; Cook et al. Reference Cook, Shi, Gardner, Pfister, Grum, Welch and Ralphs2012; Ralphs et al. Reference Ralphs, Creamer, Baucom, Gardner, Welsh, Graham, Hart, Cook and Stegelmeier2008). For example, Cook et al. (Reference Cook, Gardner and Pfister2014) reported swainsonine in aboveground tissue of silky crazyweed (Oxytropis sericea Nutt.) increasing as the host plant matured through the growing season and decreasing with the onset of senescence.
Evidence for the role of fungal endophytes in increasing tolerance to abiotic stress in locoweeds is mixed. Biomass of O. sericea seedlings exposed to water stress and low pH in sterile cultures was greater in the presence of the endophyte, and water deficit increased swainsonine production (Oldrup et al. Reference Oldrup, McLain-Romero, Padilla, Moya, Gardner and Creamer2010). In contrast, under greenhouse conditions, repeated water deficit did not affect the biomass of O. sericea or bigbend loco (Astragalus mollissimus Torr.) plants regardless of each taxon’s ability to produce swainsonine (Vallotton et al. Reference Vallotton, Murray, Delaney and Sterling2012). Water deficit increased swainsonine levels in greenhouse-grown woolly loco (Astragalus mollissimus Torr. var. mollissimus) and Astragalus mollissimus var. bigelovii (A. Gray) Barneby ex B.L. Turner plants, but not in Astragalus mollissimus Torr. var. thompsoniae (S. Watson) Barneby or O. sericea plants (Klypina et al. Reference Klypina, Pinch, Schutte, Maruthavanan and Sterling2017; Vallotton et al. Reference Vallotton, Murray, Delaney and Sterling2012). When endophyte-free and endophyte-infected O. sericea or A. mollissimus plants were exposed to sublethal drought under greenhouse conditions, endophyte presence did not influence photosynthetic gas exchange or leaf pigments in either taxon; however, the endophyte did induce antioxidant defenses in A. mollissimus (Klypina et al. Reference Klypina, Pinch, Schutte, Maruthavanan and Sterling2017).
Evidence or any effect of endophyte presence on locoweed plant growth is also mixed. Freckled milkvetch (Astragalus lentiginosus Douglas ex Hook.) plant volume and leaf %N was reduced when grown with the Alternaria endophyte compared with endophyte-free plants in 19-L pots in the field (Harrison et al. Reference Harrison, Beltran, Buerkle, Cook, Gardner, Parchman, Poulson and Forister2021). However, nitrogen fertilization that enhanced plant growth did not influence swainsonine production in aboveground tissues (Delaney et al. Reference Delaney, Klypina, Maruthavanan, Lange and Sterling2011), nor did CO2 fertilization (Cook et al. Reference Cook, Gardner, Pfister, Lee, Welch and Welsh2017), suggesting that although the endophyte must use carbon and nitrogen provided by the plant for growth and swainsonine synthesis, any drain on the host is asymptomatic, with no resource trade-off.
Given the conflicting results reported by various researchers, how Alternaria endophytes affect their obligate Astragalus and Oxytropis hosts remains unclear. More crucially, we do not know whether plants that host the swainsonine-producing fungus—and are therefore toxic to livestock—possess a selective advantage over plants that lack the endophyte. To address this question, we established a long-term common garden to directly compare survival, growth, and fecundity of A. mollissimus well outside its native range and O. sericea within its native range in the presence or absence of their Alternaria endophyte. We hypothesized the endophyte would confer a growth and fecundity advantage for host plants compared with those with no endophyte when challenged by various abiotic stresses under field conditions. To the best of our knowledge, the research we report here represents the first field-based, multiyear study to test this hypothesis across multiple generations.
Materials and Methods
Plant Materials
We compared three taxa from the two closely related genera Astragalus and Oxytropis which grow in unique geographical niches in the western USA (Fox et al. Reference Fox, Allred and Roalson1998) and vary in their endophyte’s ability to synthesize swainsonine (Delaney et al. Reference Delaney, Klypina, Maruthavanan, Lange and Sterling2011; Vallotton et al. Reference Vallotton, Murray, Delaney and Sterling2012). Astragalus mollissimus var. mollissimus is widespread throughout the varied soils of the prairies, plains, mesas, roadsides, and pine forests from New Mexico to the north central United States (elevation: 1,400 to 2,500 m asl), has a short life history for a perennial (Soltani et al. Reference Soltani, Benakashani, Baskin and Baskin2021), and is a high producer of toxic swainsonine. Astragalus mollissimus var. thompsoniae has a more limited range in the southwestern United States, where it grows in sandy soils of the mesas, grasslands, and butte foothills (elevation: 1,400 to 2,150 m asl). Although A. mollissimus var. thompsoniae possesses the Alternaria endophyte (Ralphs et al. Reference Ralphs, Creamer, Baucom, Gardner, Welsh, Graham, Hart, Cook and Stegelmeier2008), it produces little or no detectable swainsonine, possibly due to host modification of the endophyte (Kulshreshtha et al. Reference Kulshreshtha, Creamer and Sterling2004; Vallotton et al. Reference Vallotton, Murray, Delaney and Sterling2012). Therefore, we included A. mollissimus var. thompsoniae in this study to separate the effects on the host of endophyte presence from those of swainsonine production. Oxytropis sericea inhabits gravelly, sandy, and loam soils of the mountains, plains, prairies, sagebrush, oak, and mixed conifer communities from northern Mexico, through much of the western United States into Canada (elevation: 1,000 to 3,300 m asl), typically lives much longer than Astragalus species (Fox et al. Reference Fox, Allred and Roalson1998; Welsh Reference Welsh2007), and produces intermediate levels of swainsonine (Vallotton et al. Reference Vallotton, Murray, Delaney and Sterling2012). Seeds used to establish this study were collected from northern New Mexico as described in Delaney et al. (Reference Delaney, Klypina, Maruthavanan, Lange and Sterling2011) for O. sericea and A. mollissimus var. mollissimus and Vallotton et al. (Reference Vallotton, Murray, Delaney and Sterling2012) for A. mollissimus var. thompsoniae.
Generation of E+ and E− Seedlings
Endophyte-free (E−) plants were generated through mechanical removal of the seed coat and residual parenchymatous tissue using sterile tweezers; the resulting embryos were surface sterilized with 1% bleach solution for 30 s and rinsed in sterile de-ionized water (Klypina et al. Reference Klypina, Pinch, Schutte, Maruthavanan and Sterling2017). Mechanical and fungicide removal yield similar results in terms of endophyte removal with seedlings unaffected by either treatment (Grum et al. Reference Grum, Cook, Gardner, Roper, Pfister and Ralphs2012); we chose mechanical removal to reduce any toxicological effect of the fungicide on the embryo. Whole E+ seeds and E− embryos were germinated at room temperature in a petri dish on water-moistened sterile filter paper; transplanted to a Jiffy 7 peat pellet (Plantation Products, Norton, MA, USA) until emergence of the first true leaf; then repotted in three parts standard greenhouse soil mix (1:1:1 [by vol] soil:potting soil:perlite; soil was a Bozeman silt loam [fine-silty, mixed, superactive Pachic Argicryolls]; potting soil was Sunshine Mix no. 1, Sun Gro Horticulture, Bellevue, WA, USA) to one part washed, fine sand; and grown in the greenhouse under a 16-h photoperiod of natural sunlight supplemented with mercury vapor lamps (165 μmol m−2 s −1) at 25 ± 4 C. Plants were fertilized biweekly with Jack’s Classic 20-20-20 (N-P-K) All Purpose fertilizer (J. R. Peters, Allentown, PA, USA) at 100 ppm. Seedlings emerged similarly regardless of seed coat manipulation.
Endophyte Detection
Endophyte status (E+ or E−) was confirmed for each plant using DNA and swainsonine analyses before transplanting. Genomic DNA was extracted using a modified cetyltrimethylammonium bromide (CTAB) protocol (Doyle and Doyle Reference Doyle and Doyle1987). Mechanically ground (Mini-Beadbeater, Biospec, Bartlesville, OK, USA) dried plant tissue was suspended in CTAB extraction buffer (55 mM CTAB, 0.1 M Tris-HCL pH 8.0, 20 mM ethylenediaminetetraacetic acid pH 8.0, 1.4 M NaCl, 2% β-mercaptoethanol), incubated at 65 C for 1 h, and pelleted; the supernatant was treated with 1 µl 10 mg ml−1 RNase at 37 C for 30 min, then extracted twice with equal volume of chloroform:isoamyl alcohol (24:1); and DNA was precipitated with 0.1 vol 3M NaOAc in 100% ethanol with a final 70% ethanol wash. Endophyte presence or absence was determined through PCR analysis using primers amplifying the internal transcribed spacer region of Alternaria sect. Undifilum fungal endophytes as described previously (Ralphs et al. Reference Ralphs, Creamer, Baucom, Gardner, Welsh, Graham, Hart, Cook and Stegelmeier2008). Endophyte status was also confirmed by measuring plant swainsonine content at the USDA-ARS Poisonous Plant Laboratory, Logan, UT, USA, before transplanting and again in 2018 after growing in the field. Swainsonine was extracted from dried, ground leaf material (50 mg) in 2% acetic acid for 18 h with agitation. After extraction, samples were analyzed by liquid chromatography–mass spectrometry as previously described (Gardner et al. Reference Gardner, Molyneux and Ralphs2001). Detection limit of swainsonine was 0.001% dry mass. The concentrations of swainsonine detected in our E+ plants (data not shown) were at the level that will cause vertebrate toxicity (Pfister et al. Reference Pfister, Stegelmeier, Gardner and James2003; Stegelmeier et al. Reference Stegelmeier, James, Panter, Gardner, Ralphs and Molyneux1999). For E− plants, swainsonine content remained zero when plants were tested as seedlings and again as transplants after several years of growth; therefore, horizontal transmission of the endophyte was never detected.
Establishment and Maintenance of a Common Garden
Four-month-old, greenhouse-grown seedlings were transplanted into a common garden (15.2 m by 4.6 m) at the Montana State University Post Farm near Bozeman, MT, USA (45.67537°N, 111.1559°W; elevation 1,450 m). The soil was an Amsterdam silty loam (fine-silty, mixed, superactive, frigid Typic Haplustolls) (Miller et al. Reference Miller, Jones, Zabinski, Tallman, Housman, D’Agati and Holmes2023) with pH 7, 2.1% organic matter, and nitrate-nitrogen at ca. 7 mg kg−1. Mean annual precipitation was 384 mm, with about half received each year during locoweed’s reproductive growth period from April through June (Supplementary Table S1) (U.S. National Centers for Environmental Information 2023). Mean annual temperature for April to June was 10 C, with average low of −8 C in January and average high of 28 C in July. Seedlings were planted as pairs with endophyte (E+) and endophyte removed (E−) in a randomized nested design across nine blocks located along a slight elevation gradient from east to west within the garden plot. As plants died, new pairs were transplanted into the garden with ca. 35 pairs of each taxon and generation planted into each block. Astragalus mollissimus seedlings were planted in the common garden from 2011 to 2015 (A. mollissimus var. mollissimus, 96 pairs total) and 2012 to 2015 (A. mollissimus var. thompsoniae, 74 pairs total). Most O. sericea seedlings were planted in 2011 (18 pairs) and 2013 (24 pairs), with additional pairs planted in 2012 (2 pairs), 2014 (3 pairs), and 2015 (3 pairs) to replace deceased plants (50 pairs total as Gen 0). Most Oxytropis plants died, and all A. mollissimus var. mollissimus and A. mollissimus var. thompsoniae plants died during the 2013 to 2014 winter regardless of endophyte status, and replacement pairs of greenhouse-grown seedlings were planted as feasible.
As already described, seeds used to initiate this experiment were collected in the field from plants growing in their native ranges in New Mexico. This raises the possibility that data for the first generation of plants established in our common garden (Gen 0) might be biased by maternal effects reflecting the different environments experienced by the original New Mexico seed parents and their offspring grown in Montana. To address this for O. sericea, we established 50 E+/E− pairs for each of two successive generations after Gen 0 as follows: Parent plants (Gen 0: plants grown from seed field collected in New Mexico) were established in the common garden in 2013. Seeds collected in the common garden from 10 E+ and 10 E− of these 1-yr-old Gen 0 plants were used to establish first-generation (Gen 1) plants: five seedlings from each Gen 0 parent were transplanted into the garden as 50 E± pairs in fall 2015. Second-generation (Gen 2) plants were grown from seed collected in the common garden in 2016 from 1-yr-old Gen 1 plants representing 5 E+ and 5 E− lines established in Gen 1. Seedlings of 50 E ± pairs of Gen 2 were established in the garden in 2017. Endophyte presence and absence was confirmed for all plants at each stage by PCR and swainsonine analyses as previously described. This design also allowed us to monitor transgenerational transmission of the endophyte and any of its associated effects in O. sericea.
Seedlings were hand watered as needed during their establishment year only, and all weeds—including volunteer locoweed seedlings—were mechanically removed from the garden throughout the study period. Similar to earlier reports (Ida et al. Reference Ida, Harder and Kudo2012; Wyka Reference Wyka1999), there were few instances of herbivore or pathogen damage detected on the common-garden plants during this 9-yr field study. We observed occasional minor invertebrate and vertebrate herbivory regardless of plant endophyte status, but given the low levels of herbivore activity and its random distribution across E+ and E− plants, we did not attempt to manage or measure it.
Data Collection
The following measurements were collected on all plants until their death, or for surviving plants until termination of the common-garden experiment in July 2020.
Gas Exchange
We obtained gas exchange measurements over 5 yr for A. mollissimus var. mollissimus and A. mollissimus var. thompsoniae plants and 8 yr for O. sericea Gen 0, 5 yr for Gen 1, and 3 yr for Gen 2. Gas exchange was measured using a LI-6400XT portable photosynthesis system (Li-Cor, Lincoln, NE, USA) between 10:00 AM to 2:00 PM from a single leaf located within the top quarter of a plant as described previously (Delaney et al. Reference Delaney, Klypina, Maruthavanan, Lange and Sterling2011; Klypina et al. Reference Klypina, Pinch, Schutte, Maruthavanan and Sterling2017). Responses were measured over three locoweed phenological stages (May, vegetative; June, flowering; July/August, full pod/senescence). Net photosynthesis (P net: μmol CO2 m−2 s−1), stomatal conductance (g s: mol m−2 s−1), and transpiration rate (E: mmol H2O m−2 s−1) were measured under ambient light (photosynthetically active radiation, approximately 1,500 µmol m−2 s−1) with a flow rate of 400 µmol s−1 and internal CO2 concentration of 400 µmol mol−1. After each measurement, the leaf was excised and mounted within a paper frame equal to the chamber area and photographed. The leaf area was determined by scanning the leaf image and analyzing the pixel count using the open-sourced Java image processing and analysis program ImageJ (Schneider et al. Reference Schneider, Rasband and Eliceiri2012). Gas exchange measurements from each leaf were adjusted for leaf area measured.
Fecundity
The following components of plant reproductive output were measured each year of the experiment across taxa and endophyte status.
Reproductive Stems
A reproductive stem (“stem” throughout) in these locoweed taxa is the inflorescence on a leafless scape emerged from the crown. The total number of stems per plant was counted twice during each growing season, once in full bloom (May) and then again after initial bloom had ended (June). A subsample of 10 stems plant−1 was randomly selected, and for these stems, the number of flowers per stem (May) or seed pods stem (June) was determined. The highest number of stems counted from the two independent counts is reported.
Pod Collection
Mature seed pods were randomly collected from as many stems as possible beginning in early July. A minimum of 25 pods plant−1 were collected over the course of 2 wk. Pods were considered mature if they were beginning to show signs of splitting open at the beak, and were selected from the top, middle, and bottom of a stem. Pods that had split open more than 1 mm were not included to ensure collected pods had not lost seeds. If plants did not produce 25 pods, as many pods as possible were collected. All seed pods were placed in a −20 C freezer to prevent destruction of seeds by insect herbivory (Loganathan et al. Reference Loganathan, Jayas, Fields and White2011).
Mature Seed Count and Mass
Seed pods were split open and scraped to remove any lodged seeds. Seeds that appeared underdeveloped (if seed size < 1.5-mm length by 1.4-mm width) as measured with digital calipers (#700-126, Mitutoyo, Kawasaki, Japan) or ovaries that had not been fertilized were considered immature and excluded from the seed count. At least 100 seeds were weighed when possible and mean mass per seed was recorded.
Seed Germination and Viability
For seed germination analysis to determine fecundity, seeds were surface sterilized by successive washes with 70% ethanol (1 min), sterile de-ionized water (30 s), and 10% bleach (2 min), and rinsed three times in sterile deionized water for 5 min. Air-dried seeds were scarified with 100-grit sterile sandpaper, placed in a petri dish on filter paper saturated with sterile deionized water, and incubated at room temperature in the dark for 2 d, then examined daily. Seeds were considered germinated when the radicle was at least 6 mm long and were considered ungerminated if no radicle had emerged or the radicle failed to elongate beyond 6 mm after 10 d. Seed viability was determined using methods for Fabaceae plant families described by the Association of Official Seed Analysts (Peters and Lanham Reference Peters and Lanham2005). Scarified seeds were incubated in the dark at 30 C overnight (12 to 16 h) on saturated filter paper containing 1% (v/v) 2, 3, 5-triphyenyl tetrazolium chloride; seeds with red color were deemed viable.
Final Biomass
Above- and belowground tissues were collected in July 2020 from all surviving O. sericea Gen 0 (5 E−, 4 E+), Gen 1 (21 E−, 15 E+), and Gen 2 (16 E−, 14 E+) plants. Biomass measurements were determined by separating, drying, and weighing stem, root, and crown tissue. Crown tissue was defined as the connective tissue between the aboveground rosette of leaves and inflorescences and the belowground taproots (Striker et al. Reference Striker, Manzur and Grimoldi2011). Tissue was oven-dried to constant mass at 50 C for a minimum of 120 h before weighing. Numbers of roots and stems were counted and recorded. Root and crown diameters were measured with an electronic caliper (Fowler High Precision, Ultra-Cal V, Canton, MA, USA). We were unable to collect root fines or roots deeper than 0.66 m, and no nodules were observed on any roots, either because the appropriate nitrogen-fixing bacteria were not present or the soil nitrogen content inhibited the symbiosis.
Data Analysis
The main effects of taxon, variety (for A. mollissimus), generation (for O. sericea), endophyte presence, and their interactions were analyzed. Year sampled (or year planted for overwinter mortality) and plant age (number of winters survived) were treated as fixed effects in linear or generalized linear mixed models (see Tables 1–4), using the R software environment (R Core Team, Reference Core Team2024), specifically the lme4 package for fitting generalized and linear mixed models (Bates et al. Reference Bates, Machler, Bolker and Walker2015). Random effects accounted for the nine blocks of each E± pair. For datasets with repeated measures over time (overwinter mortality, reproductive stems, flowers, seed pods, mature seeds, seed viability, germination after 10 d, photosynthesis, and mean seed mass), a random effect for plant nested within pair, nested within block, was also included. Data were visualized using ggplot2 (Wickham Reference Wickham2016) and model estimates were displayed using the ggeffects package (Lüdecke Reference Lüdecke2018).
Table 1. Two-way interaction ANOVA estimating the effect of (A) endophyte (+/−), Astragalus mollissimus var. mollissimus or Astragalus mollissimus var. thompsoniae, and their interaction, and (B) endophyte (+/−), Oxytropis sericea generations, and their interaction, on overwinter mortality a .
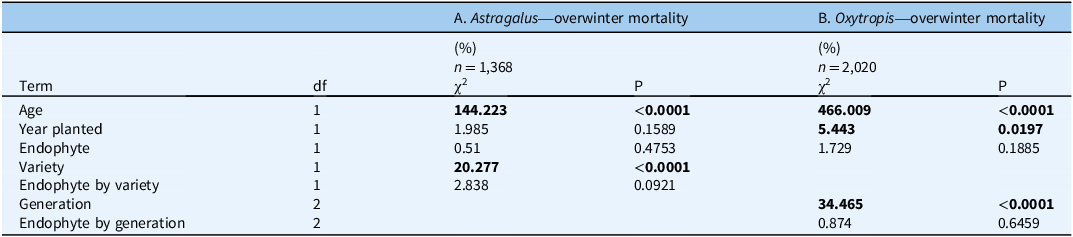
a These variables were measured for plants grown as pairs with or without the endophyte near Bozeman, MT, USA. The dataset includes 33 E± pairs of A. mollissimus var. mollissimus, 13 pairs of var. thompsoniae, and 127 pairs of Oxytropis sericea plants grown in a common garden from 2011 to 2020 near Bozeman, MT, USA.
Bold values indicate p-value ≤ 0.05.
Table 2. Two-way interaction ANOVA estimating the effect of endophyte (+/−), Astragalus mollissimus var. mollissimus or Astragalus mollissimus var. thompsoniae), and their interaction on physiology, growth, and fecundity variables a .
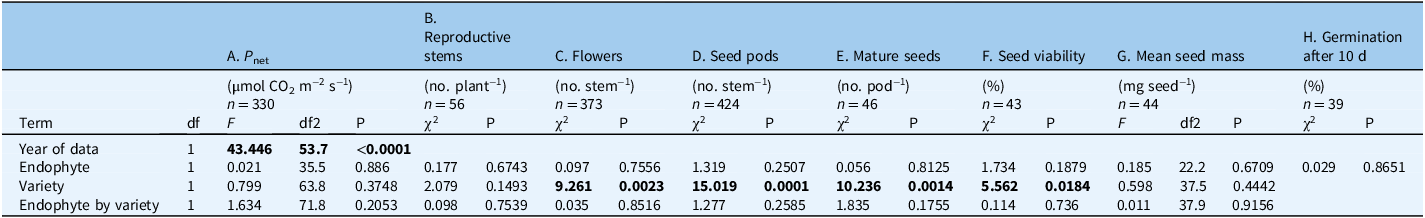
a These variables were measured for plants grown as pairs with or without the endophyte near Bozeman, MT, USA. Models A and G: P-values and F-statistics were obtained by running a linear mixed model; interactions tested included endophyte by generation. The numerator degrees of freedom are in the df column and the denominator degrees of freedom are in the df2 columns. Models B–F and H: P-values and χ2 values were obtained by running a generalized linear mixed model with a Poisson response distribution and log link for count responses, and a grouped binomial response distribution with a logit link for proportion out of total (%) responses. The dataset includes 33 E± pairs of A. mollissimus var. mollissimus and 13 pairs of A. mollissimus var. thompsoniae grown in a common garden from 2011 to 2020 near Bozeman, MT, USA.
Bold values indicate p-value ≤ 0.05.
Table 3. Two-way interaction ANOVA estimating the effect of endophyte (+/−), Oxytropis sericea generations, and their interaction on growth and fecundity variables a .
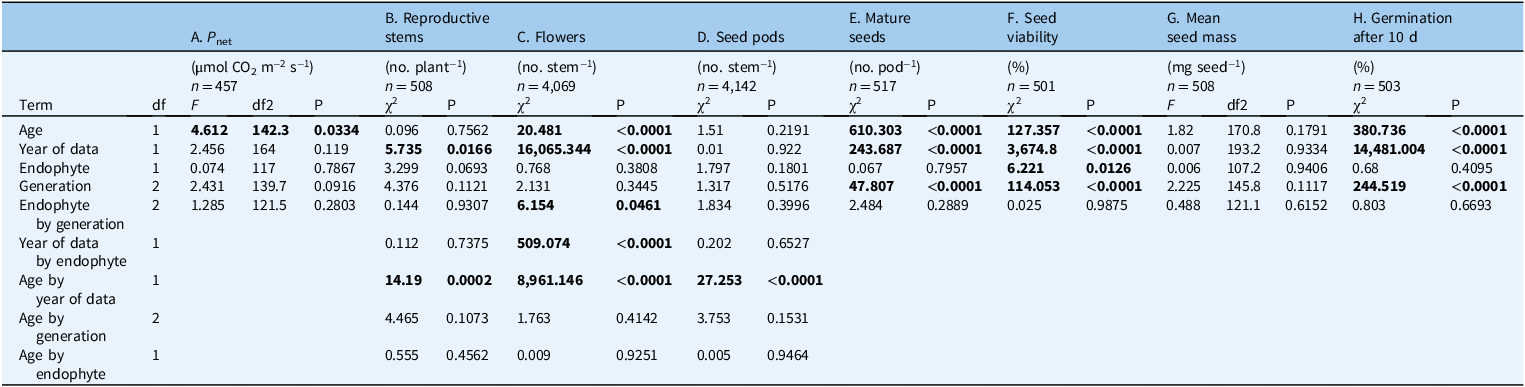
a These variables were measured for plants grown as pairs with or without the endophyte near Bozeman, MT, USA. Models A and G: P-values and F-statistics were obtained by running a linear mixed model; interactions tested included endophyte by generation. The numerator degrees of freedom are in the df column and the denominator degrees of freedom are in the df2 columns. Models B–F and H: P-values and χ2 values were obtained by running a generalized linear mixed model with a Poisson response distribution and log link for count responses and a grouped binomial response distribution with a logit link for proportion out of total (%) responses; interactions tested included endophyte by generation, year of data collection by endophyte, age by year of data collection, age by generation, and age by endophyte. The dataset includes 127 E± pairs of Oxytropis sericea plants grown in a common garden from 2011 to 2020 near Bozeman, MT, USA.
Bold values indicate p-value ≤ 0.05.
Table 4. Two-way interaction ANOVA estimating the effect of endophyte (+/−), Oxytropis sericea generations, and their interaction on final crown and root size count variables a .
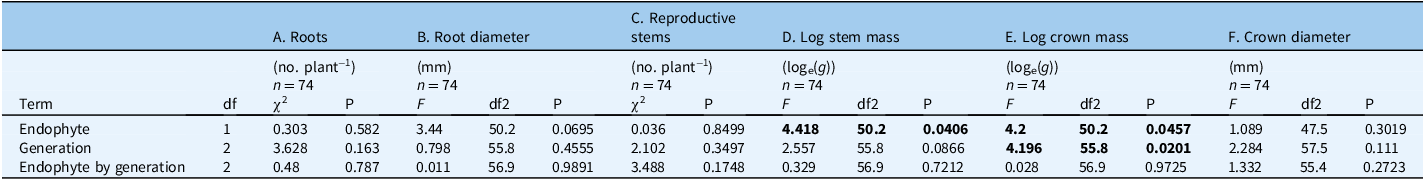
a These variables were measured for plants grown as pairs with or without the endophyte near Bozeman, MT, USA. Models B and D−F: P-values and F-values were obtained by running a linear mixed model; interactions tested included endophyte by generation. The numerator degrees of freedom are in the df column and the denominator degrees of freedom are in the df2 columns. Models A and C: P-values and χ2 values were obtained by running a generalized linear mixed model with a Poisson response distribution and log link for count responses; interactions tested included endophyte by generation. The dataset includes 127 E± pairs of Oxytropis sericea plants grown in a common garden from 2011 to 2020 near Bozeman, MT, USA.
Bold values indicate p-value ≤ 0.05.
Generalized linear mixed models (Tables 2–4) with a Poisson response distribution and log link were used for count responses (reproductive stems, seed pods, flowers, mature seeds, roots, maximum stems). A grouped binomial generalized linear mixed model with a logit link was used for proportion out of total responses (successes and failures of seed viability or germination). For continuous measurements, photosynthesis, mean seed mass, root diameter, log stem mass, log crown mass, and crown diameter (Table 2 for A. mollissimus; Tables 3 and 4 for O. sericea), we used a linear mixed effects model with the same fixed and random effects structure. For overwinter mortality analysis (Table 1; Supplementary Table S2), a Gompertz discrete proportional hazards model was used to compare the probability of overwinter mortality for the species-appropriate interaction term, specified below, after adjusting for the fixed effects of year planted and age of the plant and accounting for the random effects structure. The main effects for the interaction terms were considered in an additive model if there was no interaction with endophyte.
We tested for a two-way interaction between the two varieties of the A. mollissimus and endophyte status (Table 2; Supplementary Table S3). If there was no endophyte by variety interaction, main effects of endophyte and varieties were evaluated after accounting for the effects of the other fixed effects in the model (Supplementary Tables S2 and S4). For most models, there were no other fixed effects included due to varieties aliasing (being indistinguishable) with the other variables; however, models for overwinter mortality (age, year planted) and gas exchange responses of photosynthesis, conductance, and transpiration (year of collection) included additional fixed effects. The random effects structure did not change for these models.
For O. sericea, we tested for a two-way interaction between generation and endophyte (Tables 2–4; Supplementary Table S5). If there was no endophyte by generation interaction, main effects of endophyte were evaluated after accounting for the main effects of generation and adjusting for endophyte as well as the random effects from the nested and repeated-measurements design (Supplementary Tables S6–S8). Fixed effects for models not involving final harvest outcomes included the year planted (overwinter mortality) or year of data collection (all other models) and age of plant (all models). There were no other fixed effects for models involving final harvest outcomes besides endophyte, generation, and their interaction; these were included due to the aliasing of generation with the other variables. The same random effects structure for blocks and pairs in blocks was used for all models.
Results and Discussion
Astragalus mollissimus var. mollissimus and Astragalus mollissimus var. thompsoniae
Survival
Regardless of endophyte status, the majority of Astragalus plants grew as annuals in our common garden ca. 1,500 km north of their native range. Less than 3% of 194 A. mollissimus var. mollissimus transplants (6 = 5 E−, 1 E+) and <1% of 148 A. mollissimus var. thompsoniae transplants (1 E+) survived two winters in Montana; therefore, the Astragalus analysis focused on 1-yr-old plants regardless of response variable. Winter survival was not affected by endophyte for either variety but was greater for A. mollissimus var. mollissimus than A. mollissimus var. thompsoniae, with 44% of A. mollissimus var. mollissimus and 17% of A. mollissimus var. thompsoniae plants surviving at least one winter (χ2 = 2.838, df = 1, P = 0.092; Figure 1A and 1C; Table 1A; Supplementary Table S2A; Supplementary Figure S1A).
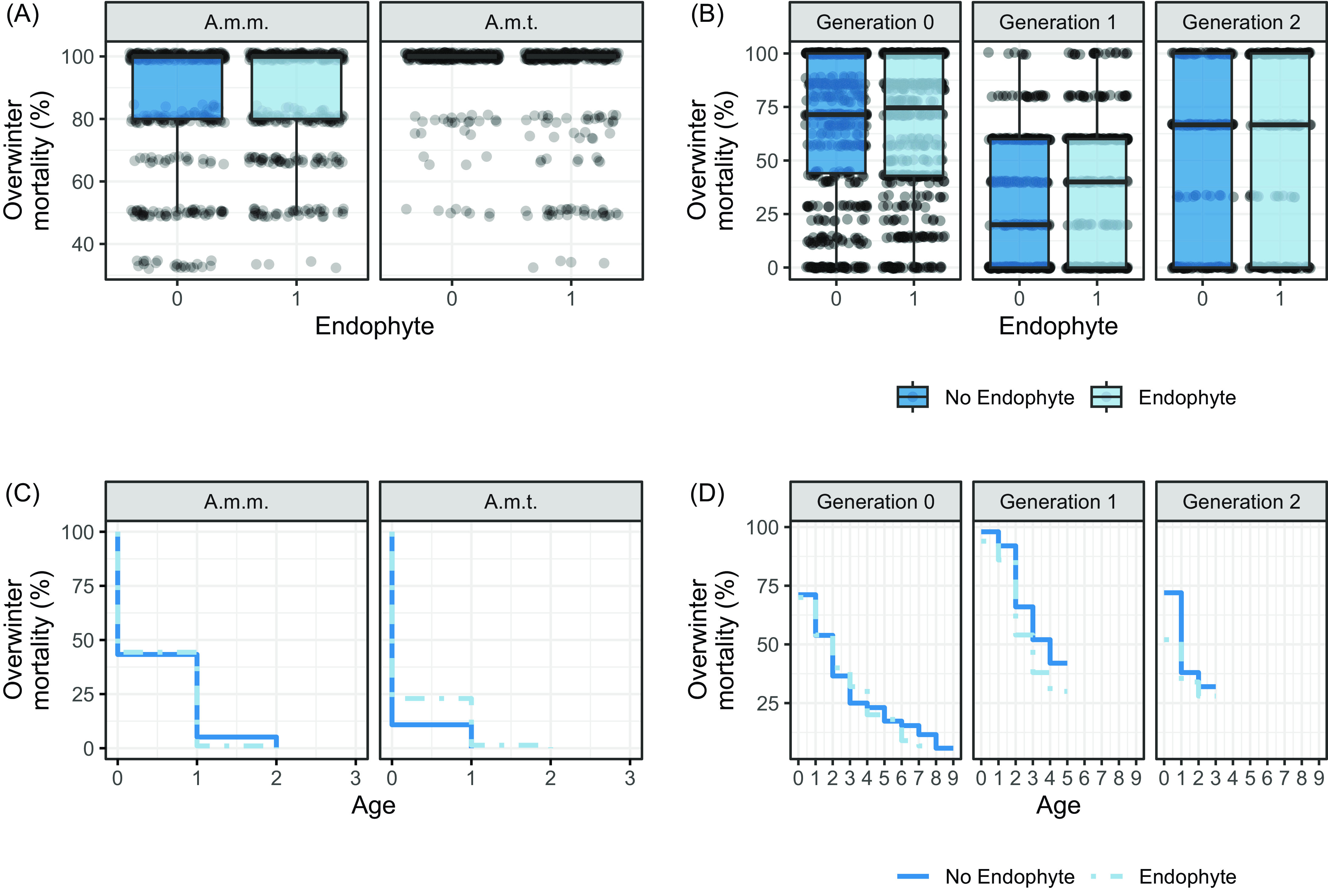
Figure 1. Box plots and Kaplan-Meier curves of overwinter mortality of Astragalus mollissimus var. mollissimus, Astragalus mollissimus var. thompsoniae, and Oxytropis sericea generation 0, 1, and 2 plants in response to the locoweed endophyte Alternaria sect. Undifilum (E−, dark blue; E+, light blue) when grown as pairs in a common garden from 2011 to 2020 near Bozeman, MT, USA. The dataset includes 33 pairs of A. mollissimus var. mollissimus, 13 pairs of A. mollissimus var. thompsoniae, and 127 pairs of Oxytropis sericea plants. The plots are (A) average overwinter mortality (%) by endophyte status and Astragalus mollissimus variety; (B) average overwinter mortality (%) by endophyte status and Oxytropis sericea generation; (C) Kaplan-Meier curve for overwinter mortality (%) by age of plant, endophyte status, and Astragalus mollissimus variety; (D) Kaplan-Meier curve for overwinter mortality (%) by age of plant, endophyte status, and Oxytropis sericea generation 0, 1, and 2 plants. A.m.m., A. mollissimus var. mollissimus; A.m.t., A. mollissimus var. thompsoniae.
Physiology
We found no variety by endophyte interactions for photosynthesis (F(1, 71.8) = 1.634, P = 0.205; Figure 2A; Table 2A; Supplementary Figure S4A), stomatal conductance (F(1, 69.8) = 1.161, P = 0.285; Supplementary Figures S2 and S3; Supplementary Table S3A), or transpiration (F(1, 76.5) = 2.626, P = 0.109; Supplementary Figures S2 and S3; Supplementary Table S3B).
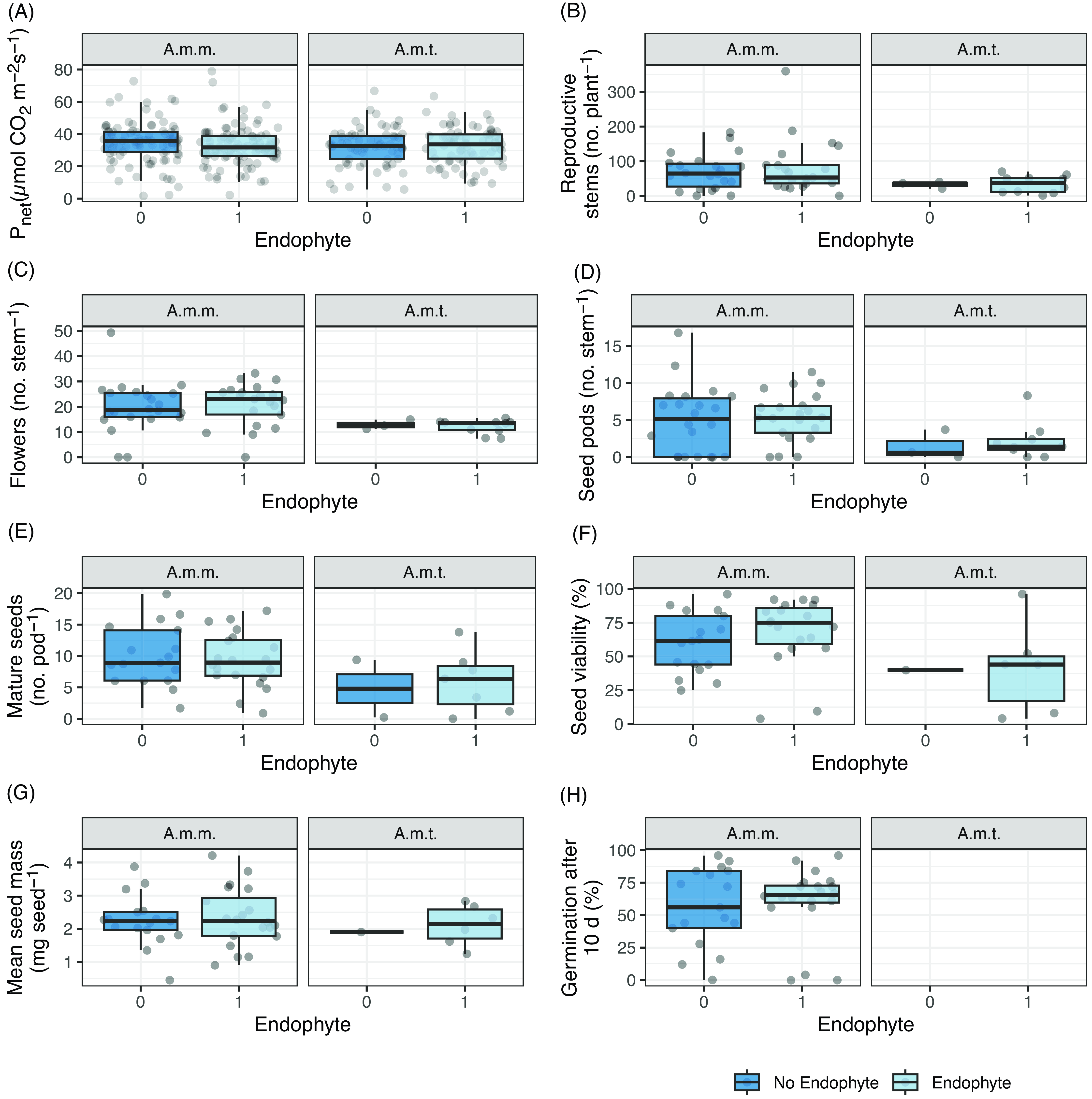
Figure 2. Box plots of physiology, growth, and fecundity of Astragalus mollissimus var. mollissimus and Astragalus mollissimus var. thompsoniae plants in response to the locoweed endophyte Alternaria sect. Undifilum (E−, dark blue; E+, light blue) when grown as pairs in a common garden from 2011 to 2020 near Bozeman, MT, USA. The dataset includes 33 pairs of A. mollissimus var. mollissimus and 13 pairs of A. mollissimus var. thompsoniae. Variables collected are (A) P net, (B) reproductive stems per plant, (C) flowers per stem, (D) seed pods per stem, (E) mature seeds per pod, (F) percent seed viability, (G) mean seed mass, and (H) percent germination after 10 d (note that germination was not tested for A. mollissimus var. thompsoniae). Box edges represent the 0.25 and 0.75 quartiles; solid line represents the median value; whiskers extend to the minimum and maximum value or 1.5× the interquartile range; outliers are not identified. Points are the original data values with horizontal noise added to aid in visibility. A.m.m., A. mollissimus var. mollissimus; A.m.t., A. mollissimus var. thompsoniae.
Reproductive Traits
Reproductive traits of A. mollissimus varieties were unaffected by endophyte. For example, there was no variety by endophyte interaction detected for reproductive stems per plant (χ2 = 0.1, df = 1, P = 0.7539; Figure 2B; Table 2B) or main effect for endophyte (χ2 = 0.181, df = 1, P = 0.670; Supplementary Table S4B). Similarly, no variety by endophyte interactions were detected for fecundity response variables, although A. mollissimus var. mollissimus produced more flowers, seed pods, and mature seeds than A. mollissimus var. thompsoniae (Figure 2C–E; Supplementary Tables S2C–E and S4C–E), and its seeds were on average more viable (68%) compared with A. mollissimus var. thompsoniae seeds (41%) (Figure 2F; Table 2F; Supplementary Table S4F). Mean seed mass did not differ by endophyte or A. mollissimus variety (Figure 2G; Table 2G; Supplementary Table S4G). For germination, A. mollissimus var. thompsoniae did not produce enough seeds to test; however, ca. 60% of A. mollissimus var. mollissimus seeds germinated within 10 d regardless of endophyte (χ2 = 0.029, df = 1, P = 0.865; Figure 2H; Table 2H). Similarly, there was no effect of endophyte on the rate of germination (data not shown).
Oxytropis sericea
Survival
Oxytropis sericea plants grew as perennials in our southwest Montana common garden, reflecting the more extensive natural range of this species; one individual (E−) survived nine winters (data not shown). Endophyte status had no effect on survival. A total of 55 (E+ and E− plants combined) out of 100 Gen 0 transplants survived two winters, and 39% of all Gen 0 plants survived three winters. Again, regardless of endophyte, 89% and 60% of the 100 Gen 1 transplants survived two and three winters, respectively, and 36% and 30% of the 100 Gen 2 transplants survived two and three winters, respectively. For those that survived more than one winter, there was no generation by endophyte interaction for winter mortality by year of collection (χ2 = 0.874, df = 1, P = 0.646; Figure 1B and 1D; Table 1B; Supplementary Table S2B; Supplementary Figure S1B). The model estimated similar survival regardless of endophyte for each generation, with estimated proportion surviving 3 yr for Gen 0, 1, and 2 being on average 89% versus 88%, 93% versus 69%, and 3% versus 2%, respectively, for E+ versus E−.
Physiology
Overall, there were no generation by endophyte interactions for photosynthesis (F(2, 121.5) = 1.285, P = 0.280; Figure 3A; Table 3A), stomatal conductance (Supplementary Figures S5A and S6A; Supplementary Table S6A), or transpiration (Supplementary Figures S5B and S6B; Supplementary Table S6B). The physiological parameters of Gen 1 and 2 offspring were similar to those of the Gen 0 parent generation, regardless of endophyte when accounting for the year of data collection and the age of the plant (Supplementary Figures S5–S7; Supplementary Tables S5–S7). Also, gas exchange rates in O. sericea were similar relative to year sampled (Figure 3; Table 3; Supplementary Table S7) and age of plant (Supplementary Figures S5–S9).
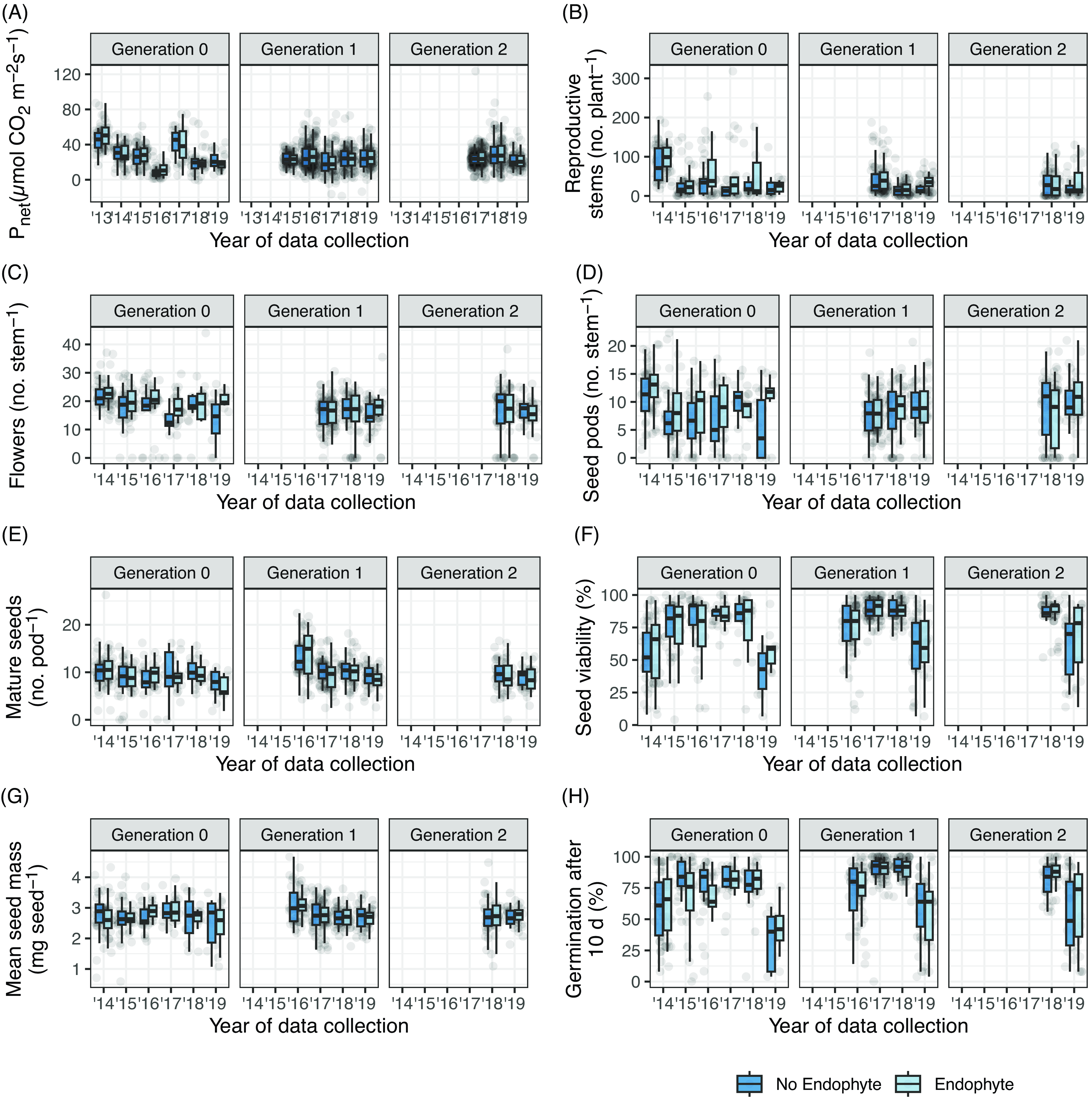
Figure 3. Box plots of physiology, growth, and fecundity of Oxytropis sericea generation 0, 1, and 2 plants in response to the locoweed endophyte Alternaria sect. Undifilum (E−, dark blue; E+, light blue) when grown as pairs in a common garden from 2011 to 2020 (year of data collection) near Bozeman, MT, USA. The dataset includes 127 pairs of plants. Variables collected are (A) P net, (B) reproductive stems per plant, (C) flowers per stem, (D) seed pods per stem, (E) mature seeds per pod, (F) percent seed viability, (G) mean seed mass, and (H) percent germination after 10 d. Box edges represent the 0.25 and 0.75 quartiles; solid line represents the median value; whiskers extend to the minimum and maximum value or 1.5× the interquartile range; outliers are not identified. Points are the original data values with horizontal noise added to aid in visibility.
Reproductive Traits
Few reproductive trait responses in O. sericea plants were influenced by endophytic effects. The maximum number of reproductive stems per plant was highly variable, and while the generation by endophyte interaction was not significant (χ2 = 0.144, df = 2, P = 0.931; Figure 3B; Table 3B), the main effect for endophyte (χ2 = 3.327, df = 2, P = 0.068; Supplementary Table S7) suggests Gen 0 E+ plants compared with Gen 0 E− plants produced more reproductive stems in certain years (Figure 3B; Table 3B) and ages (Supplementary Figure S7D; Table 3B; Supplementary Table S7B) as reflected in interactions, possibly due to the maternal environment or ages of Gen 0 plants. Additionally, there was a generation by endophyte interaction for flowers per stem (χ2 = 6.154, df = 2, P = 0.046; Table 3C; Figure 3C); however, this interaction does not appear biologically relevant, as subsequent seed pods per stem had no evidence of a generation by endophyte interaction (χ2 = 1.834, df = 2, P = 0.400; Table 3D; Figure 3D).
Generation by endophyte interactions in O. sericea were not different for fecundity traits (Table 3): seed pods (Figure 3D), mature seeds per pod (Figure 3E), seed viability (Figure 3F), seed mass (Figure 3G), percentage of total seeds germinated after 10 d (Figure 3H), or rate of germination (data not shown). Seed viability had a significant main effect for endophyte (χ2 = 6.213, df = 1, P = 0.013; Supplementary Table S7F), although the mean seed viability values from the data are 76% and 75% for E+ and E−; we believe that a difference was detected because the model is able to identify small differences after accounting for the random effects structure and the other fixed effects in the model, including a significant difference among the three generations (χ2 = 113.02, df = 2, P < 0.0001; Supplementary Table S7F).
Final Biomass
At final harvest of surviving plants in 2020, there were no endophyte by generation interactions (Table 4), nor were there any endophyte or generationmain effects for final root count or diameter or stem count per plant (Figure 4A–C; Table 4A–C; Supplementary Table S8A–C; Supplementary Figure S10A-C). Plants with endophyte had slightly larger stem mass (F(1, 51.9) = 4.477, P = 0.039; Supplementary Table S8D; Supplementary Figure S10D; Figure 4D) and slightly greater crown mass (F(1, 51.9) = 4.297, P = 0.043; Supplementary Table S8E; Figure 4E) than E− stems and crowns. There was also a generation main effect for log crown mass (F(2, 57) = 4.32, P = 0.018; Supplementary Table S8E; Supplementary Figure S10E; Figure 4E), holding all other variables at their marginal means, with larger crowns in Gen 0 because they were older at the time of harvest in 2020. This result also suggests the maternal effects in Gen 0 were no longer detectable in Gen 1 and 2.
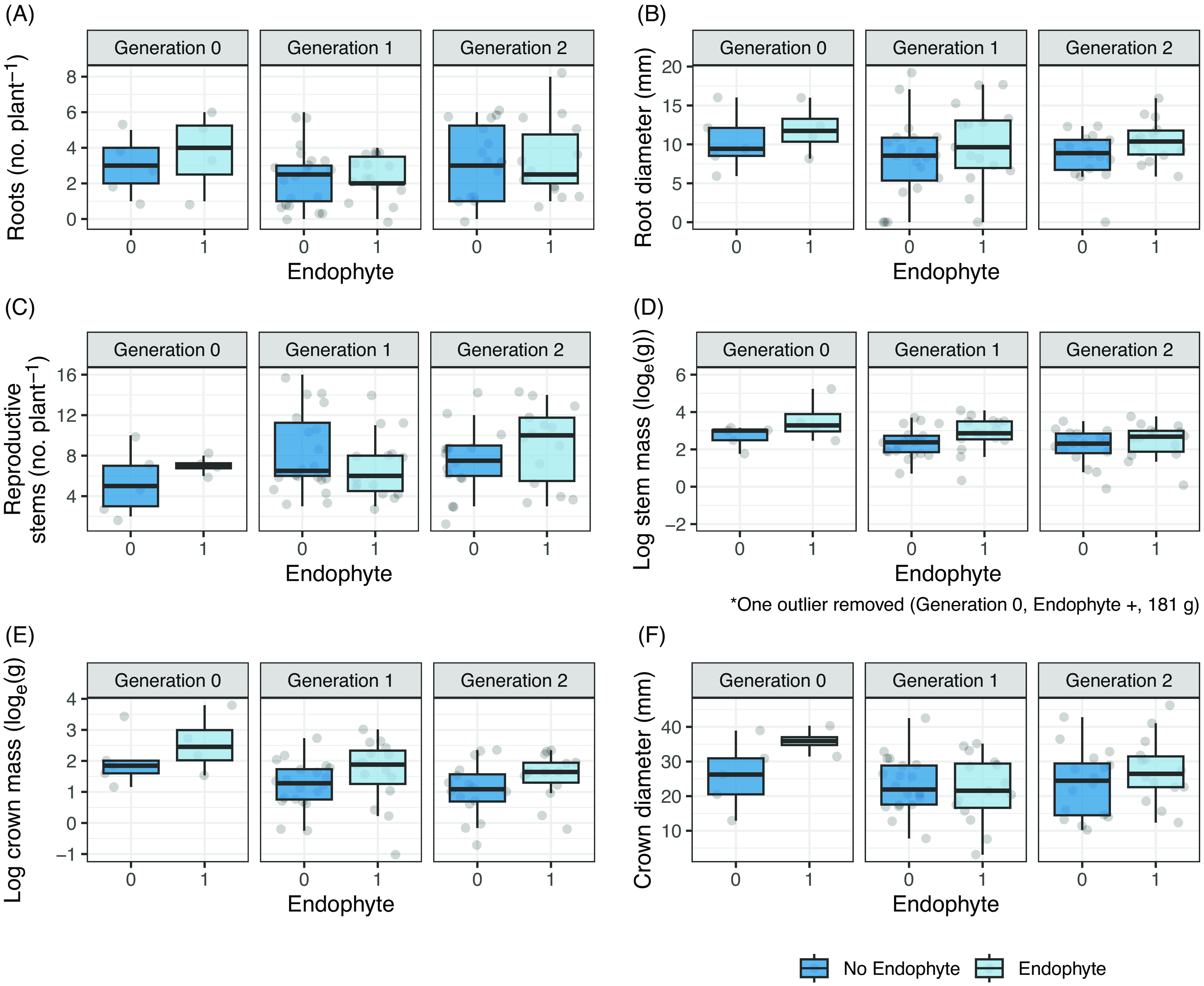
Figure 4. Box plots of final crown and root size parameters in 2020 of Oxytropis sericea generation 0, 1, and 2 plants in response to the locoweed endophyte Alternaria sect. Undifilum (E−, dark blue; E+, light blue) when grown as pairs in a common garden from 2011 to 2020 near Bozeman, MT, USA. The dataset includes 127 pairs of plants. Variables collected are (A) root number per plant, (B) root diameter, (C) reproductive stems per plant, (D) log (base e) stem mass, (E) log (base e) crown mass, and (F) crown diameter. Box edges represent the 0.25 and 0.75 quartiles; solid line represents the median value; whiskers extend to the minimum and maximum value or 1.5× the interquartile range; outliers are not identified. Points are the original data values with horizontal noise added to aid in visibility. One outlier was removed from D, as noted under the plot.
We hypothesized that under field conditions, the Alternaria endophyte would confer a growth and fecundity advantage for Astragalus and Oxytropis host plants compared with those with no endophyte. However, our results show very little evidence that the locoweed–endophyte symbiosis enhances functional traits that might increase fitness in the host plants. Endophyte presence did not alter survival, photosynthetic physiology, or fecundity in any of the locoweed taxa that we investigated, even when exposed to variable weather conditions when established and throughout their lifetime (Supplementary Table S1). We did detect some enhancement of crown and reproductive stem tissue in Oxytropis plants containing the endophyte, and the final biomass measurements for Oxytropis plants that had survived multiple years indicated that reproductive stem and log crown mass were larger in E+ plants in each generation (Figure 4; Table 4). Following overwintering, most of the regrowth of locoweed plants in spring occurs from the crown via basal buds using remobilized carbohydrates and nitrogen from the crown (Wyka and Galen Reference Wyka and Galen2000). This capacity for regrowth also contributes to Oxytropis surviving stressful environments, including drought (Ralphs et al. Reference Ralphs, Gardner, Graham, Greathouse and Knight2002; Vallotton et al. Reference Vallotton, Murray, Delaney and Sterling2012). Photosynthate reserves stored as greater crown and stem mass in E+ plants could improve Oxytropis overwintering and survival in successfully perennating older plants in some environments, although Oxytropis winter survival in our Montana common garden was not affected by endophyte status.
With short growing seasons at higher latitudes, reproductive success of locoweeds depends on rapid flowering and seed set (Ida et al. Reference Ida, Harder and Kudo2012; Soltani et al. Reference Soltani, Benakashani, Baskin and Baskin2021). Ida et al. (Reference Ida, Harder and Kudo2012) found that even under defoliation or shading, Oxytropis could maintain production of reproductive tissue and seeds due to its ability to mobilize carbohydrates from storage tissue. Although our study found no differences between E+ and E− plants in overall measures of fecundity, the increase in crown mass and reproductive stems we observed in E+ plants suggests that investigation of endophytic effects on photosynthate distribution or pollination ecology could be worthwhile. Another possibility is the potential for the endophyte to trigger transmissible epigenetic effects in an E+ host plant that persist through subsequent E− generations after the endophyte is removed (Asgari Reference Asgari2014; Yin et al. Reference Yin, Zhou, Lin, Li and Zhang2019); however, we found no endophyte phenotype when comparing three generations of E+ and E− plants. Additional research might usefully compare field-collected E− plants with those newly generated by artificial removal of the endophyte (Ueno et al. Reference Ueno, Gundel, Seal, Ghersa and Martínez-Ghersa2020, Reference Ueno, Gundel, Molina-Montenegro, Ramos, Ghersa and Martínez-Ghersa2021).
It has been suggested that the locoweed–endophyte symbiosis coevolved to help the host respond to stress through the dual role of lysine as a stress modulator in plants and as a precursor to the swainsonine biosynthesis pathway (Guan et al. Reference Guan, Liu, Mur, Fu, Wei, Wang and He2021). However, we found no swainsonine growth or fecundity trade-off for the taxa in this study, similar to results reported by Vallotton et al. (Reference Vallotton, Murray, Delaney and Sterling2012). As already noted, we included A. mollissimus var. thompsoniae in our study because the Alternaria endophyte in this locoweed variety does not produce detectable swainsonine, allowing us to separate effects on the host of endophyte presence from those of swainsonine production. However, although being far north of its native range reduced A. mollissimus var. thompsoniae fecundity and survival, this reduction was equally true for both E+ and E− A. mollissimus var. thompsoniae, and we detected no differences in growth parameters associated with presence or absence of the non–swainsonine producing endophyte. Taken together, these results do not suggest any cost or benefit of endophyte presence or swainsonine production to the host plant. We did not measure the success of the Alternaria endophyte as quantitative mycelial production, but we confirmed it was vertically transmitted by multiple E+ plants through three generations in O. sericea. This consistent presence of the endophyte is further evidence of its neutral effects on the host.
As noted in the “Introduction,” studies on the Poaceae have led to a general assumption of endophyte–host mutualism when an endophyte produces a toxin. If a similar trade-off occurs in Astragalus and Oxytropis species, the nitrogen cost of synthesizing swainsonine could be offset by swainsonine’s benefit to the plant as an anti-herbivory tactic. However, despite swainsonine’s severe effects on vertebrate herbivores, it does not appear to have a general defensive role. For example, swainsonine content does not protect A. mollissimus varieties from herbivory by the specialist weevil Cleonidis spp. (Thompson et al. Reference Thompson, Knight, Sterling and Murray1995). Livestock generally show no preference for plants that contain or do not contain swainsonine (Pfister et al. Reference Pfister, Cook, Lee, Gardner and Riet-Correa2020). Locoweeds green up early in the spring before much other forage is available, and persistent mixtures of E+ and E− plants within populations reduce the likelihood that grazing animals could learn to avoid these plants. Field studies have shown that livestock can habituate to eating these plants and may even train other animals to acquire a preference for them (Molyneux and Ralphs Reference Molyneux and Ralphs1992; Pfister et al. Reference Pfister, Stegelmeier, Gardner and James2003; Ralphs et al. Reference Ralphs, Panter and James1990).
Although our study was conducted across multiple years, it was limited to a single location. It is possible the endophyte provides other benefits to the host plant in different environments. However, it is unlikely that endophyte presence with or without swainsonine production will aid Astragalus in any migration north of its native range, consistent with the lack of such migration to date (Soltani et al. Reference Soltani, Benakashani, Baskin and Baskin2021) and the annual nature of the Astragalus plants in our Montana common garden. The general lack of locoweed response to the endophyte in our study challenges the view that an endophyte increases the adaptative capacity of its host to improve migration success: our results indicate there is no selective advantage associated with endophyte symbiosis when these locoweeds adapt to new environments. We also note that any fitness advantage should increase the frequency in field populations of plants containing the endophyte, but this does not appear to be the case: plants lacking the endophyte can comprise up to 10% to 60% of individuals within Oxytropis populations (Cook et al. Reference Cook, Shi, Gardner, Pfister, Grum, Welch and Ralphs2012).
Our long-term field study demonstrates the importance of investigating multiple fitness traits at the individual level when assessing the importance of endophytes to host plants. Based on these results, we conclude that the locoweed–endophyte complex is best defined as a “no-effects” commensalism, with growth and reproductive capacity of the three host taxa investigated being unaffected by the presence or absence of Alternaria. Given the resulting lack of selection, it is likely that mixtures of E+ and E− plants will persist in Astragalus and Oxytropis populations, and swainsonine poisoning will continue to threaten grazing livestock that cannot distinguish between plants with and without the toxin.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/wsc.2025.10026
Data availability statement
Data necessary to reproduce the analyses are available in the Dryad repository: https://doi.org/10.5061/dryad.ghx3ffbzf.
Acknowledgments
The authors thank Stephen Lee and Dale Gardner at the USDA-ARS Poisonous Plant Lab, Logan, UT, for swainsonine analyses; R. K. D. Peterson at Montana State University for Licor 6400 training; Rosie Wallander for technical assistance; and the following undergraduate students for their assistance in data collection: Talinna Appling, Frida Isaksen-Swensen, Raeleigh Price, Frank T. Dougher, Stacey Robbins, and Kaylee Schmidt.
Funding statement
This research was supported by the NIFA/USDA Hatch/Multi-state project W-1193/2193/3193 “Locoweed and Its Fungal Endophyte: Impact, Ecology and Management” under 1009744, the Montana Agricultural Experiment Station, and the MSU Vice President for Research and Economic Development.
Competing interests
The authors declare no conflicts of interest.










