Introduction
Organic and inorganic amendments are commonly used in agricultural systems to supply crops with essential nutrients and enhance soil fertility (Gullino et al. Reference Gullino, Garibaldi, Gamliel and Katan2022). Compost is a frequently used soil amendment to increase organic matter content and fertility and improve physical, chemical, and biological soil conditions (Hoitink and Fahy Reference Hoitink and Fahy1986). The speed of the composting process depends on the composition of the compost, the C/N ratio, the moisture content, the temperature, and the microbial activity in the material to achieve a uniform product (Kuhlman Reference Kuhlman1990). The temperatures recommended for the composting process depend on the need to eliminate pests and, at the same time, avoid undesirable detrimental effects on essential microorganisms (Grundy et al. Reference Grundy, Green and Lennartsson1998).
Weed seeds are a potential contaminant of composts derived from plant waste material. Weed seeds may enter the compost via the base material or external mechanisms. For example, seeds of species belonging to Asteraceae (e.g., common groundsel (Senecio vulgaris L.); spiny sowthistle [Sonchus asper (L.) Hill]) are spread by the wind and can easily contaminate nearby masses. Composting may destroy some weed seeds, while others may survive in the final product (Grundy et al. Reference Grundy, Green and Lennartsson1998). The seeds’ ability to survive the composting process has shown to be species specific, and for some species, sublethal temperatures promote germination (Grundy et al. Reference Grundy, Green and Lennartsson1998). Inadequate temperature development during composting may result in viable weed seeds in the final compost and constitute a risk of spreading invasive and noxious weeds to new areas (Bernal et al. Reference Bernal, Sommer, Chadwick, Qing, Guoxue, Michel and Sparks2017). Significant spatial differences in temperature can occur within a compost, which may influence the processes that affect weed seeds (Salisbury Reference Salisbury1961).
Barnyardgrass [Echinochloa crus-galli (L.) P. Beauv.] is one of the most troublesome grass weeds in the warmer areas of southeast Norway. The species has been a weed problem since the 1970s, typically in open-row crops like carrots (Daucus carota L.), onions (Allium spp.), and potatoes (Solanum tuberosum L.). It is now also a weed in spring cereals. Relocation of soil, compost soil, contaminated crop seeds, untreated manure, slaughterhouse waste used as fertilizer, and seeds on machinery and from feeding places for wild birds are reported as possible pathways for its spread (VKM 2016). The effects of steaming and composting on E. crus-galli seeds in biowaste have to our knowledge never been investigated.
In this project, we studied the possibility of using steam to destroy E. crus-galli seeds in the biowaste mixture (Study 1) and before composting (Study 2) to (1) avoid seed spread via biowastes when it is transported to other locations for composting; and (2) increase the probability of destroying the seeds, for example, if the composting process does not reach the necessary temperature to kill the seeds. For the steam treatment, we used a prototype steaming device resembling commercial products (Bitarafan et al. Reference Bitarafan, Kaczmarek-Derda, Brandsæter and Fløistad2021, Reference Bitarafan, Kaczmarek-Derda, Berge, Tørresen and Fløistad2022). The experiments are crucial for assessing the viability of scaling up the prototype to a potentially valuable tool for treating plant waste during relocation and reuse processes. We hypothesized that steaming the biowaste with increasing temperatures from 60 to 100 C would significantly increase seed death. If some seeds survived, we hypothesized that all seeds would die after 1 yr of composting. The study does not include the calculation of energy consumption and costs, because we used a brand-new prototype of a steaming device that is not yet available commercially.
Materials and Methods
Two studies were conducted in 2022 and 2023 in Ås, Norway. We assessed the effect of steam treatments alone (Study 1) and in combination with composting (Study 2) on the germination of E. crus-galli seeds in a biowaste mixture, which consisted of onion waste (60%), horse (Equus spp.) manure (20%), and wood shavings (20%). Wood shavings were between 2 and 25 mm in length and about 0.5 mm in thickness (Figure 1). In both studies, six populations of E. crus-galli were included.

Figure 1. Box with compost consisting of onion waste (60%), horse manure (20%), and wood shavings (20%) and thermocouples mounted at different places in the box.
Study 1. Steaming of Biowaste Mixture
In the first study, a seed-containing biowaste mixture was steamed to various target temperatures to estimate the temperature that killed the seeds. The target temperatures of 60, 70, 80, 90, and 100 C were followed by a 3-min residence time. Afterward, seeds were collected and tested for germinability. For the target temperature of 60 C, the experiment was done with two residence times: 3 min and 24 h. The 24-h residence time should imitate a natural cooling-down period. Seeds were exposed to heat during the steaming process, in which the duration varied for each target temperature and in the following residence time. Hence, the total thermal treatment duration always exceeded 3 min. A temperature of 25 C was used for untreated control samples. The experiment was conducted on September 1, 2022, and repeated on September 2, 2022. Four replications were used for each of the seven treatments (including the untreated control).
Study 2. Steaming to a Target Temperature of 60 C and Subsequent Composting of the Biowaste Mixture
In the second study, the following six treatments were included: (1) control (no treatment); (2) compost-treated seeds moved from the compost chambers at the end of the thermophilic stage (no steam, short-term composting for 14 d with temperature peaks above 60 C in 10 d and above 50 C in 4 d); (3) compost-treated seeds moved from the compost chambers after composting was stopped, that is, at the end of maturation stage (no steam, long-term composting for a total of 6 mo, including 3 mo with temperature above 12 C); (4) seeds steamed without being composted (steam, no composting); 5) steamed and compost-treated seeds moved from the compost chambers at the end of the thermophilic stage (steam followed by short-term composting); and (6) steamed and compost-treated seeds moved from the compost chambers when the composting was stopped (steam followed by long-term composting). For Treatments 4, 5, and 6, the target temperature in the steamed biowaste mixture with the seeds was 60 C (followed by a residence time of 3 min). Seed germination was assessed after the treatments. The experiment was conducted on November 15, 2022, and repeated on October 19, 2023. Four replications (seed bags) were used for the six treatments (including the untreated control). A completely randomized design was used.
Seed Samples (Studies 1 and 2)
Seeds from six E. crus-galli populations were included: one population collected in September 2021 from the Ås region in Akershus County, Norway (Akershus); four populations from the east and west sides of the Oslo fjord, Norway (East1, East2, West1, and West2) collected in September and October 2019; and one population sampled in August to September 2019 in the Radocza region, Poland. Seeds were collected from many plants and stored in paper bags under dry conditions at room temperature. Four replications, each of 50 seeds of each population, were placed into polypropylene fleece bags (9 cm by 7 cm) for all treatments.
Steaming Treatments
The steaming treatment was done using a prototype device (Soil Steam International AS, Sandefjord, Norway) with the following specifications: 190 cm by 144 cm by 88 cm; 130-kg weight; an effective steaming area of 120 cm by 80 cm. The steam generator has a production capacity of 250 kg h−1 with an effective calorific rating of 167,000 kcal h−1 and a working pressure of 50 kPa. The fuel consumption (diesel) was 19.2 L h−1. When the steam enters the non–airproof chamber, it is distributed over the biowaste mixture. If the temperature reaches 98 to 100 C in the whole mixture, the steam consumption is reduced significantly, causing an imbalance between steam supply and demand. In such a case, excessive steam exits from the top of the chamber, causing no significant pressure change.
Dry seeds were put into seed bags. All seed bags (one bag per E. crus-galli population) in the same replication of a target temperature were placed at the bottom of a plastic perforated basket (60 cm by 40 cm by 20 cm) and covered with a 7-cm layer of biowaste mixture. Excluding the controls, a total of 24 baskets in the first study (6 combinations of target temperatures by 4 replications) and 4 baskets in the second study (1 target temperature by 4 replications) were steamed. Each basket was placed in the steaming chamber repeatedly, and 15 thermocouples were placed in different places in the mixture. When the container lid was closed, steam with a constant temperature of approximately 150 C was released from the top and vacuumed from the bottom of the steaming chamber. The temperatures in the mixture were recorded every second using PT1000 sensors connected to a cRIO-9073 data logger (National Instruments, Austin, TX, USA). A custom-made program was used to record the temperatures, the duration of the steaming, and the duration of the post-steaming residence time. Steaming and vacuuming were stopped when the average temperature was about 5 to 10 C below the target temperature in the biowaste mixture, because the average temperature usually continued to increase for some seconds after the steam was stopped. The basket was removed from the steaming chamber when the post-steaming period of 3 min was completed, and the seed bags were immediately removed from the basket and placed at ambient air temperature. For the target temperature of 60 C followed by a resting period of approximately 24 h in the first study, the basket was removed from the steaming chamber after the completion of the post-steaming residence time of 3 min and placed in a closed polystyrene box (59.5 cm by 38.8 cm by 26.2 cm) for about 24 h. Four baskets (one target temperature by four replications), smaller than the baskets in the other treatments, were used. A separate polystyrene box was used for each basket. The mixture temperature was recorded by two thermocouples in each replication of the 24-h treatment.
The mean temperature curve was calculated for each target temperature and replication based on the temperatures recorded by the individual thermocouples. Due to malfunctioning of some thermocouples, the number of individual sensors used to calculate the mean temperature curve varied. Eleven (Experiment 1) and seven to nine (Experiment 2) thermocouples were used In Study 1. Fifteen (Experiment 1) and 12 (Experiment 2) thermocouples were used in Study 2. An example of one replication with a target temperature of 60 C is shown in Figure 2. The maximum values of each of the mean temperature curves during the thermal treatment period (steaming process plus the residence time) were extracted and used in the statistical analysis. It has previously been shown that during short periods of steam exposure, the maximum temperature is a valid explanatory variable for weed seed germination (Melander and Jørgensen Reference Melander and Jørgensen2005). For the approximately 24-h treatment, the mean temperature was calculated from the temperatures recorded by two thermocouples per replication. Generally, it was not easy to reach the exact target temperatures. However, the actual temperatures (54.8 to 99.6 C) were closely aligned with the target range (60 to 99 C). The post-steaming period of 3 min (180 s) was achieved in all replications except in one case (210 s). For the 24-h treatment, the actual lengths of the prolonged residence time were in the range of 23.4 to 24.3 h. However, the time differences did not affect the results significantly. In the second study with a target steam temperature of 60 C, the actual temperatures achieved were 58.2 to 63.2 C (Experiment 1) and 59.2−72.9 C (Experiment 2).
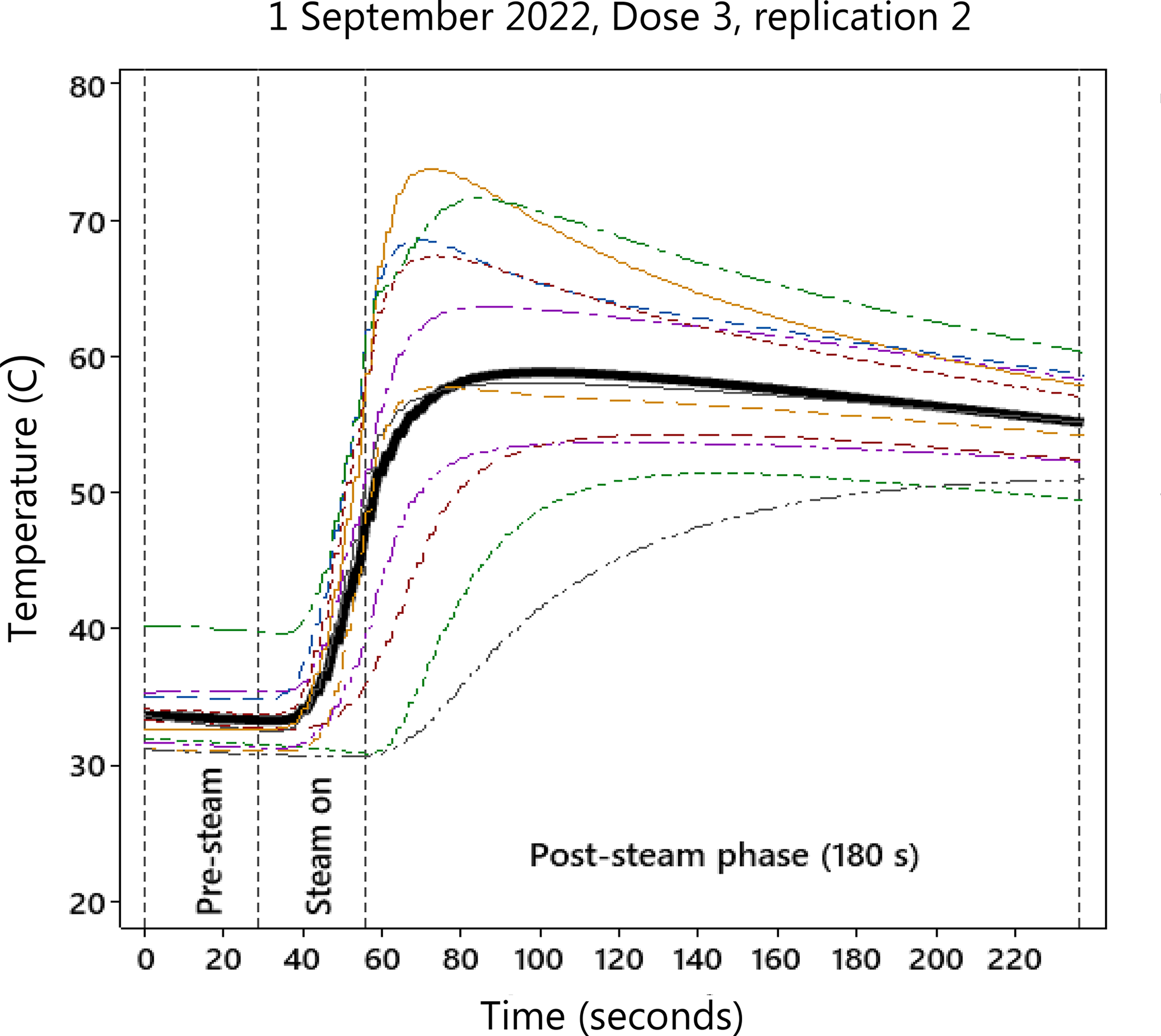
Figure 2. Example from Study 1 of temperature curves measured in the biowaste mixture in one basket (replication) during steaming at a target temperature of 60 C. The mean mixture temperature (black line) was calculated from temperatures recorded by 11 thermocouples (colored lines). The maximum mean temperature was extracted and used to estimate the parameters of the dose–response curves (Figure 4). After the steaming period (3 min), the basket with the seed samples was removed from the steaming container.
Composting Treatments
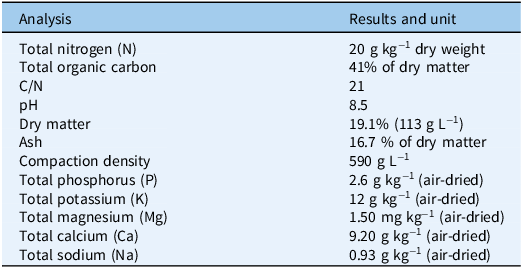
The composting (Weldon et al. Reference Weldon, Rivier, Joner, Coutris and Budai2023) was conducted in rotating composting units (tumblers), each consisting of two separate 135-L chambers with insulated walls (Joraform 270, Jora Composters, Palm Desert, CA, USA). The chamber side walls had two sections of 10-cm2 aeration holes. Four chambers from two tumblers were used for each of the four replications of treatments. A mixture of 80 to 135 L was placed in each experimental unit (compost chamber). All seed bags from the same replication of the treatments were put in a larger bag and then in the chamber with the composting substrate. The onion waste degradation released water at the start of the composting process, resulting in a moisture content too high to keep an aerobic process. Therefore, 10 L of dry wood shavings were added to each chamber on days 5 and 8 to keep the moisture content between 45% and 55%. After 14 d, all seed bags of the same replication of the treatments were removed at the thermophilic stage. The composts, with the rest of the seed bags, were left to mature for 6 mo in the tumblers, being turned once every second week during the whole period for mixing and aeration. The seed bags were removed at the end of the maturation phase. One representative compost sample from each of the two experiments was analyzed for physical and chemical parameters by the commercial analytical laboratory (Eurofins Agro Testing Norway AS, Moss, Norway) (Table 1). Each compost sample was a mixture of four subsamples, each from one replication.
Table 1. An example from Study 2 of a sample description of the final composted mixture (Experiment 1) analyzed by Eurofins Agro Testing Norway AS, Moss, Norway.
Temperatures inside the composting chambers were recorded every 15 mins using Decagon’s ECH2O data loggers (Meter, Münich, Germany). During the 14 d, the temperature in all chambers exceeded 60 C, except in one replication in the first experiment that reached 55 C. The temperature range within all replications in this stage ranged from 12.3 to 64.5 C and 5.9 to 66 C in the first and second experiments, respectively (Figure 3). During the 6 mo of composting, the temperature only exceeded 12 C in three of the months.
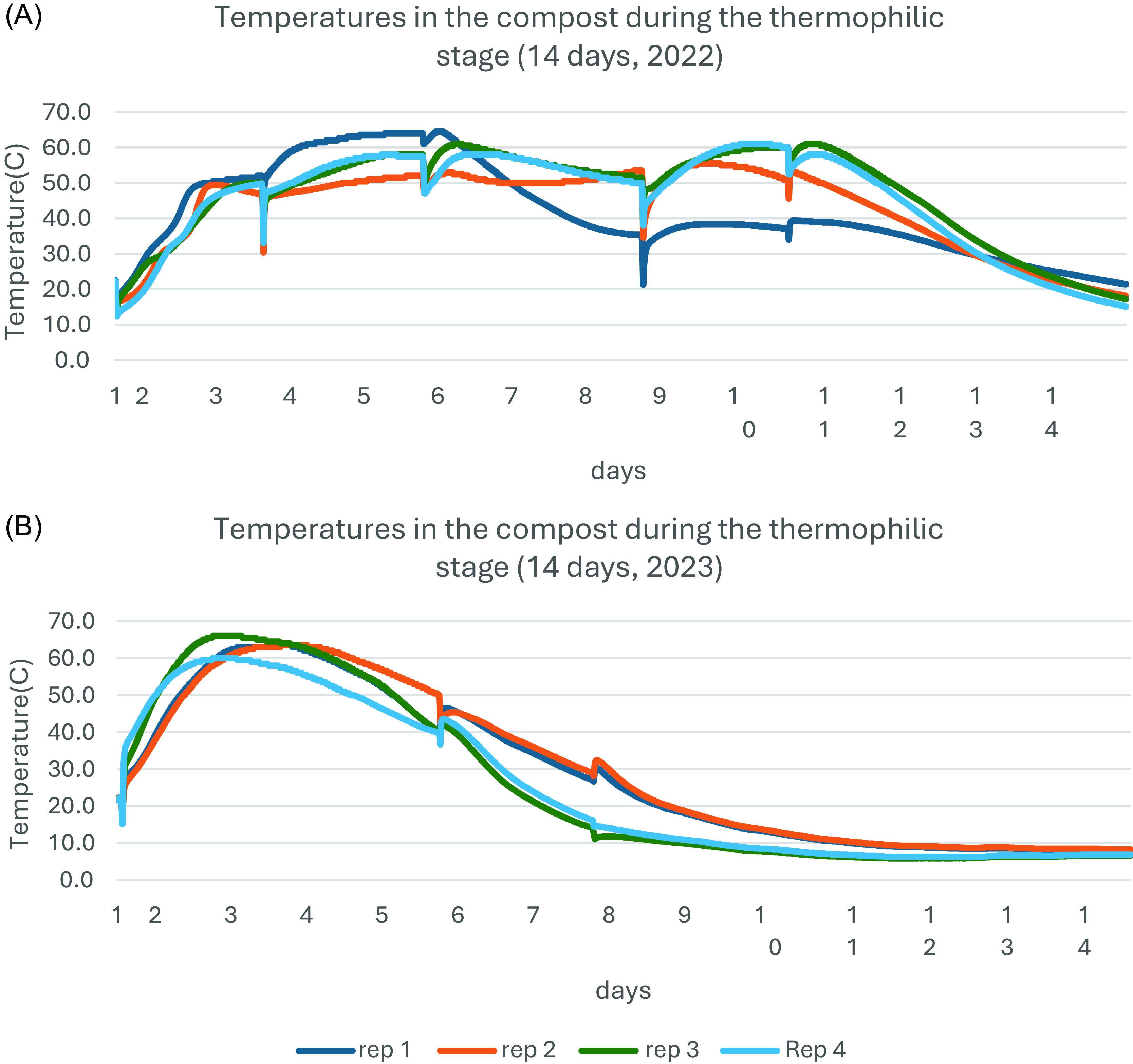
Figure 3. The recorded temperature inside the composting chambers during the 14 d of the thermophilic stage in the first (A) and second (B) experiments in Study 2, respectively. Each color represents measurements from a thermocouple. The temperature was recorded every 15 min during the 2 wk of the thermophilic stage. The temperature in all chambers went above 60 C, except in one replication in the first experiment that reached 55 C.
Germination Tests after Steaming and Composting
Echinochloa crus-galli seed bags were moved to a greenhouse right after the treatments. Each bag was opened carefully, placed on the soil surface in pots (top diameter = 12 cm), and covered by a thin layer of soil. Commercial potting soil was used (Tjerbo torvfabrikk AS, Rakkestad, Norway). It was composed of 80% sphagnum peat, 10% composted bark, and 10% sand (v/v/v), limed, and fertilized with NPK (950:40:220 mg L−1). Soil pH was 5.5 to 6.5. Pots were placed randomly in a greenhouse (day/night: 21/16 C; 14/10 h; relative humidity: 68%) and watered from the bottom with tap water during the experimental period to ensure water was not a limiting factor for seed germination, which was recorded. Germination tests of untreated samples were done the same way. We did not test whether streaming and composting may have induced seed dormancy in some seeds in some of the treatments.
Statistical Analyses
Study 1
Quasi-binomial generalized linear models were fit to the germination proportion data for target temperatures of 60 to 99 C, followed by a 3-min residence time, including different linear predictors for each population and experiment combination across temperatures. F-tests were used to assess the significance of the effects. The effective doses to reduce the seed germination 50% (ED50) and 90% (ED90) were calculated for each population by pooling the data from the two experiments, because the treatment by experiment interaction was not significant at a 5% level. Due to the low germination in control samples of East2 and West1, a model could not be fit. The Monte Carlo method, alongside the High Density Interval-type intervals (Mainguy et al. Reference Mainguy, Bélanger, Ouellet-Cauchon and de Andrade Moral2024), was used to construct the 95% confidence intervals for the ED50 and ED90.
Study 2
A quasi-binomial generalized linear model was fit to the germination proportion data, initially including the main effects and the two-way and three-way interactions between experiment, population, and treatments. F-tests were used to assess the significance of the impact. Because there were only marginally significant interactions with experiment at a 5% level, the model was refit using experiment only as an additive term.
In both cases, quasi-binomial models were used to accommodate overdispersion (Demétrio et al. Reference Demétrio, Hinde, Moral, Ferreira and Godoy2014). Model goodness of fit was assessed using half-normal plots with a simulated envelope (Moral et al., Reference Moral, Hinde and Demétrio2017). All analyses were carried out in R (R Core Team 2024).
Results and Discussion
Study 1
The temperature during the steaming process (Study 1) varied significantly from one place to another in the relatively small steaming chamber because of the heterogeneous substrate and compaction (cf. Figure 2). Therefore, we also expected a significant variation in the germination response to the same steaming temperatures, because some parts of the biowaste mixture may have been heated better and faster than others. Hence, the estimated maximum temperatures used in the statistical analyses were based on substantial temperature differences in the substrate. Similarly, a significant temperature variation was found in the composted material (Study 2), but the variation was less pronounced, and the temperature curves were more alike (Figure 3).
Compost material is often heterogeneous organic wastes of various plant parts with different textures. It mainly contains easy (e.g., disaccharides, starch) and difficult to degrade carbohydrates (e.g., lignin, cellulose), various other chemical components, and water. The material may also be dissimilarly compacted, which can affect the steaming and composting processes.
Seeds were placed in bags to ensure they were easy to find after the treatments. The bags were in close contact with the compost to mimic a natural mixture of compost and seeds. While directly mixing with the compost would have been more realistic, it would be time-consuming or impossible to find all seeds after the treatment, which would also have reduced the precision of estimating germination percentages.
The increased temperature in the steamed mixture decreased the seed germination of all samples (Figure 4). The mean germination percentages of untreated seed samples were: 68.00%, 75.00% (Akershus), 45.00% (East1), 0.25% (East2), 4.50% (West1), 35.75% (West2), and 88.00% (Poland). Due to the low germination in control samples of East2 and West1, a model could not be fit. Populations responded differently to temperatures (Figure 4; Table 2). The estimated ED50 and ED90 varied between 17.7 and 50.3 C and 50.0 and 78.6 C, respectively (Table 2). Lower ED50 and ED90 values were associated with populations for which the control treatment exhibited poorer germinability than populations with higher germinability (Figure 4). To ensure an almost 100% kill, a temperature of 100 C was necessary. Similar results have been observed for E. crus-galli and other weed species in two seed-infested soil types (Bitarafan et al. Reference Bitarafan, Kaczmarek-Derda, Berge, Tørresen and Fløistad2022, Reference Bitarafan, Kaczmarek-Derda, Berge, Fløistad and Andreasen2024). However, those authors also found the invasive species garden lupine (Lupinus polyphyllus Lindl.) required temperatures larger than 100 C to reduce seed germination by 90%.
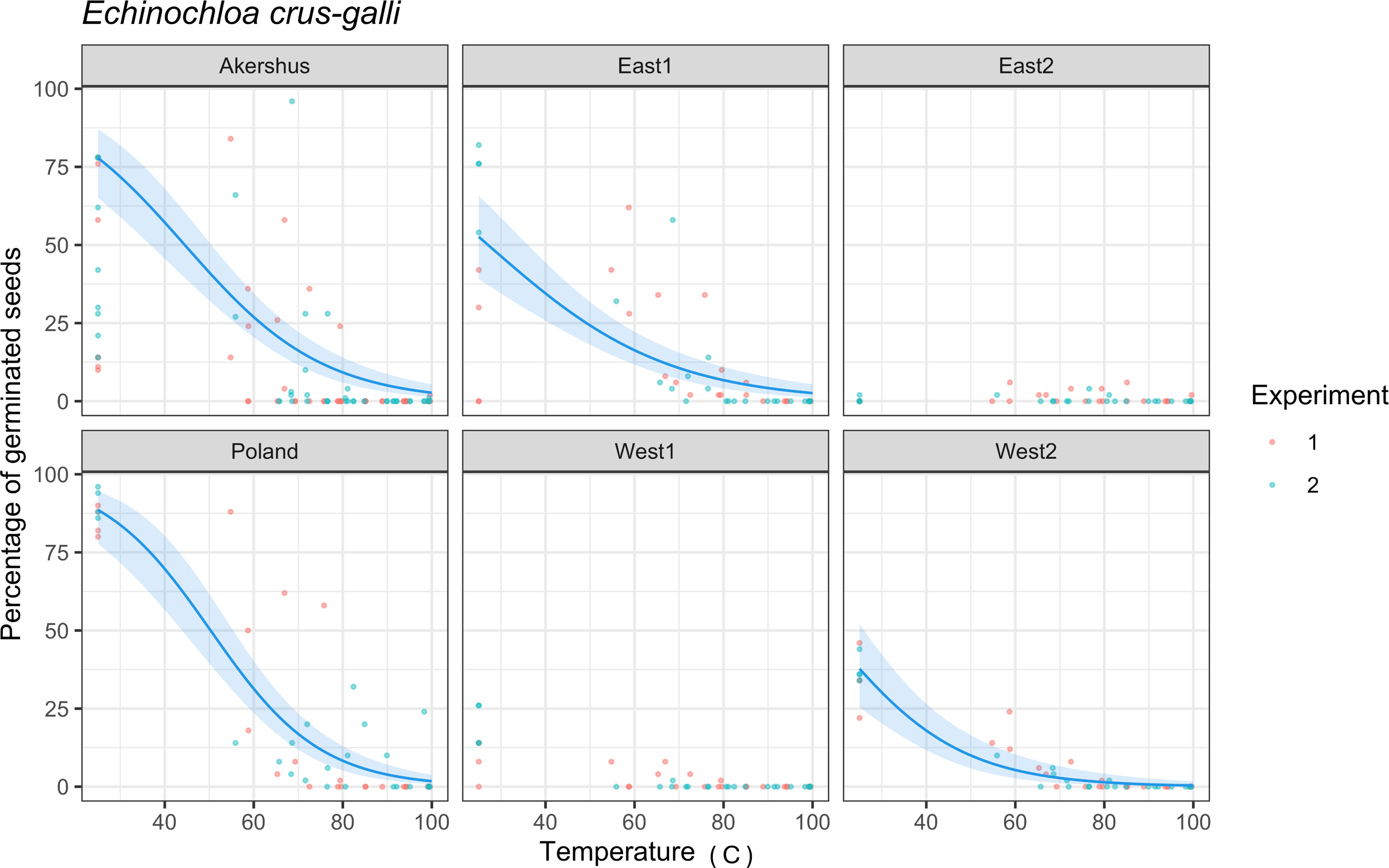
Figure 4. Dose–response relationship between germination percentage of Echinochloa crus-galli seeds and maximum temperature in the steamed mixture of onion waste, horse manure and wood shavings in Study 1. Observations from Experiments 1 and 2 are represented by red and blue dots, respectively. The fitted curve estimated by the quasi-binomial generalized linear model based on all data is displayed in blue, whereas the 95% confidence intervals for the true mean proportion of germinated seeds are represented as a shaded area. Due to the low germination in control samples of East2 and West1, a model could not be fit.
Table 2. Estimated effective dose to reduce the seed germination by 50% (ED50) and 90% (ED90) calculated for each population of Echinochloa crus-galli in Study 1. a
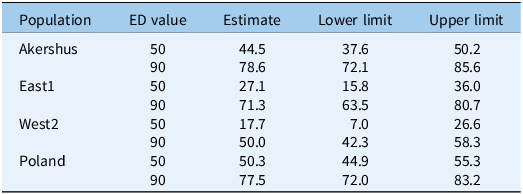
a Lower and upper limits for the 95% confidence intervals for the true effective doses are also shown. Due to the low germination in untreated control samples of East2 and West1, a model could not be fit.
All seeds were dry when placed in the steamer. Substrate and seed moisture affect the seeds’ susceptibility to heating (Verdù and Mas Reference Verdù and Mas2004), because water is a better heat conductor than dry tissue. Therefore, dry seeds heat up more slowly than imbibed seeds. Due to the high moisture content in some substrates for compost, some E. crus-galli seeds may have undergone imbibition, and we would therefore expect a lower temperature to be sufficient to kill imbibed seeds than dry seeds. Seeds that have a thick seed coat or a seed coat impenetrable to water or are protected by other means may be more resistant to hot steam than others. Echinochloa crus-galli seeds are known to be able to withstand high temperatures, because the caryopsis is protected by its lemma and palea, sterile floret, and the first and second glumes (Vidotto et al. Reference Vidotto, De Palo and Ferrero2013).
In the 24-h treatment, only five seeds (2.5%) from the East1 population were germinated and two seeds (1%) from (one replication) the West1 population. No seeds germinated from the four other populations.
Several factors affect a seed’s germinability during steaming: temperature regimes (Andreasen et al. Reference Andreasen, Rossing and Ritz2020; Melander and Kristensen Reference Melander and Kristensen2011; Thompson et al. Reference Thompson, Jones and Blair1997); heat duration (Van Loenen et al. Reference Van Loenen, Turbett, Mullins, Feilden and Wilson2003); water content in the seeds and substrate moisture (Egley Reference Egley1990); seed dormancy (Thompson et al. Reference Thompson, Jones and Blair1997); and seed structure, anatomy, and morphology (e.g., seed coat, size) (Dahlquist et al. Reference Dahlquist, Prather and Stapleton2017). Maximum temperature and heat duration are the most essential factors for reducing seed germination. A study showed that the duration of exposure required for weed seed thermal death varied from 0.17 h at 70 C to 672 h at 39 C (Dahlquist et al. Reference Dahlquist, Prather and Stapleton2017). However, there was a clear negative relationship between the estimated maximum temperature achieved during steaming and the following germination percentage of seeds (Figure 4).
Study 2
While 89.8%, 92.5%, 84.0%, 95.0%, 25.3%, and 83.0% germination was observed in the untreated control samples of Akershus, East1, East2, West1, West2, and the Polish population, respectively, none of the seeds survived the composting process regardless of pre-steam treatment (Figure 5). Steaming before compositing was not necessary. As for the first study, steaming at 60 C was insufficient to kill the seeds. The germination percentages were 70.3%, 54.0%, 42.0%, 54.5%, 15.0%, and 57.3% for the Akershus, East1, East2, West1, and West2 seed samples and the Polish population, respectively (Figure 5).
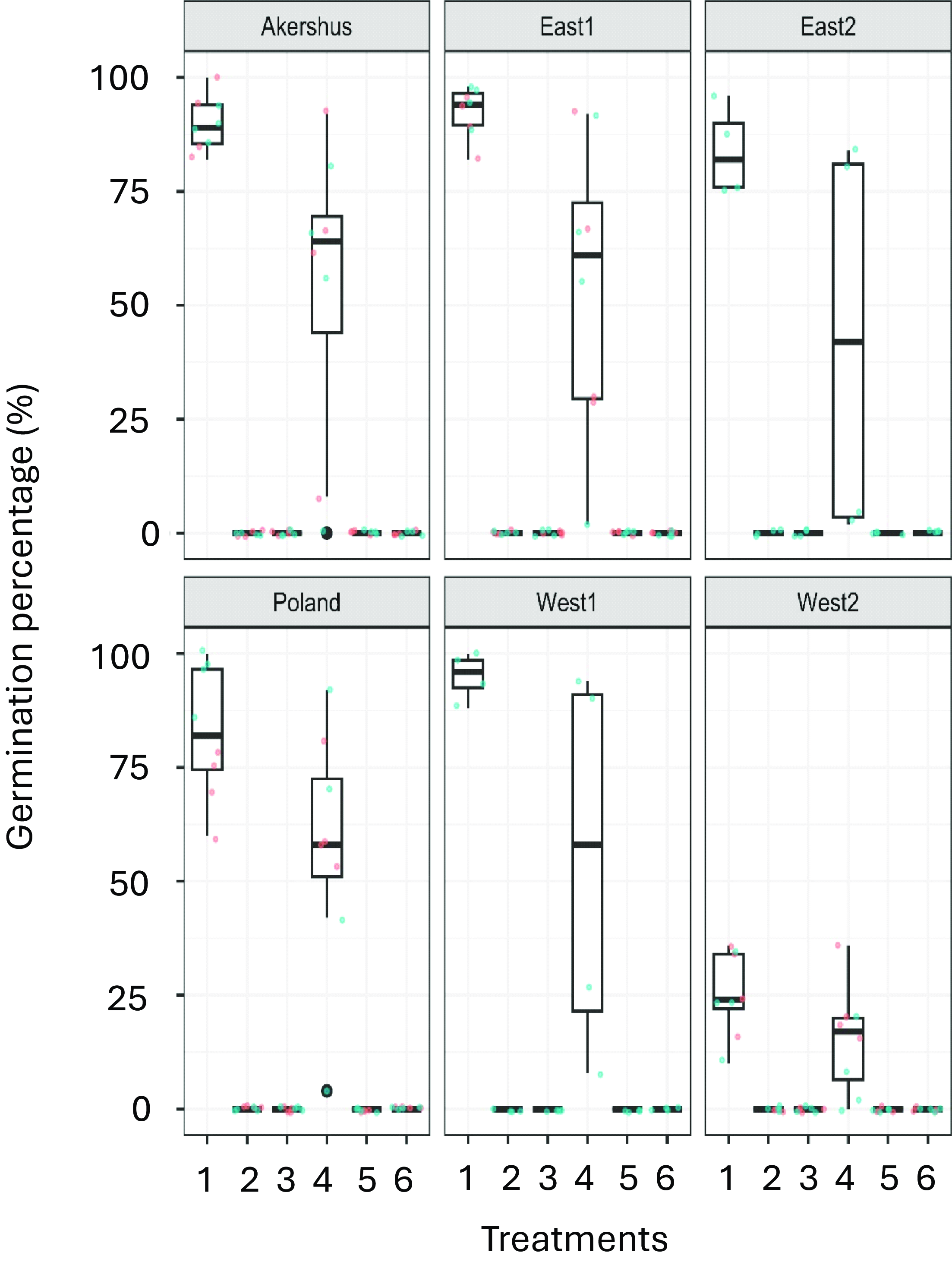
Figure 5. Box plots showing the resulting germination percentage of Echinochloa crus-galli seeds for each of the six populations after the six treatments based on both experiments in Study 2: (1) control (no treatment); (2) compost-treated seeds moved from the compost chambers at the end of thermophilic stage; (3) compost-treated seeds moved from the compost chambers when composting was stopped, i.e., at the end of maturation stage; (4) seeds steamed (approx. 60 C) without being placed in compost; (5) steamed (approx. 60 C) and compost-treated seeds moved from the compost chambers at the end of thermophilic stage; and (6) steamed (approx. 60 C) and compost-treated seeds moved from the compost chambers when the composting was stopped. Observations from Experiments 1 and 2 are represented as red and blue dots, respectively, horizontally jittered to ease visualization.
The thermophilic composting phase is defined by high temperatures, and organoheterotrophic bacteria carry out the transformation. The thermophilic microorganisms degrade fats, cellulose, hemicellulose, and some lignin. During this phase, the maximum degradation of organic matter occurs, together with the destruction of pathogens and weed seeds (Bernal et al. Reference Bernal, Sommer, Chadwick, Qing, Guoxue, Michel and Sparks2017). Echinochloa crus-galli seeds will not survive composting conditions according to the EPPO prescription for effective composting, which is either at least 55 C for a continuous period of 2 wk or at least 65 C for a continuous period of 1 wk. It is important to rotate the windrow regularly (OEPP/EPPO 2008). Wiese et al. (Reference Wiese, Sweeten, Bean, Salisbury and Chenault1998) found that seeds of E. crus-galli were killed after a minimum of 3 d of exposure to 49 C when buried in compost (Wiese et al. Reference Wiese, Sweeten, Bean, Salisbury and Chenault1998).
In conclusion, although E. crus galli seeds are considered well protected against heat damage, they were not able to survive the composting process when they were exposed to a temperature of 60 C for several days. The long-term exposure to steamed warm soil (in the 60 C by 24-h treatment) also killed almost all the seeds. However, killing the seeds in the biowaste substrate only by a short steaming and residence time (3 min) required high temperatures for the populations with the highest germination percentage before the treatment, while most seeds in populations with lower germination percentages could be killed at lower maximum temperatures (Figure 4).
Steaming is an energy-demanding process, especially if large amounts of material must be steamed at high temperatures to kill weed seeds. Therefore, steaming of biowaste should only be done if there is a significant risk of spreading invasive weeds. There is a need to calculate the exact energy consumption and costs of the steaming. Composting was an efficient method to kill E. crus galli. Future research should investigate the necessary composting time to kill weed seeds.
Acknowledgments
We thank the technical staff at the Norwegian Institute of Bioeconomy Research (NIBIO), Soil Steam International AS, Lindum AS, Toten Løkpakkeri AS, and Larvik Løk AS, for practical help and their collaboration during the experiments.
Funding statement
The funding for this project was provided by the Norwegian Institute of Bioeconomy Research (NIBIO) base funds from the Ministry of Agriculture and Food; and the project “ResourceReturn—New Steam Technology Converts Biologically Contaminated Soil Masses and Plant Wastes into New Resources (grant number 321616)” financed by the Research Council of Norway, Soil Steam International AS, Lindum AS, Toten Løkpakkeri AS, Larvik Løk AS, and the Norwegian Public Roads Administration.
Competing interests
The authors declare no conflicts of interest.









