The marine black tiger shrimp Penaeus monodon is the world's second most cultured crustacean species(1). For crustacean shrimp, as for farmed finfish, plant protein sources are increasingly included in feeds in order to reduce the reliance on wild-caught marine protein sources. However, the replacement of fish or shrimp meal by plant protein sources changes the amino acid (AA) profile of the diet, with methionine (Met) as one of the first-limiting essential AA(Reference Nguyen and Davis2, Reference Davis and Arnold3). In P. monodon fed an optimal crude protein level, we have previously noted that a 30 % Met deficiency diminished protein accretion(Reference Richard, Blanc and Rigolet4) and increased deamination(Reference Richard, Vachot and Brèque5), suggesting a change in AA catabolism in shrimp receiving an imbalanced dietary Met supply. In P. monodon, or crustacean species in general, not much is known on the metabolic utilisation of Met besides its need for protein synthesis. In contrast, the importance of Met as a methyl-group donor for methylation reactions and as a precursor of other sulphur-containing compounds, such as cysteine or taurine, is well recognised in vertebrates(Reference Espe, Hevroy and Liaset6–Reference Baker10). As such, homocysteine (Hcy), at the branch point of the three major pathways of Met metabolism (transmethylation, remethylation and trans-sulfuration), is often regarded as a regulatory component of Met metabolism since Hcy can be either trans-sulfurated for cysteine production (catalysed by cystathionine β-synthase; CBS) or remethylated into Met. In the rat liver, the remethylation of Hcy into Met occurs by two different pathways using either the folate–vitamin B12-dependent enzyme methionine synthase (MS) or the betaine–homocysteine methyltransferase (BHMT)(Reference Stipanuk9), which appear to contribute equally to the regeneration of Met(Reference Finkelstein and Martin11).
We have recently evaluated the Met requirement for maximal protein gain in P. monodon to be 0·56 g/kg body weight per d, corresponding to a dietary level of 0·8 % Met (% DM)(Reference Richard, Blanc and Rigolet4). This value is close to the Met requirement value of 0·9 % found for post-larval P. monodon (Reference Millamena, Bautista-Teruel and Kanazawa12), but lower than the 1·3–1·4 % Met requirement values reported for juvenile P. monodon (Reference Liou and Yang13) or kuruma shrimp Marsupenaeus japonicus (Reference Teshima, Alam and Koshio14, Reference Michael, Koshio and Teshima15). The total sulphur amino acid (SAA) requirements estimated at 1·1 % (0·8 % Met) by us in an earlier study(Reference Richard, Blanc and Rigolet4) and 1·3 % (0·9 % Met) by Millamena et al. (Reference Millamena, Bautista-Teruel and Kanazawa12) included 0·3 and 0·4 % of cystine, respectively. Little attention has been paid to the interaction between dietary Met and cyst(e)ine when determining Met requirements in shrimp, despite the ample evidence of the Met-sparing effect of cystine in vertebrates such as teleosts(Reference Nguyen and Davis2, Reference Walton and Cowey16, Reference Cowey, Cho and Sivak17), birds(Reference Graber and Baker18, Reference Sasse and Baker19) or mammals(Reference Teeter, Baker and Corbin20, Reference Chung and Baker21), where 50 % or more of the requirement for Met can be covered by a dietary supply of cystine.
Dietary choline has been found to improve growth, providing free methyl groups(Reference Dilger, Garrow and Baker22–Reference Emmert, Webel and Biehl24), thus exerting a sparing effect on Met utilisation(Reference Michael, Koshio and Teshima15, Reference Wu and Davis23, Reference Simon25). As BHMT is directly involved in the remethylation of Met through betaine, this enzyme is suggested to have a dual role: in the catabolism of choline (betaine) and/or the conservation of Met depending on the dietary level of Met(Reference Simon25).
For crustaceans, there is no information on the nutritional regulation of enzymes involved in SAA metabolism. In the present study, we examined the potential sparing effect of dietary choline and cystine on the utilisation of Met for protein accretion in juvenile shrimp P. monodon and their effect on the activity of two enzymes of Met metabolism involved in remethylation (BHMT) and trans-sulfuration (CBS).
Materials and methods
We investigated in shrimp the Met-sparing effect (i) of dietary choline when Met was either adequate (control diet; CTL) or limiting (30 and 50 %) in the diet (diets CTL, CTL+choline chloride (CC), 30 % Met- or sulphur amino acid-limiting diet (DEF30), DEF30+CC, 50 % Met- or sulphur amino acid-limiting diet (DEF50) and DEF50+CC) and (ii) of cystine added to the 30 and 50 % Met-limiting diets (diets DEF30, DEF30+cystine (Cyss), DEF50 and DEF50+Cyss). The first series of the diets was formulated to contain decreasing levels of SAA with a constant cystine:Met ratio. The second series contained similar levels of total SAA by modifying the cystine:Met ratio.
Experimental diets
We formulated eight semi-purified isonitrogenous diets to supply three dietary SAA levels and two choline levels (Tables 1 and 2). The dietary crude protein level was based on our previous study with juvenile P. monodon (Reference Richard, Blanc and Rigolet4) and formulated to be 35 % crude protein as fed (38 % diet DM). N was supplied by casein and a crystalline AA blend at a ratio of 43:57 (Table 1). The diets were manufactured by Institut National de la Recherche Agronomique at the experimental facility of Donzacq (Donzacq, France). The crystalline AA blend was coated with 2 % agar dissolved in warm water (pH 7, 40°C). Glucosamine and fish protein-soluble concentrate (CPSP 90) were added to improve feed palatability. Casein, cholesterol, soyabean lecithin, sodium alginate, cellulose, CPSP 90, starch, glucosamine, minerals and vitamins were first mixed together and homogenised before adding the coated AA, fish oil and CC. After thorough mixing, feed was pelleted (3 mm), dried at 40°C and shipped to the experimental facility in Madagascar, where it was stored at 4°C.
Table 1 Formulation of the experimental semi-purified diets fed to juvenile Penaeus monodon for 5 weeks
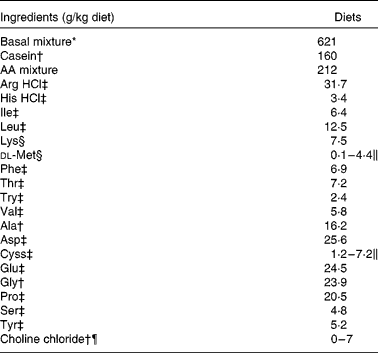
AA, amino acids.
* Basal mixture supplied (in g/kg diet as fed): gelatinised starch, 310; fish oil, 59; soya lecithin, 20; sodium alginate, 49; cellulose, 20; agar, 20; stabilised cholesterol, 15; fish protein concentrate (CPSP 90), 20; d-glucosamine 98 % HCl, 8; mineral mixture, 50; vitamin mixture, 50. Mineral and vitamin mixtures were as presented in Richard et al. (Reference Richard, Blanc and Rigolet4, Reference Richard, Vachot and Brèque5) and supplied 167 g/kg mixture of choline chloride (60 %).
† Acros (Illkirch, France); 95 % pure casein (CAS 9000-71-9).
‡ Jerafrance (Jeufosse, France).
§ Eurolysine (Paris, France).
∥ The crystalline AA mixture supplied methionine (Met) at 4·5, 2·3 and 0·1 g/kg feed and cystine (Cyss) at 2·9, 2·0 and 1·2 g/kg feed in the control, 30 and 50 % Met-limiting diets, respectively. For the Cyss-enriched diets, the AA mixture supplied 5·1 and 7·2 g/kg feed in the 30 and 50 % Met-limiting diets, respectively. An increase in the amount of non-essential AA compensated for varying Met and Cyss levels.
¶ Choline chloride (99 %) was supplemented at 7 g/kg feed in the choline-enriched diets. Gelatinised starch (in basal mixture) compensated for the choline addition.
Table 2 Analysed chemical composition of the experimental semi-purified diets fed to juvenile Penaeus monodon for 5 weeks

CTL, control diet adequate in Met or sulphur amino acid (SAA); CC, choline chloride; DEF30, 30 % Met- or SAA-limiting diet; DEF30+CC, DEF30 with excess CC; DEF50, 50 % Met- or SAA-limiting diet; DEF50+CC, DEF50 with excess CC; DEF30+Cyss and DEF50+Cyss, with extra Cyss in order to adjust the SAA level to that in CTL; Cyss, cystine.
Study of the methionine-sparing effect of choline
We formulated three diets to be adequate (diet CTL) or 30 or 50 % limiting in Met (diets DEF30 and DEF50, respectively). In these diets (CTL, DEF30 and DEF50), the ratio of cystine:Met was kept constant at 0·4, so that total SAA levels decreased according to the Met level (from 1·2 to 0·6 g/100 g DM; Table 2). The adequate Met level (0·8 g/100 g DM) corresponds to the requirement value determined in our previous study(Reference Richard, Blanc and Rigolet4). We formulated three supplemental diets (CTL+CC, DEF30+CC and DEF50+CC) to supply choline in excess (7000 mg/kg dry feed). CC, an efficient choline source in shrimp(Reference Michael, Koshio and Teshima15), was used in the diets. The basal level of choline in the diet was 3000 mg/kg DM, as in our previous study(Reference Richard, Blanc and Rigolet4).
Study of the methionine-sparing effect of cystine
We used the same two diets as described previously (DEF30 and DEF50). In two other diets, Cyss was added in excess (DEF30+Cyss and DEF50+Cyss) in order to adjust the total SAA supply to that of the diet CTL (1·1–1·2 g/100 g DM). In these diets, the cystine:Met ratios were 0·9 for diet DEF30+Cyss and 1·6 for diet DEF50+Cyss.
Animal husbandry
Juvenile P. monodon (3·3 (0·1) g; Aqualma hatchery, Madagascar) were reared in circular 150 litre fibreglass-covered tanks (fifteen shrimp per tank) for 5 weeks, following a 7 d adaptation period during which they were fed the CTL diet at 3·5 % biomass/d. For each diet, four replicate groups were used. Average temperature, oxygen concentration, pH and salinity of the water were, respectively, 29·8 ± 1·2°C, 6·1 (SD 0·4) mg/l, 8·0 (SD 0·1) and 35·0 (SD 0·5) g/l. Water was exchanged on a daily basis, at a minimum of 40 % for each tank.
The shrimp were fed ad libitum four times per d (08.00, 13.00, 18.00 and 23.00 hours) using three circular trays in each tank. After 2 h, feed was removed, and the left-over was carefully collected and stored at − 20°C. Weekly apparent DM intake was calculated after drying the uneaten feed to a constant weight (48 h, 90°C). Every week, biomass of each tank was weighed. Mortality was checked daily, and dead shrimp were removed and weighed. Shrimp in ecdysis were not considered for final sampling. A representative sample of 12 h feed-deprived shrimp was taken at the start (twenty-five shrimp) and the end of the experiment (five shrimp from each of the four replicate tanks per dietary treatment) for whole-body composition analysis. All samples were kept at − 20°C before analyses. Feed and whole carcasses were analysed for DM (105°C, 24 h), ash (550°C, 12 h) and crude protein (N × 6·25, Kjeldahl Nitrogen Analyser 2000; Fison Instruments, Milan, Italy). Feed samples were analysed for choline content at the laboratory IPL (Bordeaux, France). Daily growth coefficient (DGC, %) was calculated as: (W f0·3333 − W i0·3333)/Δt) × 100, where W i and W f are the mean initial and final body weights (g), respectively, and Δt is the duration of the growth trial (35 d). N gain (mg/shrimp per d) was calculated as ((Nf × W f) − (Ni × W i))/Δt × 1000, where Ni and Nf are the N content of shrimp at the start and the end of the experiment (g/100 g fresh matter).
Enzyme activities
Tissue sampling and preparation
At sampling days, the shrimp were allowed to eat for 1 h before the excess feed was removed from the tank. At 3 h after feed removal, shrimp were sampled; hepatopancreas were quickly dissected out, immediately weighed and frozen in dry ice before stocking at − 80°C. Frozen tissues were homogenised in ten volumes of ice-cold phosphate buffer (0·04 m, pH 7·4, 1 mm-EDTA, 1 mm-dithiothreitol) with Ultra-Turrax (16 000 rpm). The homogenates were centrifuged at 1000 g (4°C for 10 min). The supernatant fraction was then centrifuged at 45 000 g (4°C for 20 min). Samples were analysed for BHMT (EC 2.1.1.5) and CBS (EC 4.2.1.22) activities. Protein content of the hepatopancreas was determined by the Bradford method with bovine serum albumin as the standard. From the preliminary results, we determined the optimal protein concentration to be 1 mg protein per tube for the determination of both BHMT and CBS activities, and the sample volume to be added in each tube was adjusted to this protein concentration.
Determination of the enzyme activity
Measurements of BHMT activity were based on Finkelstein & Mudd(Reference Finkelstein and Mudd26) and Lambert et al. (Reference Lambert, Titgemeyer and Stokka27). The following compounds were incubated for 90 min at 37°C in a volume of 467·5 μl: 37·5 μl of 466·6 mm-potassium phosphate buffer (pH 7·4); 35 μl of 100 mm-dl-Hcy (Sigma-Aldrich, Saint-Quentin Fallavier, France); 55 μl of 59 mm-betaine 14CH3 (ARC, Isobio, Fleurus, Belgium) at 2500 Bq. The volume of each tissue extract was adjusted according to its protein concentration and homogenisation buffer (0·04 mm-potassium phosphate buffer, pH 7·4, 1 mm-EDTA and 1 mm-dithiothreitol) was added to compensate the volume if necessary. After incubation, the reaction was stopped by adding 62·5 μl of cold water and 280 μl of the mixture were pipetted into a 10 ml polypropylene column (0·8 × 4 cm; Biorad, Marnes-la-Coquette, France) containing 2·5 ml of Dowex® ion exchange resin (1 × 4 (Cl− ), 200–400 mesh; Sigma Aldrich). The non-converted betaine was eluted with 10 ml of water, and the labelled products were eluted with 10 ml of 1 m-acetic acid (from the preliminary tests, acetic acid gave a 85 % product recovery).
Measurements of CBS activity were based on Mudd et al. (Reference Mudd, Finkelstein and Irreverre28) and Lambert et al. (Reference Lambert, Titgemeyer and Stokka27). The following compounds were incubated for 120 min at 37°C in a volume of 400 μl: 100 μl of mix reagent (buffer with 1·2 m-Tris–HCl, pH 8·3, 20 mm-EDTA, 500 mm-dl-Hcy and 0·6 mm-pyridoxal phosphate); 20 μl of 50 mm-l-serine 3-14C (ARC) at 2500 Bq. The added volume of each tissue extract was adjusted according to its protein concentration, and homogenisation buffer (0·03 mm-potassium phosphate buffer, pH 6·9, 1 mm-EDTA and 1 mm-dithiothreitol) was added to compensate the volume if necessary. After incubation, the reaction was stopped by adding 400 μl of 10 % cold TCA, and all samples were centrifuged for 5 min at 6500 rpm. The supernatant fraction (500 μl) was pipetted into 20 ml of water. The mixture was eluted to the column containing Dowex® ion exchange resin (50Wx4 (H+), 200–400 mesh; Sigma Aldrich) by rinsing consecutively with 18 ml of water, 35 ml of 0·4 m-HCl, 10 ml of water and 4 ml of 2 m-NH4OH. Preliminary tests indicated a recovery of 54 % with HCl and NH4OH. Radioactivity of samples was measured in a Packard Tri-Carb liquid scintillation counter after addition of 10 ml of Ultima-Gold™ reagent (Perkin Elmer, Les Ulis, France). All analyses were done in duplicate, and a blank value obtained from incubation of a heat-inactivated extract was subtracted for each sample. BHMT and CBS activities are expressed as unit (U)/mg protein, where 1 unit is defined as 1 nmol of betaine and serine transformed in 90 and 120 min reaction, respectively.
Amino acid analyses
Feed protein-bound amino acids
Feed (100 mg) was hydrolysed (23 h, 110°C) with 25 ml of 6 m-HCl and 12·5 ml of 2-mercaptoethanol. For the analysis of cystine (through cysteic acid measurement), mercaptoethanol was replaced by a solution of 1 % phenol and 0·2 % sodium azide. After dilution (1:20), 10 μl of the hydrolysed sample were derivatised by adding 70 μl of AccQ.Tag buffer (Waters, Guyancourt, France) and 20 μl of AccQ.Fluor reagent (6-aminoquinolyl-N-hydroxysuccinimidyl carbonate) in a 0·5 ml microtube. A diluted (1:25) standard solution of 17 AA (Sigma Aldrich)) was derivatised in the same way. Each sample (5 μl) was then analysed by HPLC (column Symmetry C18, 5 μm, 3·9 × 150 mm) using three mobile phases (AccQ.Tag buffer, 100 % acetonitrile and water, respectively) with a total elution time of 45 min. The AA separations were done using a flow rate of 1 ml/min, and the control temperature was set at 37°C. The excitation and emission wavelengths in the fluorescence detector were 250 and 395 nm, respectively.
Haemolymph free amino acids
Haemolymph of six shrimp per treatment (from those sampled for the determination of enzyme activity) was collected by puncture between the cephalothorax and the first pair of pleopods using a 1 ml syringe (needle 25G 1·59 cm, 0·5 × 16 mm). Plasma was obtained following immediate centrifugation (5 min, 5000 g) and stored at − 20°C. Each plasma sample (100 μl) was then centrifuged (1 h 30 min, 2000 g, 15°C) followed by a filtration (filters Amicon-Microcon, YM 100 kd) to eliminate protein. Samples were then derivatised following the AccQ.Tag method (Waters) and injected into the HPLC column as described previously. AA concentrations are expressed as μmol/l of haemolymph.
Statistical analysis
The effects of dietary Met and choline on metabolism and performances were analysed by a two-way ANOVA using Met (adequate, 30 % limiting and 50 % limiting) and choline (adequate or excess) as independent factors (six diets). The effect of Met and cystine dietary levels was analysed by a two-way ANOVA using the Met levels (30 and 50 % limiting) and cystine levels (normal or excess) (four diets). For the amino acid and enzyme analyses, outliers (detected by a scatterplot with box plots) were excluded from the analysis. Outlier and extreme points are described as follows:
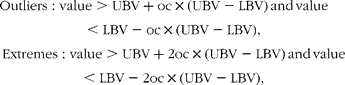
where LBV is the lower value of the box plot (25th percentile), UBV is the upper value of the box plot (75th percentile) and oc is the outlier coefficient (constant 1·5).
For body composition and performances, four replicates per treatment were considered in the analysis, except for diet DEF30 (n 3). Because the dietary treatments did not affect the hepatic somatic index and hepatic protein concentration, the enzyme activities were compared in term of units/mg protein. All analyses were performed using STATISTICA 5.0 software (StatSoft, Inc., Tulsa, OK, USA). Data were analysed by Duncan's multiple range test in the case of a significant effect (P < 0·05).
Results
Performances
The survival (91–98 %) of the shrimp was not affected by the dietary treatments (P>0·05; Tables 3 and 4). Growth parameters and feed efficiency were significantly higher in shrimp fed the excess dietary choline compared with those fed the basal choline diets (P < 0·05; Table 3). The 30 % Met-limiting diet significantly reduced the DGC (%) and feed efficiency of shrimp compared with the CTL diet (Table 3). Surprisingly, growth parameters of shrimp fed the 50 % Met-limiting diets were intermediate between those of shrimp fed the CTL and 30 % Met-limiting diets. We observed a significant interaction between Met and choline for individual N gain (P < 0·05; Fig. 1). This interaction should be attributed to the fact that the extra choline improved N gain in shrimp fed the Met-limiting diets but not in shrimp fed the Met-adequate CTL diet. The addition of cystine significantly improved the DGC and feed efficiency of shrimp, irrespective of the level of the Met limitation (P < 0·05; Table 4). N gains were significantly increased by the extra dietary cystine at both Met levels (30 and 50 % limiting) (P < 0·05; Fig. 1).
Table 3 Effect of methionine and choline on growth and metabolic parameters of juvenile Penaeus monodon fed the experimental diets during 35 d
(Mean values with their standard errors)

CTL, control diet; CC, choline chloride; DEF30, 30 % Met- or sulphur amino acid (SAA)-limiting diet; DEF50, 50 % Met- or SAA-limiting diet; BHMT, betaine–homocysteine methyltransferase; CBS, cystathionine β-synthase; ND, not detected.
* Values are mean values with their standard errors of four replicate tanks, with exception of treatment DEF30 where only three replicate tanks are considered.
† Daily growth coefficient (%): (W f(1/3) − W i(1/3)/d) × 100, where W i and W f are the mean initial and final body weights (g), respectively.
‡ Apparent feed intake: ((FG − FU) × (100 − leaching %))/(N × 35 d), where FG is the total feed gift (g DM), FU is the total amount of uneaten feed (g DM), leaching % is the percentage of feed loss after 2 h immersion in water (salinity 30 parts per thousand) N is the average number of shrimp per tank over 35 d.
§ Values are means of six shrimp haemolymph samples unless noted otherwise (in parentheses).
Table 4 Effect of methionine and cystine on growth and metabolic parameters of juvenile Penaeus monodon fed the experimental diets during 35 d
(Mean values with their standard errors)

DEF30, 30 % Met- or sulphur amino acid (SAA)-limiting diet; Cyss, cystine; DEF50, 50 % Met- or SAA-limiting diet; BHMT, betaine–homocysteine methyltransferase; CBS, cystathionine β-synthase; ND, not detected.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* Values are means of four replicate tanks (se), with exception of treatment DEF30, where only three replicate tanks are considered.
† Daily growth coefficient (%): (W f(1/3) − W i(1/3)/d) × 100, where W i and W f are the mean initial and final body weights (g), respectively.
‡ Apparent feed intake: ((FG − FU) × (100 − leaching %))/(N × 35 d), where FG is the total feed gift (g DM), FU is the total amount of uneaten feed (g DM), Leaching% is the percentage of feed loss after 2 h immersion in water (salinity 30 parts per thousand) and N is the average number of shrimp per tank over 35 d.
§ Values are means of six shrimp haemolymph samples unless noted otherwise (in parentheses).

Fig. 1 Effect of dietary levels of choline (A) and cystine (Cyss) (B) on daily individual N gain (mg/shrimp per d) of juvenile Penaeus monodon fed semi-purified diets adequate (control; CTL) or limiting (30 or 50 %) in sulphur amino acids (SAA; Met+Cyss) during 5 weeks. CC, choline chloride; DEF30, 30 % Met or SAA-limiting diet; DEF50, 50 % Met or SAA-limiting diet. Values are means (n 4 per treatment, except DEF30 where n 3), with standard errors of the mean represented by vertical bars. a,b Mean values with unlike letters are significantly different (P < 0·05; one-way ANOVA). P values of the two-way ANOVA (Met × choline) are as follows: MET, P = 0·025; choline, P = 0·023; Met × choline, P = 0·031. P values of the two-way ANOVA (Met × Cyss) are as follows: Met, P = 0·215; Cyss, P = 0·036; Met × Cyss, P = 0·313.
Methionine metabolism
Both BHMT and CBS were found to be active in the hepatopancreas of P. monodon shrimp with high intra-treatment variability. BHMT activity was significantly affected by the dietary Met or SAA level (Table 3). The DEF30-fed shrimp had a significantly lower BHMT activity compared with those fed the CTL diet (17 v. 33 U/mg protein, respectively). No significant difference in BHMT activity was found between shrimp fed the CTL and DEF50 diets (33 v. 36 U/mg protein, respectively). Surprisingly, the addition of dietary choline did not significantly affect BHMT activity, although there was a tendency (P = 0·126) for lower BHMT activity in shrimp fed the excess choline level at the adequate (CTL) and 30 % Met-limiting levels (DEF30) (Table 3). CBS activity was not affected by either the Met or choline level in the diets (P>0·05; Table 3). The activity in hepatopancreas varied from 3 to 7 U/mg protein, respectively, for DEF30+CC- and DEF50-fed shrimp. No significant effect of extra dietary cystine could be detected on BHMT or CBS activity (P>0·05; Table 4).
Free Met, Hcy and serine concentrations in the haemolymph were not significantly affected by any of the dietary treatments, although free Met slightly decreased with the Met-limiting diets (11 and 10 v. 14 μmol/l in the CTL diet) and free serine tended to decrease in the haemolymph of shrimp fed the 30 % Met-limiting diet (P = 0·072; Table 3). Cysteine was not detected by the HPLC technique, which might reflect its very low concentration. Cystine, cysteic acid and taurine concentrations were significantly affected by the dietary Met level, but not by the dietary choline level (Table 3). Concentrations of cystine and cysteic acid were significantly higher in the haemolymph of shrimp fed the CTL diet compared with those fed the DEF30 diet (3·2 v. 2·1 μmol/l for cystine and 8·5 v. 4·5 μmol/l for cysteic acid). Haemolymph taurine concentration was significantly higher with the DEF50 diet compared with the DEF30 diet (635·5 v. 521·0 μmol/l). The addition of cystine to the DEF30 diet increased haemolymph cystine and taurine concentrations in shrimp (2·2 v. 3·7 and 523·4 v. 732·3 μmol/l, respectively, for cystine and taurine). However, no increase was observed at the 50 % Met deficiency level (Table 4).
Discussion
Both choline and cystine have a methionine-sparing effect on protein accretion
Due to size-related differences in specific growth rate, with small animals having a higher specific growth rate than the larger ones(Reference Kaushik29), some authors proposed to use DGC in order to compare growth data between studies with fish(Reference Bureau, Kaushik, Cho, Halver and Hardy30) or crustaceans(Reference Bureau, Azevedo, Tapia-Salazar, Cruz-Suárez, Ricque-Marie, Tapia-Salazar, Olvera-Novoa and Civera-Cerecedo31). The present DGC values (0·82–0·93 in Met adequate treatments) are similar to those found in other studies with shrimp fed semi-purified diets, in P. monodon (maximum DGC of 0·76–0·86)(Reference Chen, Leu and Roelants32–Reference Millamena, Teruel and Kanazawa34) or M. japonicus (maximum DGC of 0·68–0·89)(Reference Teshima, Koshio and Ishikawa35, Reference Alam, Teshima and Koshio36). The growth rate as observed in the present study was also similar to that of P. monodon (recalculated DGC of 0·75–1·18 %) having, as in the present study, a relatively high initial body weight (approximately 3 g) and being fed a practical diet(Reference Glencross, Smith and Tonks37).
As anticipated from our previous study on the determination of Met requirements in juvenile P. monodon (Reference Richard, Blanc and Rigolet4), the 30 % SAA (Met and cystine) limitation significantly decreased protein accretion. This reduction in protein accretion ( − 29 %) due to the SAA limitation as well as the daily N gains of the control group is similar to data obtained in our previous study(Reference Richard, Blanc and Rigolet4). However, it remains unclear why N gain did not further decrease by the 20 % extra deficiency (DEF50 v. DEF30). This contrasts with findings in other growing animals such as chickens(Reference Sekiz, Scott and Nesheim38), pigs(Reference Chung and Baker39) or fish(Reference Simmons, Moccia and Bureau40–Reference Ruchimat, Masumoto and Hosokawa42), where a severe Met deficiency induces a higher N loss than a marginal Met deficiency.
As with vertebrates, shrimp have a choline requirement for maximal weight gain, which seems to vary among shrimp species, being eight times superior in P. monodon compared with M. japonicus (0·47 v. 0·06 %, respectively)(Reference Shiau and Lo43). Since Met and choline share a common role as methyl donor, the requirement for Met is influenced by that for choline and vice versa(Reference Wu and Davis23, Reference Kasper, White and Brown41). The interactive effect of choline and Met on growth has been examined in only one penaeid shrimp, M. japonicus (Reference Michael, Koshio and Teshima15). Using a 2 × 3 design with two Met concentrations (0·30 and 1·65 %) and three choline levels (0·03, 0·08 and 0·14 %), the foresaid authors have found that choline in excess (0·14 %) improved the weight gain of M. japonicus only when fed the low-Met diet. The present results suggest a similar interaction between choline and Met since extra choline significantly improved protein deposition when added to both Met-limiting diets, but not when added to the Met-adequate CTL diet. The latter observation not only validates that the basal Met supply (0·8 %) was appropriate for normal growth in P. monodon, but also suggests that the basal choline supply (about 0·3 %) fulfilled the requirements. This choline level is, however, lower than the requirement value (0·47 %) previously reported in P. monodon (Reference Shiau and Lo43), possibly because the authors used a low Met level (0·49 %) close to that of the present 50 % Met-limiting diet.
The dietary supplementation of cystine, at both limiting Met levels, increased the N gain of the shrimp up to that obtained with the CTL. In other words, keeping the total SAA level constant at 1·1 % of the diet, while exchanging (on a weight basis) 30 or 50 % of Met by cystine, resulted in a similar protein accretion. Although the sparing effect of cyst(e)ine on Met requirements for protein accretion has not been examined before in shrimp, our data agree with studies in other animal species, showing that cystine can replace about 50 % of Met in growing pigs(Reference Chung and Baker21), chicks(Reference Graber and Baker18, Reference Sasse and Baker19) or rainbow trout(Reference Walton and Cowey16, Reference Cowey, Cho and Sivak17). The observation that the 1·1 % SAA diets with the cystine:Met ratio of 0·4, 0·9 and 1·6 led to equivalent N gain in juvenile P. monodon, as well as recent results in fish, which reported no reduction in protein accretion when feeding diets low in Met but not in cyst(e)ine (e.g. Atlantic salmon)(Reference Espe, Hevroy and Liaset6, Reference Sveier, Nordas and Berge44), underline the importance of estimating requirements for protein gain in terms of total SAA rather than for Met alone.
Regulation of methionine metabolism by methionine, cystine and choline
In crustaceans, so far, no study has examined the functionality of the pathways of Met metabolism and the dietary regulation of remethylation (BHMT or MS) and trans-sulfuration (CBS and cystathionase) enzyme activities, both being well documented in mammalian vertebrates(Reference Stipanuk9). The present study is thus the first ever to examine the ability of shrimp to regulate Met utilisation at the biochemical level.
Using the classical methodology developed for mammals(Reference Finkelstein and Mudd26–Reference Mudd, Finkelstein and Irreverre28), we demonstrated the presence of enzyme activity for both BHMT and CBS in P. monodon hepatopancreas, the major site for digestive enzyme secretion and nutrient absorption. In rats, activity of BHMT is principally detected in the liver(Reference Stipanuk9), and that of CBS mostly in the liver and pancreas(Reference Mudd, Finkelstein and Irreverre28). The specific BHMT activities in the present study varied between 13 and 42 U/mg protein (90 min reaction) and are within the range of those observed in cattle (17–22 U/mg protein per 90 min)(Reference Lambert, Titgemeyer and Stokka27), but two- to eightfold lower than in rats(Reference Finkelstein, Martin and Harris7, Reference Finkelstein, Martin and Harris45, Reference Finkelstein, Harris and Martin46) or chickens(Reference Emmert, Garrow and Baker47). Specific CBS activity (3–9 U/mg protein) was slightly below the hepatic CBS activity in growing cattle (15–20 U/mg protein per 60 min)(Reference Lambert, Titgemeyer and Stokka27) but more than tenfold lower than in the rat liver (with or without Met supply)(Reference Finkelstein and Mudd26).
The present results show a significant effect of the dietary SAA content on BHMT remethylation activity, being the lowest in shrimp fed the 30 % SAA-limiting diet and back to control levels with the 50 % SAA-limiting diet. Whereas BHMT activity has been reported to be unaffected by a reduction in dietary Met in pigs(Reference Emmert, Webel and Biehl24), comparable quadratic BHMT responses have been observed in the liver of cattle(Reference Lambert, Titgemeyer and Stokka27) and rats(Reference Finkelstein, Harris and Martin46), following more extreme variations in Met supply (from excessive to quasi-absent), than in the present study (from adequate to highly limiting). Remethylation of Hcy to Met occurs by two pathways, using either BHMT or MS as the catalysing enzyme. Although the relative contribution of both enzymes has been suggested to be equal in the rat liver(Reference Finkelstein and Martin11), recent data from broilers indicate that Hcy fluxes through BHMT and MS change according to the dietary SAA level, with an almost equal contribution at adequate SAA supply and a lower relative contribution of BHMT (compared with MS) at excess and limiting SAA supply(Reference Pillai, Fanatico and Beers48). The latter observation possibly explains the reduced BHMT activity observed in the present study with the 30 % SAA-limiting diet compared with SAA-adequate CTL. Regarding choline, several studies in mammals(Reference Emmert, Webel and Biehl24, Reference Finkelstein, Martin and Harris45) and birds(Reference Emmert, Garrow and Baker47) have found that hepatic BHMT activity increases by adding choline to diets either adequate or limiting in Met, consistent with the use of choline-derived methyl groups for remethylation. In contrast, BHMT activity in the present study tended (P = 0·126) to decrease in shrimp fed the extra choline at the adequate or 30 % limiting SAA supply. That BHMT activity may not be as responsive to dietary choline as concluded previously has recently been underlined in a study with broilers following similar observation as in the present study(Reference Dilger, Garrow and Baker22). Moreover, analysis of both remethylation pathways in terms of Hcy fluxes showed that excess choline in chicks (regardless of the dietary SAA level) decreases the relative contribution of BHMT to remethylation while increasing that of MS(Reference Pillai, Fanatico and Beers48, Reference Pillai, Fanatico and Blair49). Several hypotheses may explain the apparent inconsistencies in the effect of choline on the regulation of Hcy remethylation(Reference Pillai, Fanatico and Beers48). First, an excess of methyl groups may inhibit BHMT due to an excess of dimethylglycine, a by-product of BHMT reaction(Reference Stipanuk9, Reference Finkelstein and Martin11, Reference Pillai, Fanatico and Blair49). Also, serine may be used for the production of more 5-methylenetetrahydrofolates, increasing remethylation through folate-dependent MS(Reference Pillai, Fanatico and Beers48).
Following the addition of cystine in both SAA-limiting diets, shrimp did not respond by increasing BHMT remethylation or decreasing CBS activity, which catalyses the first step of trans-sulfuration. This was surprising, as the effect of cysteine on hepatic Met metabolism was earlier found to be related to dietary Met level, with a down-regulation of CBS only in rats fed a low-Met diet with extra cysteine(Reference Finkelstein, Martin and Harris7, Reference Finkelstein, Martin and Harris8). Based on these results, we expected the CBS activity to decrease with the addition of dietary cystine, in line with the enhanced protein accretion observed in these shrimps. Hence, the constant low CBS activity, irrespective of the cystine or Met supply, seems to reflect a poor trans-sulfuration, with Met being directed mostly towards protein synthesis. However, metabolic fluxes have previously been reported to be affected by the catalytic constant rate, the enzyme concentration or the substrate concentration(Reference Stipanuk and Dominy50). This was already observed in the rat liver in which the flow through the BHMT reaction increased while enzyme activity decreased(Reference Finkelstein and Martin11). In the present study, the absence of change in haemolymph concentrations of Met and Hcy implies that the pathways controlling intracellular Met/Hcy metabolism were effectively regulated in juvenile P. monodon. These observations emphasise the need to undertake complementary studies on the effect of dietary SAA levels on the flux of Hcy through remethylation and trans-sulfuration.
Apart from being needed for protein synthesis, Met and cyst(e)ine are precursors of biologically important S-containing molecules such as glutathione or taurine(Reference Stipanuk9). Among its many physiological functions, taurine plays a major role in cellular osmoregulation(Reference Jacobsen and Smith51), which is of particular importance in P. monodon and other euryhaline animals living in intertidal ecosystems. In terrestrial vertebrates, taurine is synthesised mostly via cysteine sulphinic acid, involving cysteine dioxygenase and cysteinesulphinate decarboxylase(Reference Jacobsen and Smith51, Reference Goto, Matsumoto and Takagi52). However, the capacity for taurine synthesis appears to be species-dependent as illustrated by the dietary taurine requirement in cats, due to a lack of cysteinesulphinate decarboxylase(Reference Knopf, Sturman and Armstrong53). Among teleost species, some debate remains regarding the capacity of some marine fish to synthesise taurine(Reference Espe, Hevroy and Liaset6, Reference Goto, Matsumoto and Takagi52, Reference Kim, Matsunari and Takeuchi54–Reference Kaushik and Luquet57). This field of research has importance especially in the context of replacing the fish and shrimp meal (rich in taurine) by plant protein sources (lacking in taurine) in the feeds. Literature on taurine synthesis capacity of crustacean species is scarce. While the freshwater prawn Macrobrachium rosenbergii appears able to cover its taurine requirement by biosynthesis(Reference Smith, Miller and Mead58), the enhanced growth of P. monodon following the addition of taurine to purified diets (4–8 g/kg diet) suggests limited taurine synthesis in P. monodon (Reference Shiau and Chou59). Nevertheless, in the present study, the growth of the shrimp fed diets that were devoid of crystalline taurine was not lower than that of shrimp receiving semi-purified diets with 5 g/kg diet of taurine as part of the attractant blend(Reference Richard, Blanc and Rigolet4). Furthermore, the taurine concentrations in the haemolymph of shrimp in the present study were within the range of values reported before in P. monodon reared at a salinity of 30 g/l(Reference Fang, Tang and Lee60), even with the 50 % SAA-limiting diet. Moreover, the increase in haemolymph taurine concentration following cystine addition to the 30 % Met-limiting diet suggests that P. monodon has a capacity to regulate taurine synthesis in relation to dietary cystine levels. However, the role of taurine and the regulatory pathways of taurine synthesis in P. monodon, and aquatic crustacean species in general, needs further investigation.
Conclusions
Our data demonstrate the sparing effect of choline and cystine on Met requirements for protein accretion in juvenile P. monodon, and suggest that growth requirements should be expressed in terms of total SAA rather than Met alone. The present study shows for the first time the existence and functionality of enzymes involved in Met metabolism (i.e. BHMT and CBS) located in shrimp hepatopancreas. BHMT, but not CBS, which had an overall low activity, was found to respond to the dietary SAA deficiency. The addition of choline and cystine to the SAA-limiting diets did not stimulate remethylation by BHMT nor did they down-regulate CBS trans-sulfuration activity. However, the constant Met and Hcy concentrations in the haemolymph, independent from dietary treatment, suggest that the shrimp were able to maintain remethylation/trans-sulfuration equilibrium. Finally, our data suggest the capacity of shrimp to synthesise taurine. However, further research is needed to better understand the sparing effects of choline and cystine on Met requirements and to characterise the pathways regulating taurine synthesis.
Acknowledgements
The authors acknowledge Frédéric Terrier and Peyo Aguirre for their help during diet manufacturing and Marie Jo Borthaire for her assistance with the laboratory analyses. Special thanks are due to Christian Ramamonjisoa (Aqualma facility) for his technical assistance. L. R. designed the study and did the data analysis. L. R., S. J. K. and I. G. contributed to the drafting of the manuscript. C. V. and L. R. performed the enzymatic analyses. A. S. performed the AA analyses. L. R. and V. R. contributed to the organisation of the study in Madagascar. There are no contractual agreements for the presented data, which might cause conflicts of interest. The authors acknowledge UNIMA and institutional funds from INRA for funding the present study and Agence National de la Recherche et de la Technologie (ANRT; France) for the scholarship to L. R. (CIFRE PhD Research Grant).






