Primary angiitis of the central nervous system (PACNS) is a vasculitis isolated to the central nervous system (CNS). Although timely identification and treatment are critical to prevent morbidity and mortality, its variable manifestations make this challenging. Patients most frequently develop headache, cognitive impairment, and/or focal neurological deficits with or without infarction.Reference Salvarani, Brown and Calamia 1 , Reference Salvarani, Brown and Christianson 2 We present the case of Newfoundland and Labrador’s (NL) first patient with angiography-negative, biopsy-positive PACNS, who presented with headache and ataxia.
A 63-year-old female presented with 9 days of gait disturbance, incoordination, vertigo, nausea, and vomiting. She also reported 3 years of sporadic, mild headaches that were never associated with any neurological changes. She had a history of osteopenia, reflux, a benign thyroid nodule, and benign femur and skull lesions. Her medications included rabeprazole and acetaminophen.
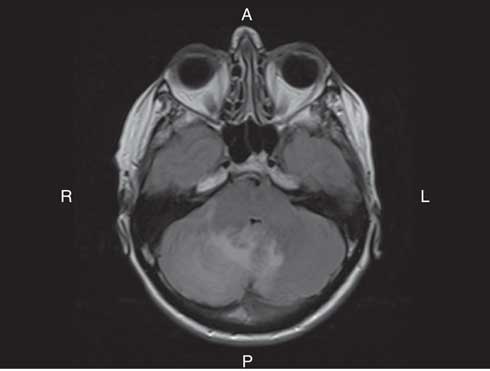
On examination, she had bilateral appendicular ataxia, a positive Romberg sign, and an ataxic gait. The remainder of her examination was normal. CT of her head showed right cerebellar edema. MRI showed cerebellar leptomeningeal enhancement with edema and localized mass effect (Figure 1). Her C-reactive protein and erythrocyte sedimentation rate were elevated at 13.3 mg/L and 25 mm/hour, respectively. Complete blood count (CBC), blood chemistry, creatinine, and glucose were normal. Infectious and inflammatory workups were negative. A CT of the chest, abdomen, and pelvis and a bone scan showed no malignancy.
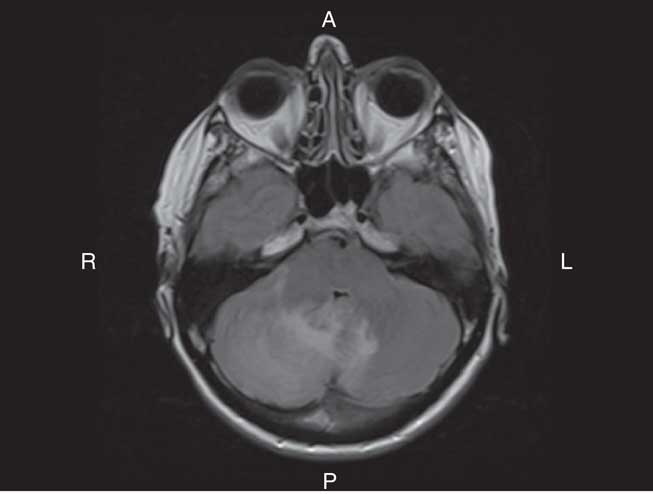
Figure 1 Axial T2 FLAIR MRI of the brain with Gadolinium showed hyperintensity in keeping with edema within the right lobe of the cerebellum. The edema extended across the vermis into the left cerebellar hemisphere. There was associated regional mass effect on the brainstem and effacement of the fourth ventricle. There was also linear enhancement along the folia of the cerebellum, which was in keeping with leptomeningeal enhancement. The leptomeningeal enhancement was greater on the right than the left.
A cerebellar biopsy on Day 5 revealed vasculitis and lymphocyte-predominant inflammatory infiltration of the meninges and grey and white matter (Figures 2A-2B). There were no granulomatous or necrotic changes. Gram, kinyoun, periodic acid-Schiff, and β-amyloid stains were negative.
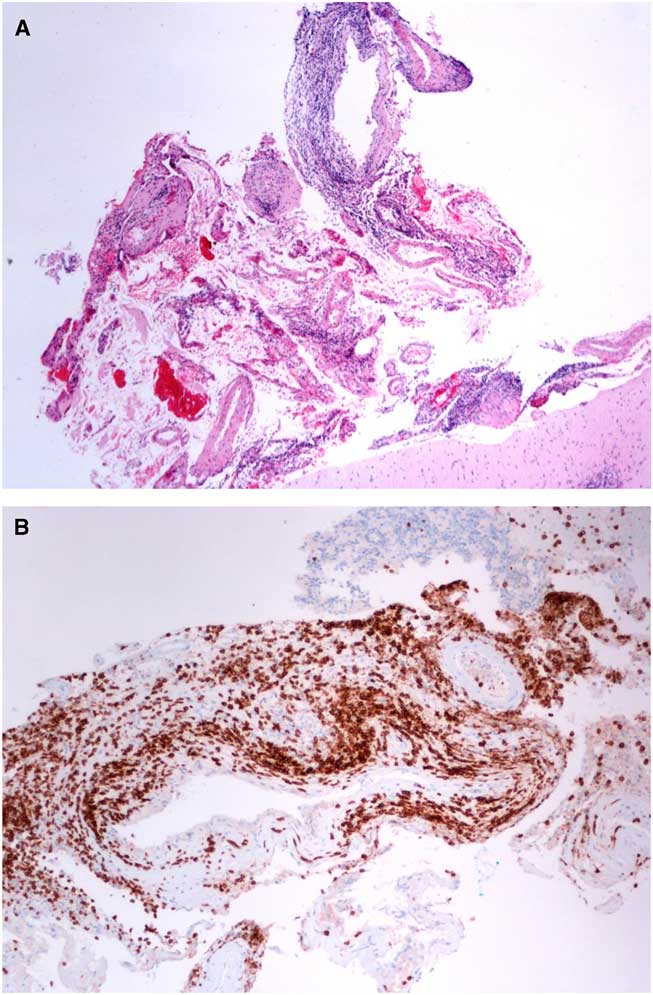
Figure 2 (A) Hematoxylin and eosin stain of the biopsy from the right cerebellum showed vasculitis. The vasculitic inflammatory infiltrate consisted mostly of lymphocytes, plasma cells, macrophages, and a rare eosinophil (4× magnification). (B) Immunohistochemistry confirmed that the inflammatory cells were predominantly CD3+ lymphocytes. They were mainly localized to the meninges and meningeal vasculature (10× magnification).
The patient was started on dexamethasone 4 mg per os (PO)/intravenous q8h. Cerebellar edema delayed a lumbar puncture, which was done on Day 13. CSF analysis showed normal glucose and protein, but 16×106/L white blood cells with 69% neutrophils, 29% lymphocytes, and 2% monocytes. CT angiography on Day 14 was normal.
She was diagnosed with PACNS. Her symptoms and elevated inflammatory markers resolved by Day 15. She was discharged home on prednisone 60 mg PO daily. During follow-up at 16 months, she had started taking methotrexate 15 mg PO weekly and tapering prednisone. She remained neurologically normal.
PACNS is an idiopathic disorder characterized by inflammation and destruction of blood vessels isolated to the CNS. It is extremely rare, with an estimated incidence of 2.4/1,000,000 person-years.Reference Salvarani, Brown and Calamia 1 Our patient was the first in NL to have biopsy-proven PACNS.
The etiology and pathogenesis of PACNS remain elusive. Proposed theories suggest a relationship with infection, an immune response to an antigen within cerebral vasculature, and an association with cerebral amyloid angiopathy.Reference Salvarani, Brown and Hunder 3 Our patient had no infectious symptoms and her biopsy was negative for amyloid staining.
Much of the uncertainty in diagnosing PACNS lies in its variable clinical manifestations. Most patients report an insidious onset; however, it may be acute. Common symptoms include headache, cognitive dysfunction, persistent neurologic deficits, or stroke. Unlike other vasculitides, constitutional symptoms are uncommon. Seizures and intracranial hemorrhage are considered rare, as are ataxia and “dizziness,” as seen in our patient.Reference Salvarani, Brown and Calamia 1 , Reference Salvarani, Brown and Christianson 2 , Reference De Boysson, Zuber and Naggara 4 Her 3-year history of mild headaches without neurological changes may have been an early manifestation of her disease and have not recurred since treatment.
The differential diagnosis for PACNS is broad. Infection was ruled out based on her negative cultures, brain biopsy, and response to steroids. Her presentation was not in keeping with reversible cerebral vasoconstriction syndrome. No malignancy was detected on imaging. Although lymphoma was considered, the biopsy negated it. Secondary CNS vasculitis was unlikely in the absence of rheumatologic symptoms and negative serology. Ultimately, PACNS was diagnosed based on her biopsy and the absence of another more likely diagnosis.
Although there are no highly specific or sensitive tests for PACNS, various investigations can support the diagnosis. The CBC, metabolic profile, autoinflammatory serology, and infectious and malignant workup are typically normal or negative. Acute phase reactants may be elevated. CSF usually shows lymphocytosis, elevated total protein, and normal glucose.Reference Salvarani, Brown and Hunder 3 , Reference Hajj-Ali, Singhal and Benseler 5 , Reference Hajj-Ali and Calabrese 6 Our patient’s CSF had neutrophilia, but this may be explained by her treatment with dexamethasone. The remainder of her biochemical investigations were normal.
A normal MRI can almost always exclude PACNS. In PACNS, it most often demonstrates ischemic infarctions. Meningeal enhancement occurs in 8% of cases. Gadolinium-enhancing lesions, as in our patient, are also more likely in small-vessel disease. The CTA may be normal or show nonspecific areas of stenosis and dilatation.Reference Hajj-Ali and Calabrese 6 Our patient’s CTA was negative, which could be due to isolated small vessel involvement or because she was already being treated with steroids. Conventional angiography was not pursued once the biopsy results had confirmed a diagnosis.
Meningeal and brain biopsy is the gold standard to confirm PACNS. It most often demonstrates granulomatous vasculitis, with 50% of these cases also having β4 amyloid deposition. Lymphocytic vasculitis, as with our patient, is the second most common histopathological pattern. Necrotizing vasculitis is least common.Reference Salvarani, Brown and Hunder 3 Isolated small-sized PACNS is more likely to be angiography-negative but biopsy-positive. Similarly, only our patient’s biopsy showing vasculitis and lymphocyte-predominant infiltration confirmed the diagnosis of PACNS despite negative angiography.
There are few studies examining optimal management of PACNS. The most recent and largest cohort study demonstrated favorable responses in the majority of patients treated with prednisone alone or prednisone plus cyclophosphamide. However, there was a higher relapse rate in the prednisone-only group.Reference Salvarani, Brown and Christianson 2 A poorer prognosis is associated with large vessel lesions and infarctions on MRI. Those with small vessel involvement, leptomeningeal enhancement, and lymphocytic instead of granulomatous and/or necrotizing vasculitis on histopathology have better outcomes. These patients may only require glucocorticoids for induction, with additional immunosuppressants reserved for relapses or maintenance.Reference Salvarani, Brown and Calamia 1 - Reference Salvarani, Brown and Hunder 3 Our patient had multiple favorable factors, including negative angiography, leptomeningeal enhancement, and lymphocytic vasculitis on biopsy. This could explain her rapid response to high-dose prednisone alone, with no relapse at 16 months while on maintenance methotrexate and tapering steroids.
Our patient demonstrates the diagnostic difficulty posed by patients with PACNS, especially when early symptoms may be only sporadic, self-limited headaches. PACNS should be considered in patients with unexplained neurological deficits and headache.
Statement of Authorship
Study concept and design: LC, ME, and NP; data acquisition and drafting of manuscript: LC; revising for content and study supervision: ME and NP.
Conflicts of Interest
None.