Introduction
Worldwide, over 35 million individuals are infected with HIV, of whom nearly 70% live in sub-Saharan Africa (SSA) (UNAIDS, 2017). In high-income countries (HICs), combination antiretroviral therapy (cART) has near-normalized life expectancy (Deeks et al., Reference Deeks, Lewin and Havlir2013). Consequently, people living with HIV (PLWH) are an aging population in which chronic complications of HIV are increasingly recognized (Deeks, Reference Deeks2011). Of these, HIV-associated neurocognitive disorders (HANDs) are highly prevalent (affecting 20–59% worldwide) (Habib et al., Reference Habib2013; Heaton et al., Reference Heaton2011; Negin et al., Reference Negin2012) and associated with higher mortality, morbidity, and significant disability (Deeks et al., Reference Deeks, Lewin and Havlir2013; Sevigny et al., Reference Sevigny2007).
HANDs are defined by current international consensus criteria as a spectrum of disorders including HIV-associated dementia (HAD), mild neurocognitive disorder (MND), and asymptomatic neurocognitive impairment (ANI) (Antinori et al., Reference Antinori2007). These criteria encompass the milder clinical phenotypes seen post-cART (Cysique and Brew, Reference Cysique and Brew2009; Heaton et al., Reference Heaton2011) which appears to ameliorate, but not prevent HAND (Gray et al., Reference Gray, Chrétien, Vallat-Decouvelaere and Scaravilli2003; Heaton et al., Reference Heaton2011) including HAD, which is now less common. Unlike neurodegenerative dementias, HIC studies suggest a high incidence, but a degree of reversibility (Valcour, Reference Valcour2013). The etiology of HAND is complex and poorly understood but includes opportunistic infections of the central nervous system (CNS), direct neurotoxic effect of the HIV virus, long-term CNS inflammatory processes, and neurotoxic effects of cART (Gray et al., Reference Gray, Chrétien, Vallat-Decouvelaere and Scaravilli2003). Older PLWH appear more vulnerable to HAND with up to 50% affected in HIC studies and high annual incidence rates seen (Deeks et al., Reference Deeks, Lewin and Havlir2013; Hardy and Vance, Reference Hardy and Vance2009). Additional hypothesized etiologies include accelerated Alzheimer’s disease and vascular changes (Saylor and Sacktor, Reference Saylor and Sacktor2016).
In SSA, as in HICs, increased cART coverage is resulting in a rapidly aging population of PLWH, with over half now treated (Negin and Cummings, Reference Negin and Cumming2010). Consequently, HIV prevalence is also increasing with an estimated 3 million additional PLWH seen in the last 5 years (Dwyer-Lindgren et al.., Reference Dwyer-Lindgren2019). Those aged ≥50 years will be disproportionally affected, with prevalence predicted to almost triple from 3.1 million to 9 million between 2011 and 2040, while prevalence in younger adults will decrease (Hontelez et al., Reference Hontelez2012). Despite the likely increase in the number of people with HAND in SSA, studies of the impact of HIV on older PLWH in SSA are very few and, to date, no epidemiological studies of HAND in older people in SSA have been published (Guerchet et al., Reference Guerchet2017). Existing studies focus on younger adults (mean age 29.5–42.7 years) and frequently exclude older PLWH (Habib et al., Reference Habib2013) or include populations not on cART. Therefore, these data may not be reflective of the current clinical picture (specifically, the pattern and severity) of HAND in older PLWH in SSA.
Although routine screening for neurocognitive disorders in PLWH is recommended in current UK and other guidelines (Angus and Awosusi, Reference Angus and Awosusi2016), this is not the case in SSA countries. Incidence of HAND is not frequently reported in SSA and to date has not been reported in older PLWH. Accurate measurement of HAND in older PLWH in SSA may allow healthcare resource planning for this growing and vulnerable age group, particularly given the shortage of human resources for mental health and chronic disease in SSA (Olayinka and Mbuyi, Reference Olayinka and Mbuyi2014).
The aim of this study was to estimate the point prevalence and subsequent 1-year incidence of HAND in individuals aged ≥50 years under regular HIV clinic follow-up according to standard guidelines in Tanzania.
Methods
Setting
This study took place at Mawenzi Regional Referral Hospital (MRRH) HIV Care and Treatment Centre (CTC), a free-of-charge Government HIV clinic in the Kilimanjaro region of Northern Tanzania (national HIV prevalence 3.9% [3.6–4.3%]) (Dwyer-Lindgren et al., Reference Dwyer-Lindgren2019). Kilimanjaro was a pioneer site for cART in Tanzania, and treatment has been available locally for over 15 years. HIV-specific patient outcome records are maintained from diagnosis and routine CD4 lymphocyte counts taken at diagnosis and annually. HIV viral load testing became available in 2017. A total of 3169 HIV-positive patients were listed within the CTC register on the 1 March 2016, of whom 820 (25.9%) were aged ≥50 years.
Ethics
The Kilimanjaro Christian Medical College Research Ethics and Review Committee (CRERC) and Tanzanian National Institute for Medical Research (NIMR) approved this study. Participants were given written and verbal information about the study before informed consent was requested. Where participants lacked capacity to consent due to cognitive impairment, consent was sought from a close relative. Consent was rechecked prior to follow-up assessments and the same process followed. Further informed consent was obtained to elicit collateral history of cognitive impairment and functional status from informants (usually one close family member, nominated by the participant) in person or on the phone. HIV status was not mentioned to informants, who were told this was a study of aging and cognitive function.
Sampling and baseline data collection
Baseline data collection took place daily over a 10-week period from March to June 2016. Previously diagnosed HIV-positive individuals aged ≥50 years registered with MRRH-CTC were systematically sampled at the point of attendance for routine follow-up appointments in strict order of arrival. Age and CTC file number were cross-checked against patients’ handheld clinic registration cards. Depending on expected patient numbers, CTC capacity, and availability of clinical rooms, a decision to approach every second or third individual was made prior to commencement of each day, in collaboration with the senior nurse in charge of the CTC. Individuals were assessed in rooms within or directly adjoining the CTC before or after their CTC appointment after discussion with them and the senior nurse on shift.
Exclusions were those attending for emergency appointments, or where in the opinion of the study doctor, they were too unwell to take part, or participation would delay necessary treatment (see Figure 1). Where sampled individuals could not take part, the subsequent individual was selected. Collected baseline demographic data included age, gender, occupation, educational background, current employment, and living arrangements. HIV-related data obtained from clinic records included date of diagnosis, most recent and nadir CD4 count, WHO and US Centres for Disease Control and Prevention (CDC) HIV stage (determined through case note review), history of tuberculosis, pneumocystis pneumonia (PCP) prophylaxis, empirical treatment for CNS infection, cART regimen, self-reported medication adherence, and body mass index (BMI).
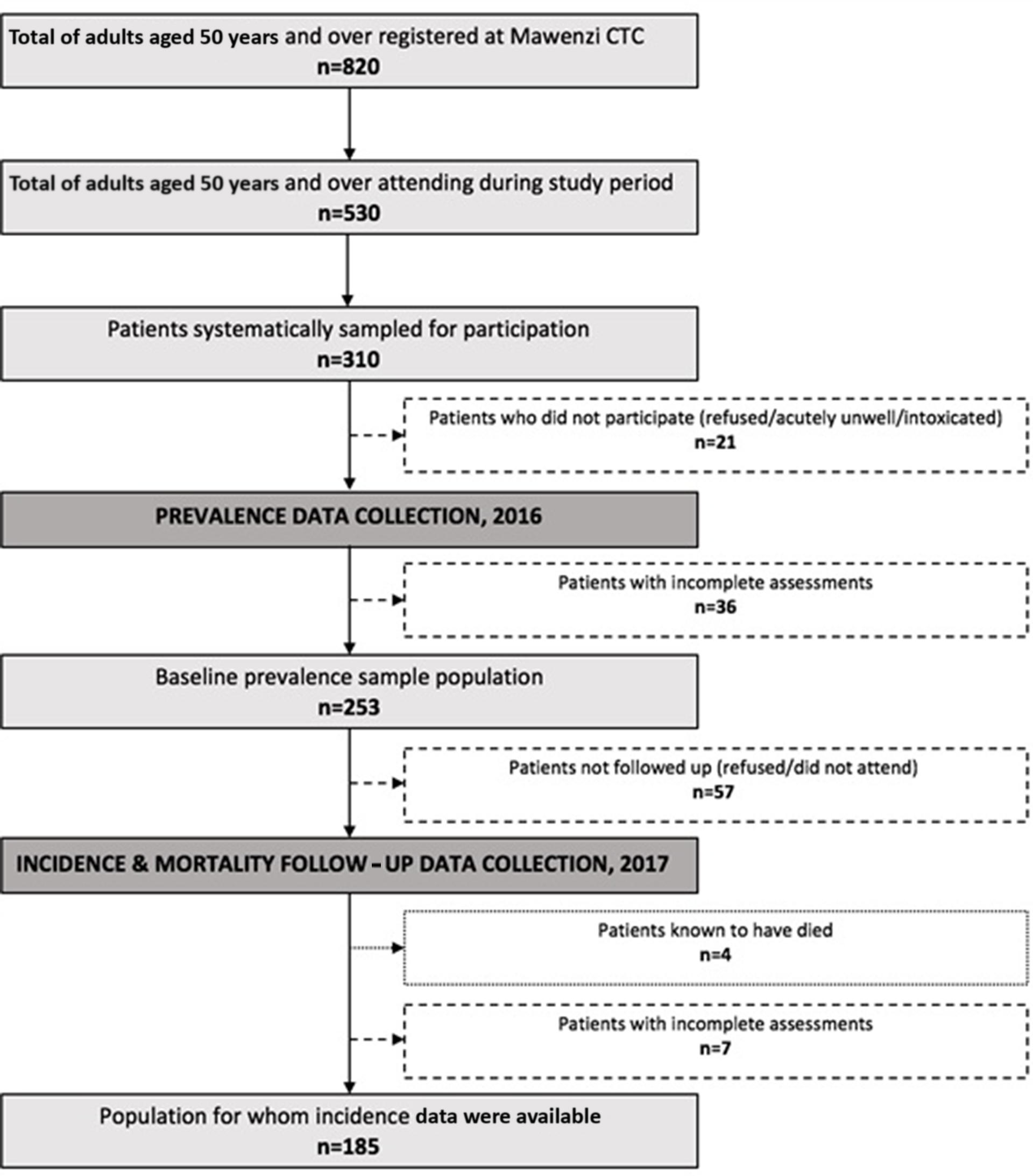
Figure 1. HIV prevalence incidence.
Assessment for HAND
All participants underwent a standardized assessment for HAND at baseline and follow-up within the CTC during their visit.
Cognitive assessment: Comprehensive neurocognitive assessment was based on the American Academy of Neurology (AAN) 2007 HAND criteria. This included a locally normed, detailed neuropsychological battery based upon that used in the original WHO cross-sectional studies of HAD conducted in SSA in the pre-cART era (Maj et al., Reference Maj1993; Maj et al., Reference Maj1991; Maj et al., Reference Maj1994) and designed for lower-literacy settings. Tests included the WHO-UCLA Auditory Verbal Learning Test (AVLT), Colour Trails I and II, Grooved Pegboard test, and forward/backward digit span. To account for the increased cortical, as well as subcortical, cognitive deficits observed in the post-cART era (Cysique and Brew, Reference Cysique and Brew2009; Hardy and Vance, Reference Hardy and Vance2009), additional lower-literacy tests prioritizing cortical function were incorporated. These included tests of visuo-construction (matchstick construction), categorical verbal fluency, alongside comprehension, and orientation selected from a locally validated ADAS-Cog adaptation (Paddick et al., Reference Paddick2017). Gross motor function (timed walk) was taken into account but not used as a core HAND criterion due to possible confounding effect of physical disabilities in this older cohort (for details of full battery, see supplementary data file 1).
Definition of cognitive impairment
Neuropsychological testing was completed by locally trained specialist nurses (AK and JR). Following AAN criteria, cognitive impairment was defined as performance one or two standard deviations below the mean in two cognitive domains (Antinori et al., Reference Antinori2007). Norms were derived from a comparison group of 85 individuals (outpatient MRRH eye clinic attendees), self-reported HIV-negative categorized into age bands 50–59 and 60 and over, with and without 4 years or more of formal education (elementary school).
Other assessments: Local translations of the Mini International Neuropsychiatric Interview (MINI) and 15-item Geriatric Depression Scale (GDS) were used to screen for psychiatric disorders. Subjective cognitive and neurological symptoms were assessed through self-report. Further clinical assessment with a research doctor (CI, JM, TL, JT, LH, and VY) aimed to confirm HAND diagnosis or determine if other psychiatric disorders better explained cognitive performance. Assessments included a standardized mental state examination and detailed bedside cognitive and neurological examination to identify other psychiatric disorders (i.e. psychosis). A structured collateral history to corroborate cognitive and functional impairment was obtained from a close informant for each participant and included a locally validated instrumental activities of daily living scale (Collingwood et al., Reference Collingwood2014). Where necessary, this took place by telephone and three attempts were made to contact each informant.
Delirium was screened using the Confusion Assessment Method (CAM), previously validated locally (Paddick et al., Reference Paddick2018) due to the possibility of ambulant delirium in rural low-resource settings and resolving delirium following acute illness. Visual acuity was assessed using a Landholt C broken-ring low-literacy logmar distance chart as a confounder for neuropsychological test performance. All clinical and neuropsychological information and multidisciplinary discussion were combined in a detailed case summary. HAND diagnosis by 2007 AAN criteria was made by consensus panel including old-age psychiatry and neurology specialists (EML, RA, SMP, and TL). Additional psychiatric diagnoses (DSM-IV criteria) were made where sufficient clinical information permitted, and individuals were classified as HAND only where this was felt to be clearly identifiable based on AAN criteria independent of comorbidities. Individuals with psychiatric comorbidities (i.e. depression) by DSM-IV criteria were included to increase generalizability and to comprehensively describe this cohort. Similarly, where individuals met criteria for HAND and other cognitive impairment (e.g. alcohol, vascular cognitive impairment), primary and secondary diagnoses were specified. Full assessment took up to 100 minutes. Cognitive testing (∼60 minutes) was generally completed before other assessments to minimize fatigue and refreshments were provided.
Data collection at 1-year follow-up
One-year follow-up assessment (February to June 2017) was identical to baseline with the addition of HIV viral load measurement, where this became available locally. All individuals assessed in 2016 were offered follow-up alongside existing clinic appointments with the exception of those listed as deceased or transferred to other regions in the CTC records. Those not attending on the expected day were invited to attend on a convenient alternative day and transport refunded. Three attempts were made to contact participants using previously recorded contact details. In addition, all individuals arriving at the clinic and the daily clinic lists were checked daily for the presence of participants assessed in 2016. Those not attending during the study period were cross-checked against clinic lists of individuals transferred, lost to follow-up, or deceased. Lists of patients known to have transferred to other clinics, died, or lost to follow-up were subsequently rechecked against hospital records in 2019 to determine last known clinic appointment date and to confirm whether those not seen during the study period did in fact attend a subsequent standard clinic appointment.
Statistical methods
Data analysis was supported by IBM SPSS (version 23; IBM, Armonk, NY, USA). Prevalence data are summarized as frequencies and incidence and mortality data as rates. Confidence intervals were calculated based on binomial (prevalence) and Poisson (incidence and mortality) distributions. Standard descriptive statistics (e.g. mean, median, standard deviation (SD), interquartile range (IQR), and frequency) and inferential tests (e.g. chi-squared, Mann–Whitney U, and t-test) were used depending on the level and distribution of the data. Statistical significance was set at 5% and two-tailed tests were used for all inferential analyses.
Results
During the baseline study period, 530 of 820 registered patients aged ≥50 years attended the clinic. Of these, 310 were systematically sampled, of whom 253 met inclusion criteria, consented, and had sufficient data available to allow a formal neurocognitive diagnosis to be assigned (for exclusions see Figure 1).
Over two-thirds (72.3%) were female, with a median age of 57 years (50–79, IQR 53–61.5) and a mean of 7.1 years since diagnosis (range 0.7–23.94). The majority (n = 218 (86.2%)) continued in employment and 40/250 (16%) considered themselves illiterate. HIV disease appeared well controlled (95.5% on cART, median BMI 21.8 (IQR 19.3 to 25.5), mean CD4 516 (98-1719)). Only 25 (9.9%) received second-line treatment (indicating prior treatment failure). Demographic and HIV-specific data on the baseline sample are summarized in Table 1 including the standard first- and second-line regimens prescribed.
Table 1. Characteristics of the baseline cohort (n = 253)
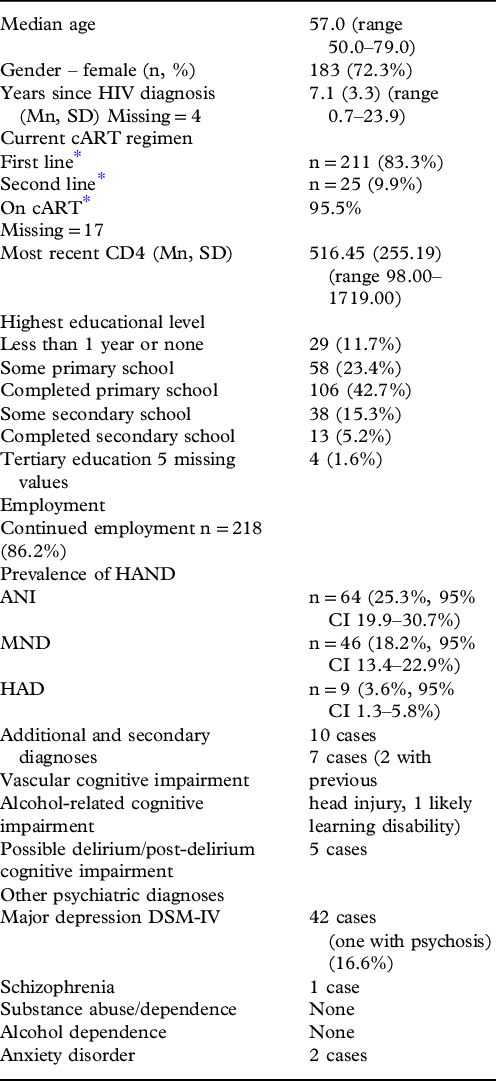
* First-line regimens (NRTI x2 + NNRTI efavirenz/nevirapine) 1 g-A (TDF, 3TC, EFV) 1b-A (AZT, 3TC, NVP/ABC, 3TC, LPV/r), 1c-A(AZT, 3TC, EFV), 1e-A (TDF, FTC, EFV) 1f-A (TDF,FTC,NVP), 1 h-A (TDF, 3TC, NVP), 1 k-A (ABC, 3TC, EFV), 1 m-A (ABC, 3TC, NVP), 1a-A (d4T, 3TC, NVP/d4T, 3TC, EFV), 1x-A (other first-line unspecified)
Second-line regimens (NRTI x2 + protease Inhibitor (PI) 2f-A (TDF, FTC, LPV/r), 2 h-A (TDF,FTC,ATV/r), 2s-A (AZT,3TC,ATC/r), 2 g-A (ABC,3TC,LPV/r), 2e-A (TDF, 3TC, LPV/r), 2 k-A (ABC/3TC, ATV/r), 2 m-A (TDF, 3TC, ATV/r), 2 n-A (AZT, 3TC, LPV/r/AZT, 3TC, EFV), 2x-A (other second-line unspecified)
Guidelines at baseline study indicated cART should commence at CD4≤350 or WHO stage 4 (NACP, 2009).
Self-reported cART adherence was good (only 43/253, 16.9%, admitted ever forgetting cART), and no participant met criteria for alcohol or drug dependence or recreational/illicit drug abuse.
Prevalence of HAND and cognitive impairment
At baseline, the prevalence of HAND was 47.0% (95% CI 40.9–53.2, n = 119). Of these, 64 (25.3%, 95% CI 19.9–30.7%) were ANI, 46 MND (18.2%, 95% CI 13.4–22.9%), and 9 HAD (3.6%, 95% CI 1.3–5.8%). The demographic and disease profile of those with and without HAND are shown in Table 2. The total number with HAND and non-HAND cognitive impairment was 137 (prevalence 54.2%, 95% CI 48.0–60.3). In addition to HAND (n = 119), this included vascular cognitive impairment (n = 9; one coexistent with MND), alcohol-related cognitive impairment (n = 7 (two with previous significant head injury); one coexistent with ANI and one coexistent with HAD), delirium or post-delirium cognitive impairment (n = 5), and one case of post-stroke cognitive impairment.
Table 2. Association of HAND with demographic and disease characteristics

Other non-HAND psychiatric diagnoses potentially affecting cognition were DSM-IV major depression (n = 42, 16.6%), schizophrenia (n = 1), and anxiety disorder (n = 2).
One-year mortality
At follow-up, four people were known to have died from those who could be traced (n = 196) (see Figure 1), a 1-year mortality rate of 2.0% (95% CI 0.5 to 5.2).
One-year incidence of HAND and cognitive impairment
One-year follow-up data were collected on 185 (73.1%) of those seen at baseline. Reasons for non-follow-up are detailed in Figure 1. Of those not assessed or recorded to have died, 6 were listed as lost to follow-up by the clinic, 3 transferred to other named health centers, and 11 files could not be traced. All other participants not undergoing follow-up assessment during the study period remained under active CTC follow-up evidenced by recorded clinic appointment attendance during 2017.
The diagnoses of the 185 people seen in both 2016 and 2017 are presented in Table 3 to illustrate the stability or otherwise of these diagnoses. At follow-up, 110 of the 185 seen (prevalence 59.5%, 95% CI 52.4–66.5) met HAND criteria. Those who were and were not followed up did not differ in age, gender, employment status at baseline, or age at diagnosis.
Table 3. Comparison of cognitive diagnoses of those fully assessed at baseline and at follow-up
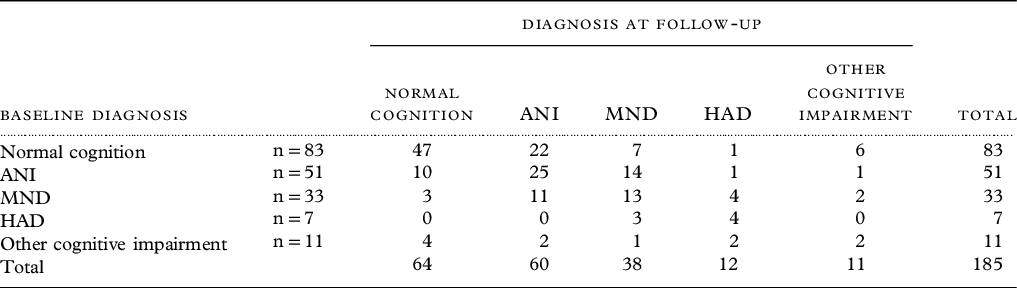
Thirty-five people who were cognitively normal at baseline met HAND criteria at 1 year, a 1-year incidence from those at risk of 37.2% (95% CI 25.9 to 51.8). Conversely, 16 PLWH with HAND at baseline no longer met criteria at follow-up (incidence of reversal 17.6%, 95% CI 10.0–28.6). For any cognitive impairment, of 83 at risk, 36 met criteria for cognitive impairment at follow-up, an incidence of 43.4% (95% CI 30.4–60.0).
Discussion
This is the first study of the epidemiology of HAND by 2007 AAN (Frascati) criteria in older PLWH receiving long-term HIV follow-up and treatment in SSA.
Almost half of our cohort met HAND criteria at baseline. Overall, the pattern of HAND identified in this cohort, with milder forms of HAND predominant, mirrors existing HIC studies using the 2007 criteria (Heaton et al., Reference Heaton2010; Sacktor et al., Reference Sacktor2016). Few comparable epidemiological studies of HAND in older PLWH exist and comparisons are challenging as demographic and HIV-specific data vary between settings.
This was a cohort where almost all were receiving cART and are therefore difficult to compare to older studies. The 2001 US Hawaii Ageing with HIV study (Valcour, Reference Valcour2013) reported a higher prevalence (25.2% HAD, 44% “minor cognitive motor disorder” [MCMD]) in PLWH aged ≥50 years, with similar severity of HIV disease (md CD4 >500, 80% cART [reflecting previous treatment guidelines]) using the older (1991) AAN criteria. Similarly, a younger Ugandan cohort (60% cART) reported an “HIV dementia” rate of 31% using the Memorial Sloane Kettering criteria (Robertson et al., Reference Robertson2007). Both these diagnostic criteria were developed for the more severe neurological impairment observed pre-CART and, although similar, are not directly comparable (Gandhi et al., Reference Gandhi2010). A recent Ugandan study (mean age 35 years) reported higher prevalence (56%) using similar neurocognitive tests (Sacktor et al., Reference Sacktor2019). These cohorts also included high proportions of untreated PLWH, and those with low CD4 counts, arguably no longer representative of HIC or many SSA settings.
We report HAND prevalence substantially higher than in the Multicenter Aids Cohort Study (MACS), a US cohort of gay and bisexual men where immunological status was similar (Mn CD4 589 cells/ml vs. 516, undetectable viral load 70 vs. 68%), but the full cohort were substantially younger (mn aged 47 vs. 57 years in Kilimanjaro), despite having a longer reported illness duration. Of the subset aged ≥50 years, 28 % met HAND criteria in 2012 (Sacktor et al., Reference Sacktor2016). Potential reasons for our increased reported prevalence include older age, demographic, and HIV-specific differences including a high prevalence of illiteracy, long duration of untreated infection, and our inclusion of individuals with comorbid conditions.
We found that HAND prevalence significantly increased with age. Existing (limited) HIC data report higher HAD prevalence in older PLWH and in older versus younger PLWH, controlling for HIV disease severity and comorbidities (OR 3.26 [1.32 to 8.07]) (Valcour et al., Reference Valcour2004). Potential mechanisms include accelerated Alzheimer’s pathology and cerebrovascular disease (Saylor and Sacktor, Reference Saylor and Sacktor2016). HIV infection increases risk of stroke, particularly in older PLWH (Martin-Iguacel et al., Reference Martin-Iguacel, Llibre and Friis-Moller2015), increasing risk of cognitive impairment. Since both stroke and hypertension are highly prevalent, underdiagnosed, and undertreated in SSA and Tanzania (Dewhurst et al., Reference Dewhurst, Dewhurst, Gray, Chaote, Orega and Walker2013; Kayima, et al., Reference Kayima, Wanyenze, Katamba, Leontsini and Nuwaha2013), this may represent another vulnerability to HAND in this setting.
Other demographic and HIV-specific differences in HAND vulnerability may be present in SSA compared to HICs. Both the MACS and the 2001 US Hawaii cohort were markedly demographically different from ours (US Hawaii 91% male, Caucasian/Asian, 74% identifying as men who have sex with men [MSM, MACS 100 male, MSM]). In SSA, HIV heterosexual transmission is most common and intravenous drug use transmission is much lower than in HICs (Avert, 2019). Similarly, hepatitis C co-infection (hypothesized to exacerbate neuronal damage and increase risk of HAND) (Vivithanaporn et al., Reference Vivithanaporn, Nelles, DeBlock, Newman, Gill and Power2012) appears low (0.6%) in PLWH in Tanzania (Platt et al., Reference Platt2016). HIV clade may confer differing vulnerability to cognitive impairment and differs between HICs and SSA (Sacktor et al., Reference Sacktor2009). Cognitive reserve, well evidenced to reduce risk of neurodegenerative dementias, may reduce risk of HAND (Foley et al., Reference Foley, Ettenhofer, Kim, Behdin, Castellon and Hinkin2012). In this cohort, only 5.2% had completed secondary school and 16% were illiterate. In contrast, 46% of MACs study participants had a college degree.
Differing cART treatment protocols may have impacted upon prevalence. Current Tanzanian guidelines (2017) state that all PLWH receive cART (NACP, 2017), whereas previously treatment was initiated when CD4 fell to ≤350–200 (NACP, 2009). A longer duration of untreated infection may have increased vulnerability to HAND in this cohort, where the median nadir CD4 was 165, well below previous treatment initiation guidelines.
Comorbid conditions are well known to complicate HAND, and the effect of these may be more pronounced in older PLWH (Milanini et al. Reference Milanini2017; Negin et al., Reference Negin2012; Saylor and Sacktor, Reference Saylor and Sacktor2016). We found a high rate (n = 18) of individuals with non-HAND cognitive disorders in addition to those with psychiatric comorbidities. Many existing studies exclude those with comorbidities as potential confounders and often report lower HAND prevalence (Sacktor et al., Reference Sacktor2016), but this may reduce generalizability to non-research clinical settings. Depression in HIV may represent progressing HIV-related brain injury as well as having a potential effect on cognitive performance (De Francesco et al., Reference De Francesco2016). It may be that pure “HAND” due only to HIV without other contributory conditions and comorbidities is relatively uncommon in older PLWH in generalized clinical settings in SSA.
Comparisons with existing SSA data are challenging since no other studies of older adult populations exist and most rely on cognitive screening rather than clinical diagnosis. A meta-analysis of SSA prevalence studies reported a pooled “HIV-dementia” rate of 30% (Habib et al., Reference Habib2013) in all age groups based on the International HIV Dementia Scale (IHDS). The IHDS is a brief, SSA-specific screening tool for “HIV dementia” and is now of limited diagnostic accuracy in milder HAND seen post-cART (Milanini et al., Reference Milanini2018). Similarly, other SSA studies report varying prevalence (24%–84%) across different clinical settings with varying cART availability and diagnostic criteria (Habib et al., Reference Habib2013). As such it is difficult to compare our findings to earlier work. The use of the 2007 criteria, in a stable cohort on cART managed according to local guidelines, allows meaningful comparison to existing post-cART and HIC studies.
Incidence and reversibility
Few studies of HAND incidence or progression have been published, particularly in older adults who may have a higher rate of progression. The US MACS study reported that 15% declined in HAND category over two waves of follow-up, but that overall HAND prevalence remained stable at 28% in those aged 50+ years, in contrast to the high rate of incidence observed in our study. Comparisons are challenging, however, as the MACS excluded those with comorbid conditions (Sacktor et al., Reference Sacktor2016).
HANDs are well recognized to fluctuate and show reversibility, compared to neurodegenerative dementias (Saloner and Cysique, Reference Saloner and Cysique2017), with a 10% improvement in HAND category (ANI/MND/HAD) reported in the MACS cohort (Sacktor et al., Reference Sacktor2016). We found a much higher (20%) improvement rate from MND or HAD to ANI and in 17% HAND resolved. This may be in part because of the short duration of follow-up (1 year) as evidence suggests fluctuations in HAND may reduce over time in stable treated populations (Saloner and Cysique, Reference Saloner and Cysique2017), but we cannot draw firm conclusions based on our existing data, particularly given the high rate of comorbid conditions such as depression and vascular impairment, improvement in which may have led to cognitive improvement also. It is notable that the median time on cART was 74 months, suggesting that recent cART initiation was not the primary driver of the reversibility observed.
The high incidence and prevalence seen may reflect suboptimal HIV disease control. Although this older PLWH cohort appeared well controlled at baseline (based on CD4 and BMI), at follow-up, 32% had a detectable HIV viral load despite high self-reported adherence. HIV viral non-suppression is likely to have been similarly high at baseline, but not measurable. HIC studies report a similar rate of non-suppression (Sacktor et al., Reference Sacktor2016). Suboptimal control may also have resulted in the higher-than-expected individuals with HAD and/or possible delirium. Since some (but not all) HIC studies of older PLWH with completely suppressed viral load (and without comorbidities) show minimal cerebral atrophy compared to matched HIV-negative controls (Haynes et al., Reference Haynes2018; Underwood, Reference Underwood2015), this may represent a potentially modifiable HAND risk factor, given that routine HIV viral load measurement is now available. This issue appears equally applicable to HIC settings.
The Frascati criteria have been viewed as overinclusive, leading to overestimates of HAND prevalence largely due to inclusion of ANI. However, ANI is well known to progress, and between 2 and 6 times increased risk of progression to symptomatic HAND (MND and HAD) is seen in large HIC studies (Grant et al., Reference Grant, Franklin, Deutsch, Woods, Vaida and Ellis2014; Heaton et al., Reference Heaton2015). ANI was highly prevalent in our cohort (25%) and of those seen for follow-up (n = 51), 16 progressed and 10 improved.
Limitations
Due to resource limitations, we were unable to carry out investigations such as CD4 count or HIV viral load and were reliant on measurements taken during routine clinical practice. Since CD4 was completed annually, the available measures may have been up to 12 months old and not an accurate reflection of the level at the time of assessment. Likewise, we were unable to test for HIV clade or comorbidities (e.g. hepatitis B and C infection) which would have assisted us in understanding to what extent this cohort differed from those previously reported in HICs. Although the detail available from documentation of history and examination was relatively extensive, it is possible that HAND and other cognitive impairments were under- or over-estimated. Neuroimaging was not locally available, and local policy was for empirical treatment of likely CNS infection. Likewise, diagnosis of vascular cognitive impairment relied upon self-report and/or clinical examination findings only. Neuroimaging would have been useful in differentiating HAND from other cognitive impairment. Likewise, though we recorded those treated for TB, we were unable to confirm where treatment had been successful. As was standard in the CTC records, in this study cART adherence was self-reported and likely to be less accurate than objective methods such as pill counts. Education and literacy status were self-reported and in a small number of cases may have been inaccurate.
It is possible that some individuals in the comparison group for cognitive testing were not HIV-negative since we relied on self-report. However, the prevalence of HIV in older adults in Tanzania remains low overall, and tests are increasingly offered as standard in people accessing general health services, so the likelihood of a substantial proportion having unknown HIV in this age group is currently low. This is, however, an acknowledged limitation of our methodology.
We were only able to follow up 73% of the baseline cohort at 1 year, resulting in wide confidence intervals for incidence which must therefore be interpreted with caution. Although those seen and not seen did not differ in age, employment status, literacy, gender, or years since diagnosis, it is still possible that the data for those lost to follow up were not missing completely at random. The follow-up coincided with unusually severe weather conditions in Tanzania and the season of planting crops. Those not attending follow-up may have been either those with agricultural responsibilities and well enough to undertake that work or living more rurally and therefore affected by road closures and/or lack of transport availability. This could have led to over- or under-estimation of incidence. We did confirm from hospital records that individuals not seen at 1 year were not necessarily lost to follow-up by the clinic. However, our data on mortality and disease progression should be viewed in this context and interpreted cautiously.
Conclusions
This is the first study of prevalence and incidence of HAND, in older cART-treated PLWH managed according to local guidelines in SSA. Despite apparently well-controlled disease, both prevalence and incidence were high, and the underlying reasons for this require further exploration. The high burden of comorbid conditions observed suggests that “pure” HAND may be uncommon in routine practice in this setting. This older cohort, demographically typical of PLWH in Tanzania, clearly differs markedly to existing cohorts reported in HICs. There are likely to be differing vulnerabilities to HAND that are not currently understood.
Our data strongly suggest that cART and regular clinic follow-up are insufficient to prevent occurrence or progression of HAND in this setting. Further work is urgently needed to identify those most at risk and to consider potential preventative strategies. It is reassuring that a degree of reversibility and clinical improvement was seen, and future work should focus on identification of factors associated with improvement. The degree to which comorbid conditions contribute to the clinical picture of “HAND” observed will be important to clarify as these may be potentially reversible etiologies.
Use of the AAN criteria for HAND requires detailed neuropsychological assessment which is useful in a research context for comparability to other studies but is not practical in resource-limited clinical settings. There is a need for measures and/or biomarkers for HAND in older PLWH in this setting and these are currently lacking.
Conflict of interest
None.
Source of funding
This study was part-funded by Grand Challenges Canada (grant number 0086-04) and Newcastle University Masters in Research Programme.
Acknowledgments
We would like to thank the patients and family members who took part in this study. We also appreciate the help of the entire clinical staff team and volunteers of the Mawenzi Regional Referral Hospital Care and Treatment Centre (CTC) and of the hospital management in enabling the smooth running of the study.
Author contributions
SMP, WKG, RW, CD, EBM-L, MD, WH, and SU led on the study conception and design. SMP supervised acquisition of data and trained the team. TL, AK, JR, LH, MY, JM, VY, CI, PM, A-LQ, JT, TG, AF, PE, and JK-W developed different aspects of the protocol and collected data. Consensus panel diagnoses were completed by EBM-L, RA, TL, and SMP. SMP, WKG, TG, and AF performed data analysis and interpretation. TG and AF completed the first draft, supervised by SMP. All authors were involved in drafting and revising the manuscript and gave their approval of the version submitted for publication.
Supplementary material
To view supplementary material for this paper, please visit https://doi.org/10.1017/S1041610221000156





