Introduction
“It has been asserted almost universally that trauma may cause epilepsy; I have never been able to understand why.”
A. Kinnier Wilson (1923)
The above quotation from a giant in clinical neurology appeared as an epigraph to Jennet’s classic “Epilepsy after non-missile head injuries.”Reference Jennet 1 Over the near half-century since the publication of Jennet’s book, much has been learned about the epidemiology of traumatic brain injury (TBI) and the risk of post-traumatic epilepsy. Specifically, TBI is now accepted as the cause of 3%-9% of all epilepsy cases.Reference Annegers, Grabow, Groover, Laws, Elveback and Kurland 2 - Reference Mahler, Carlsson, Andersson, Adelöw, Ahlbom and Tomson 5 It is also accepted that the risk of post-traumatic epilepsy is highest after severe TBI with intracranial hemorrhage and/or prolonged (e.g., >24 hours) loss of consciousness, and less elevated after moderate TBI with loss of consciousness 0.5-24 hours and Glasgow Coma Scale (GCS) 9-12 more than an hour post-injury.Reference Annegers, Grabow, Groover, Laws, Elveback and Kurland 2 , Reference Annegers, Hauser, Coan and Rocca 3 , Reference Mahler, Carlsson, Andersson, Adelöw, Ahlbom and Tomson 5 - Reference Christensen, Pedersen, Pedersen, Sidenius, Olsen and Vestergaard 7 Furthermore, it has been shown that the risk for post-traumatic seizures and epilepsy is highest during the first few years post-injury and in people with a family history of epilepsy.Reference Annegers, Grabow, Groover, Laws, Elveback and Kurland 2 , Reference Annegers, Hauser, Coan and Rocca 3 , Reference Mahler, Carlsson, Andersson, Adelöw, Ahlbom and Tomson 5 , Reference Christensen, Pedersen, Pedersen, Sidenius, Olsen and Vestergaard 7 , Reference Shorvon and Neligan 8
Mild traumatic brain injury (mTBI), or concussion, is an order of magnitude more common than moderate and severe TBI, with an annual incidence of at least 2-6/1000 individuals.Reference Cassidy, Carroll and Peloso 9 - Reference Nguyen, Fiest and McChesney 12 It has been estimated that sports-related concussions alone affect more than a million people annually in North America,Reference Langlois, Rutland-Brown and Wald 10 and interest in the acute and chronic effects of concussions, especially as related to sports, has burgeoned over the past two decades.Reference Aubry, Cantu and Dvorak 13 - Reference Tator 16 Terminologically, mTBI and concussion are typically used interchangeably,Reference McCrory, Meeuwisse and Aubry 14 , Reference McCrory, Meeuwisse and Dvorak 15 although discussants at the 5th International Conference on Concussion in Sport considered the possibility that concussion might represent a lesser degree of injury within the mTBI category, at the lowest end of a TBI spectrum from mild to severe.Reference McCrory, Meeuwisse and Dvorak 15 Notwithstanding, there are no currently accepted criteria differentiating concussion from mTBI, and the two terms will thus be used synonymously in this paper to indicate a brain injury induced by biomechanical forces that “results in a range of clinical signs and symptoms that may or may not involve loss of consciousness,” typically with a “rapid onset of short-lived impairment of neurological function that resolves spontaneously,” and “no abnormality … on standard structural neuroimaging studies.”Reference McCrory, Meeuwisse and Dvorak 15 Post-concussion symptoms usually resolve within 10-14 days in adults, and within 1 month in children, but in a minority of cases there may be persistent sequelae lasting months or even years.Reference McCrory, Meeuwisse and Dvorak 15 , Reference Hiploylee, Dufort and Davis 17 There is conflicting evidence as to whether post-traumatic epilepsy may be one of the sequelae of concussion/mTBI.
In the concussion literature, post-traumatic epilepsy has rarely been mentioned as a complication of concussion/mTBI.Reference Tator 16 , Reference Herring, Cantu and Guskiewicz 18 However, in the epilepsy literature mTBI is reported to carry an approximately twofold increased risk for epilepsy, with a sixfold increased incidence in those with a family history of epilepsy.Reference Annegers, Hauser, Coan and Rocca 3 , Reference Mahler, Carlsson, Andersson, Adelöw, Ahlbom and Tomson 5 , Reference Christensen, Pedersen, Pedersen, Sidenius, Olsen and Vestergaard 7 , Reference Annegers and Coan 19 , Reference Hung, Carroll and Cancelliere 20 Some studies reported the increased risk to persist more than 10 years after mTBI,Reference Mahler, Carlsson, Andersson, Adelöw, Ahlbom and Tomson 5 , Reference Christensen, Pedersen, Pedersen, Sidenius, Olsen and Vestergaard 7 , Reference Hung, Carroll and Cancelliere 20 however, other studies found no increased risk beyond 5 years.Reference Annegers, Grabow, Groover, Laws, Elveback and Kurland 2 , Reference Annegers, Hauser, Coan and Rocca 3 , Reference Annegers and Coan 19 In the broader epilepsy community, despite the epidemiologic literature, there is evidence of uncertainty regarding the relation between concussion/mTBI and epilepsy. For example, the public information section of the Epilepsy Foundation website states: “Mild head injuries, such as a concussion with just a very brief loss of consciousness, do not cause epilepsy. Yet the effects of repeated mild head injuries and epilepsy is unknown.” 21
The existence of different perspectives in the concussion and epilepsy literature, discrepancies in the epidemiologic literature, and ambiguity in the epilepsy community regarding the clinical validity of the epidemiologic data raises the question of possible confounding issues. Four categories of potential confounds merit consideration at the introductory stage of any investigation into the risk of epilepsy after mTBI/concussion.
Accuracy of diagnosis and classification of head injury severity
One of the problems affecting the epidemiologic studies cited above is a lack of diagnostic accuracy regarding the severity of head injury in the populations studied. The studies of cases identified through the Medical Records Linkage System of the Rochester Epidemiology Project at the Mayo ClinicReference Annegers, Grabow, Groover, Laws, Elveback and Kurland 2 , Reference Annegers, Hauser, Coan and Rocca 3 , Reference Annegers and Coan 19 included both hospitalized and non-hospitalized patients as well as clinical information on the circumstances and characteristics of the TBIs.Reference Annegers, Hauser, Coan and Rocca 3 Those studies were more likely able to reliably separate mild and moderate and severe TBI cases than the studies based entirely on registry data sets using International Classification of Diseases (ICD) codes for TBI classification, obtained principally in hospitalized patients.Reference Mahler, Carlsson, Andersson, Adelöw, Ahlbom and Tomson 5 , Reference Christensen, Pedersen, Pedersen, Sidenius, Olsen and Vestergaard 7 International Classification of Diseases codes are developed primarily for administrative purposes, and are not ideal for use in epidemiologic TBI studies,Reference Shorvon and Neligan 8 , Reference Nguyen, Fiest and McChesney 12 , Reference Pickett, Simpson and Brison 22 particularly for classification of mTBI.Reference Bazarian, Veazie, Mookerjee and Lerner 23 , Reference Carroll, Cochran, Guse and Wang 24 Another problem is that most patients with concussion/mTBI do not present to hospital and are thus not included in registry data sets limited to hospitalized patients.Reference Mahler, Carlsson, Andersson, Adelöw, Ahlbom and Tomson 5 , Reference Christensen, Pedersen, Pedersen, Sidenius, Olsen and Vestergaard 7 , Reference Nguyen, Fiest and McChesney 12 The historical lack of a universal system for diagnosis and classification of TBI has also contributed to heterogeneity in TBI classification in previous studies.Reference Nguyen, Fiest and McChesney 12 , Reference Carroll, Cassidy, Holm, Kraus and Coronado 25 - Reference Menon, Schwab, Wright and Maas 27
Accuracy of diagnosis of epilepsy and classification of seizures
As with the diagnosis and classification of TBI severity, the diagnosis of epilepsy from ICD codes in registry data setsReference Christensen, Pedersen, Pedersen, Sidenius, Olsen and Vestergaard 7 is prone to inaccuracy. However, this problem was avoided in some studies by methods that included detailed review of the medical records of identified cases.Reference Annegers, Grabow, Groover, Laws, Elveback and Kurland 2 , Reference Annegers, Hauser, Coan and Rocca 3 , Reference Mahler, Carlsson, Andersson, Adelöw, Ahlbom and Tomson 5
In the population studied by Jennet, early (within 7 days of injury) post-traumatic seizures increased the risk of late (beyond 7 days) post-traumatic epilepsy,Reference Jennet 1 and a classification that separates early and late post-traumatic seizures persists to this day.Reference Asikainen, Kaste and Sarna 28 - Reference Hunt, Boychuk and Smith 31 A subdivision of the early post-traumatic seizure category to include an “immediate” classification, as first suggested by Elvidge,Reference Asikainen, Kaste and Sarna 28 , Reference Elvidge 32 has been complicated by the existence of two different definitions: (a) a seizure occurring within 24 hours of injury,Reference Asikainen, Kaste and Sarna 28 , Reference Agrawal, Timothy, Pandit and Manju 30 , Reference Hunt, Boychuk and Smith 31 or (b) a seizure occurring immediately after impact.Reference Frey 29 This discrepancy is a source of ongoing diagnostic confusion, and only the second definition—known as a concussive convulsion—describes an entity distinct from other early post-traumatic seizures.
Concussive convulsions or impact seizures
JennetReference Jennet 1 recognized in his neurosurgical population the existence of a distinct form of seizure he called “immediate epilepsy,” and which he described as the occurrence of a generalized convulsion “within moments of injury.” He further described a benign natural history of such events in his patients: “This uncommon phenomenon consisted … exclusively of a generalised fit following a mild injury in an adult; none of the small number of such patients … had any further epilepsy.”Reference Jennet 1 Studies over the past 20 years, primarily based on video analysis of such events in elite athletes, have further refined the clinical description of the convulsions: onset within 2 seconds of impact, loss of consciousness and a brief tonic phase followed by myoclonic or clonic jerking of the extremities lasting <150 seconds; reported incidence is ~1/70 concussions.Reference McCrory, Bladin and Berkovic 33 , Reference McCrory and Berkovic 34 There may occasionally be more subtle motor manifestations, particularly brief tonic posturing without subsequent clonic movements.Reference McCrory and Berkovic 35 These events are now referred to as concussive convulsions or “impact seizures,” and they have been demonstrated to carry no risk for the later development of epilepsy.Reference McCrory, Bladin and Berkovic 33 - Reference Ellis and Wennberg 36 They may occur in children as in adults,Reference Ellis and Wennberg 36 and current practice recommendations advise against a need for neuroimaging or electroencephalography (EEG) investigations.Reference McCrory and Berkovic 34 , Reference Ellis and Wennberg 36 Failure to accurately diagnose a concussive convulsion, and to consider it the same as an early post-traumatic seizure, has been demonstrated to have the potential for adverse consequences, given the very different natural histories of the distinct entities.Reference Chadwick 37
In the sports concussion literature, concussive convulsions/impact seizures and their associated benign natural histories are well recognized and regularly included in consensus statements.Reference Aubry, Cantu and Dvorak 13 , Reference McCrory, Meeuwisse and Aubry 14 In contrast, concussive convulsions appear less well recognized in the general epilepsy literature, and have not been considered in population-based epidemiologic studies of epilepsy risk after TBI. The methods of the most recent population-based study would have excluded concussive convulsions by excluding all single seizures that occurred <7 days after a TBI,Reference Mahler, Carlsson, Andersson, Adelöw, Ahlbom and Tomson 5 however, concussive convulsions may have been included as incident cases of post-traumatic epilepsy in the other large population-based studies.Reference Annegers, Grabow, Groover, Laws, Elveback and Kurland 2 , Reference Annegers, Hauser, Coan and Rocca 3 , Reference Christensen, Pedersen, Pedersen, Sidenius, Olsen and Vestergaard 7 , Reference Annegers and Coan 19
Exacerbation of pre-existing epilepsy by mild head injury
Another potential confound for studies exploring the risk of epilepsy after mTBI is that a pre-existing epilepsy may be exacerbated, or revealed, by a concussion/mTBI. Formally described to occur in patients with focal and generalized epilepsies,Reference Tai and Gross 38 clinical experience suggests that the phenomenon may be most common in patients with primary (idiopathic) generalized epilepsy. The possibility that a mild head injury could reveal a hitherto latent primary generalized epilepsy, especially in a child or adolescent (given the childhood and adolescent onset of the typical primary generalized epilepsy syndromes), could be relevant to the sixfold increased risk of epilepsy after mTBI in children and young adults with a family history of epilepsy.Reference Christensen, Pedersen, Pedersen, Sidenius, Olsen and Vestergaard 7 , Reference Hung, Carroll and Cancelliere 20 In other words, individuals with a primary generalized epilepsy syndrome may have had their condition revealed, or unmasked, by a concussion/mTBI, rather than caused by the head injury per se. It is of course impossible to know whether or not such a primary generalized epilepsy would have remained forever latent, or would have ultimately manifested spontaneously, but it would be imprecise to attribute the fundamental cause of the genetically predetermined epilepsy to the mTBI; that is, even if the initial clinical manifestation of the epilepsy was hastened by a preceding concussion, it would be incorrect to consider the concussion responsible for a de novo epilepsy.
Primary generalized epilepsies unmasked by mTBI would understandably be captured as post-traumatic epilepsy in epidemiologic studies; exacerbation of pre-existing epilepsy by mTBI was also potentially classifiable as incident post-traumatic epilepsy in the most recent population-based study, where epilepsy patients seizure-free off medications for 5 years before TBI were declared in remission.Reference Mahler, Carlsson, Andersson, Adelöw, Ahlbom and Tomson 5
With the goal of further exploring the question of whether concussion is a risk factor for the development of de novo epilepsy, we had the opportunity to study the subject from a novel viewpoint, specifically through follow-up of a large cohort of post-concussion patients, all with a history of strictly defined concussion/mTBI. In this way, we were able to: (a) circumvent the heterogeneity of TBI classification present in previous population-based studies, and (b) ensure diagnostic accuracy of post-traumatic epilepsy by recognizing cases of concussive convulsions or pre-existing epilepsy.
Methods
This was a retrospective cohort study carried out in an academic tertiary care center (University Health Network [UHN], Toronto Western Hospital). Participants were 330 consecutive post-concussion patients (199 male, 131 female) followed by one concussion specialist (CHT) between 1997 and 2015. Concussion/mTBI was defined in accordance with the current consensus statements on concussion in sport.Reference McCrory, Meeuwisse and Aubry 14 - Reference Tator 16 The following exclusion criteria were employed to ensure accuracy of concussion/mTBI diagnosis (i.e., to exclude any case of moderate or severe TBI): abnormal brain CT/MRI, GCS<13 more than 1-hour post-injury, hospitalization >48 hours. Approximately two-thirds of the patient cohort presented with sports-related concussions. The other patients presented with concussion/mTBI related to a variety of other mechanisms, including work place injuries, motor vehicle accidents (MVAs) and falls. The independent variable in the assessment of risk factor was concussion/mTBI. Outcome measures were epilepsy incidence (dependent variable) and prevalence.
One investigator (CH) reviewed the charts and intake forms of all 330 patients and identified any patient whose medical documentation included mention of “epilepsy,” “seizure,” “convulsion,” or related terms (e.g., “black–out,” “aura”). All positively identified cases were reviewed in detail by an epileptologist (RW).
A case study of a UHN epilepsy clinic patient with a diagnosis of post-traumatic epilepsy secondary to sports-related concussion is presented to illustrate diagnostic issues that may arise in the setting of epilepsy and a prior history of concussion.
UHN Research Ethics Board approval was obtained for longitudinal study of this post-concussion patient cohort.
Results
The mean number of concussions per patient was 3.3 (±2.5); the median was 3 concussions per patient. Mean age at first clinic visit was 28 years (±14.7). Mean follow-up after last concussion was 1.4 years (±3.0). Mean follow-up after first concussion was 7.6 years (±10.8); median follow-up after first concussion was 3.1 years (interquartile range: 1.0-9.1 years). Minimum follow-up was 1 month (nine patients). Eighty-seven percent of patients had follow-up >6 months.
Eight patients were identified whose medical records included mention of the occurrence of seizures or convulsions or a possible diagnosis of epilepsy. Detailed epileptological chart review of these patients’ histories and investigations revealed none that could be considered to have a definite diagnosis of epilepsy.
Specifically, three patients had vague histories of episodes not suggestive of epileptic seizures: one with multimodality, prolonged (up to 1 hour) hallucinations and normal EEG; one with multifocal paraesthesiae, seizures endorsed on the clinic intake form and described as “sudden body spasm related to twitching muscles”; and one with a single non-convulsive “black-out” and normal EEG.
One patient had a clear history of syncope and syncopal convulsion.
Two patients had classical histories of a concussive convulsion, both hockey players: a 22-year-old with immediate loss of consciousness and convulsion on the ice; and a 15-year-old with immediate loss of consciousness and convulsion on the ice after the second of three sports-related concussions sustained over a 6-month period (the third concussion occurred 2 months after second).
The remaining two patients required more extensive review and their cases are presented in more detail.
Case 1
A 22-year-old woman, rugby player, estimated she had sustained “at least 10” concussions from grade 11 high school through first year university. Family history of two convulsions in a sibling (at ages 11 and 13 years), with unclear diagnosis and no medical treatment. The patient’s most recent concussion (rugby-related) was possibly associated with concussive convulsion on the field. Two to three months after that concussion she began to experience spells of déjà-vu lasting 30-60 seconds, followed by throbbing headaches, recurring intermittently over many months. Assessed by two different epileptologists (one UHN and one non-UHN neurologist), both unconvinced of epilepsy (differential diagnosis thought to be migraine and/or anxiety). MRIs and routine and sleep-deprived EEGs were normal. No anti-epileptic medication was prescribed. The spells ceased spontaneously 8 months before last follow-up with the second epileptologist, along with resolution of other post-concussion symptoms. Migraine, in retrospect, would seem the most likely diagnosis.
Case 2
A 17-year-old woman with a history of five sports-related concussions over 4 years (the first four concussions hockey-related, the fifth, snowboarding). No family history of epilepsy. A single witnessed convulsion occurred in the months between the third and fourth concussions, described as “tonic-clonic movements of all four limbs.” She was not seen by a neurologist or epileptologist, and no EEG was obtained. No treatment was prescribed. Four years after the most recent (fifth) concussion, a second, similar episode occurred while getting a tattoo, presumably representing reflex syncope with syncopal convulsion. The latter event makes it highly probable that the first event was also non-epileptic, and most likely a syncopal convulsion.
Thus, no patient in this large (n=330) post-concussion cohort met criteria for a definite diagnosis of epilepsy over a mean follow-up from first concussion of 7.6 years. Compared with current estimates of epilepsy prevalence (6-8/1000 individuals) and annual incidence (0.5-0.7/1000 individuals) in the general population,Reference Christensen, Vestergaard, Pedersen, Pedersen, Olsen and Sidenius 39 - Reference Fiest, Sauro and Wiebe 42 there was no difference in this post-concussion cohort (Yates’ χ2=0.472, df 1, p=0.49, using 0.5/1000/year as expected incidence).
Despite the inability to identify a single incident case of epilepsy in our large cohort of post-concussion patients, it cannot be ignored that some patients with incontrovertible seizures carry the diagnosis of post-traumatic, post-concussion, epilepsy. To illustrate this and some of the relevant diagnostic issues that may challenge the epilepsy specialist, rather than the concussion specialist, we describe a patient referred to the UHN epilepsy clinic with a long-standing diagnosis of medically refractory post-traumatic epilepsy secondary to sports-related concussion.
Case 3
A 43-year-old man with a history of soccer-related concussion at age 12 years. Family history of seizures in a cousin that remitted in later life; the same cousin’s son also has epilepsy. Approximately 1 year after the patient’s concussion he had a first generalized tonic-clonic seizure. The following year he was involved in an MVA and suffered another mTBI, possibly associated with a skull fracture but no intracranial pathology. He then began to experience generalized tonic-clonic seizures approximately once every 8 months for many years, despite anti-epileptic medication treatment with phenytoin (400-500 mg daily), carbamazepine (600 mg tid), and clobazam (30 mg daily) in combination. The presumptive diagnosis was post-traumatic epilepsy. Over the past decade, generalized tonic-clonic seizures remitted but he began to have, four or five times a year, prolonged confusional episodes lasting an hour or more, preceded by a sense of “not feeling myself” or “dizziness,” followed by deep somnolence lasting up to 24 hours, during which his wife could administer his medications, but for which time period he was subsequently entirely amnestic.
To investigate the nature of these confusional spells, the patient was admitted for video-EEG monitoring, which revealed bursts of generalized, bilaterally synchronous and symmetrical 3-4 Hz spike and wave (Figure 1) and polyspike and wave activity consistent with a primary generalized epilepsy. After reduction of anti-epileptic medications, the generalized epileptiform activity became increasingly more active, culminating in three tonic-clonic seizures occurring over 4 hours on the third day of admission, all clinically and electrographically generalized from onset. For more than 1 hour before the first motor seizure and continuing between and after each of the convulsions, abundant generalized periodic polyspike activity was recorded, during which time the patient was mildly confused and lethargic. He later remembered feeling unwell before the first tonic-clonic seizure, when the generalized spike and wave and polyspike and wave activity had become increasingly abundant. Anti-epileptic medications were resumed, substituting valproic acid for carbamazepine, and the patient was discharged from the epilepsy monitoring unit after 5 days of recording, during which time no evidence of a focal epileptiform or non-epileptiform abnormality was found.
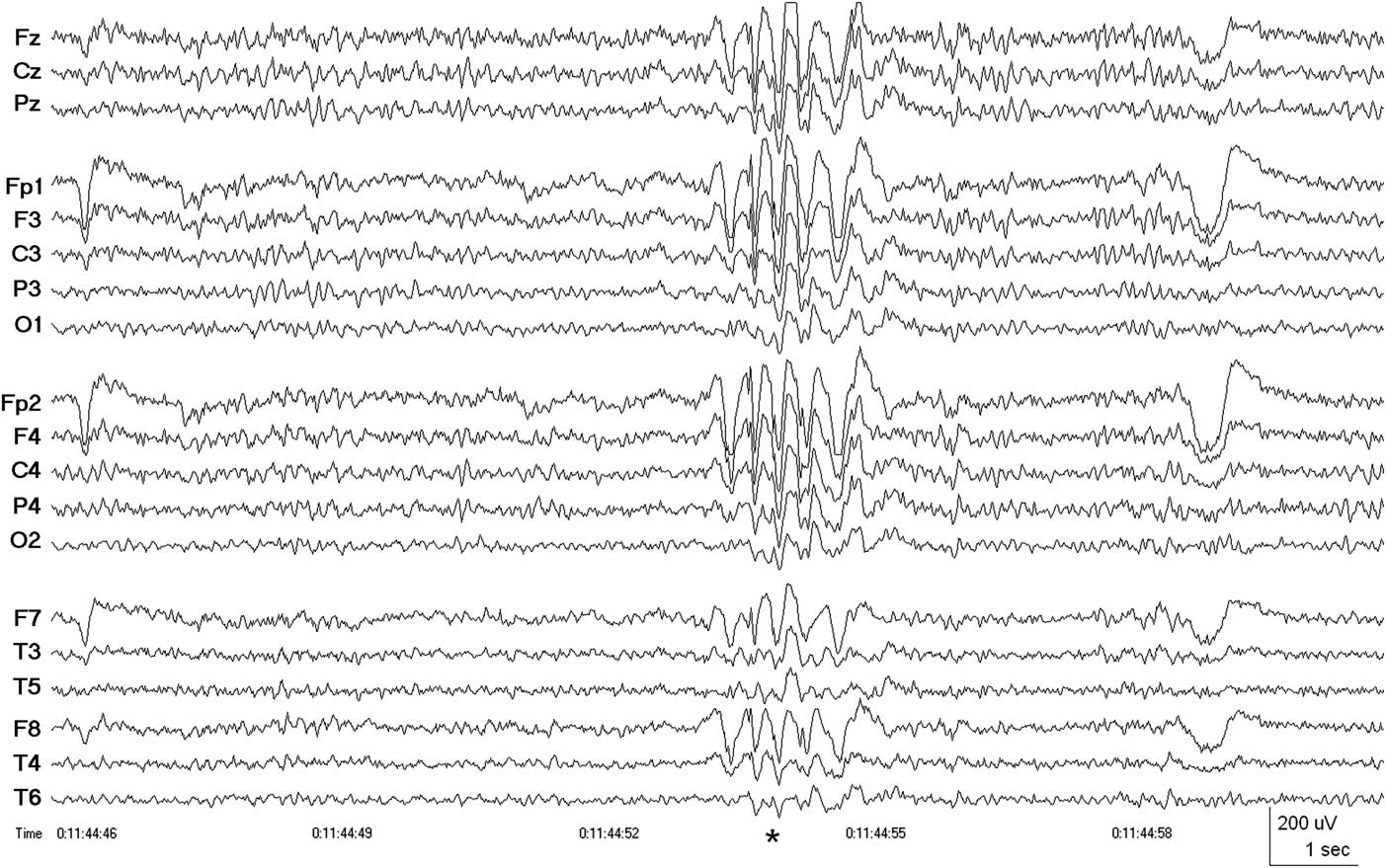
Figure 1 Case 3, patient with 30-year history of medically refractory presumed post-traumatic focal epilepsy attributed to sports-related concussion. EEG showing burst of generalized, bilaterally synchronous 3 Hz spike and wave activity (*) diagnostic of a primary (idiopathic) generalized epilepsy. There were no focal abnormalities during 5 days of continuous video-EEG monitoring. Patient seizure-free (follow-up >1 year) after initiation of treatment with valproic acid. Linked ears (A1-A2) referential montage. Low-frequency filter 0.5 Hz, high-frequency filter 70 Hz.
The patient has been seizure-free with therapeutic levels of valproic acid (1750 mg daily dosage) for more than a year since the video-EEG investigation. He continues to take phenytoin (400 mg daily) and clobazam (30 mg daily), declining for the time being a proposal to consider tapering the phenytoin dosage. Rather than post-traumatic epilepsy, this patient had suffered for three decades from idiopathic primary generalized epilepsy.
Discussion
Epidemiologic studies have indicated that mTBI, or concussion, is associated with an approximately twofold increased risk for epilepsy—sixfold in patients with a family history of epilepsy.Reference Annegers, Grabow, Groover, Laws, Elveback and Kurland 2 , Reference Annegers, Hauser, Coan and Rocca 3 , Reference Mahler, Carlsson, Andersson, Adelöw, Ahlbom and Tomson 5 , Reference Christensen, Pedersen, Pedersen, Sidenius, Olsen and Vestergaard 7 , Reference Annegers and Coan 19 , Reference Hung, Carroll and Cancelliere 20 Nonetheless, the internal validity of these findings may be open to question. In particular, classification of TBI severity in most population-based studies has been imprecise, in some based solely on registry ICD codesReference Mahler, Carlsson, Andersson, Adelöw, Ahlbom and Tomson 5 , Reference Christensen, Pedersen, Pedersen, Sidenius, Olsen and Vestergaard 7 that have been shown to have low accuracy for TBI classification, especially for mTBI.Reference Nguyen, Fiest and McChesney 12 , Reference Pickett, Simpson and Brison 22 - Reference Carroll, Cochran, Guse and Wang 24 Furthermore, the methods of some epidemiologic studies would have identified patients with concussive convulsionsReference Annegers, Grabow, Groover, Laws, Elveback and Kurland 2 , Reference Annegers, Hauser, Coan and Rocca 3 , Reference Christensen, Pedersen, Pedersen, Sidenius, Olsen and Vestergaard 7 , Reference Annegers and Coan 19 or exacerbation of pre-existing epilepsyReference Mahler, Carlsson, Andersson, Adelöw, Ahlbom and Tomson 5 as incident cases of post-traumatic epilepsy.
No incident case of epilepsy was identified among our 330 post-concussion patients during the mean follow-up period of 7.6 years, a finding indicating no difference in the annual incidence of epilepsy between our patient cohort and the general population (p=0.49). Nevertheless, it must be acknowledged that our cohort study was slightly underpowered to detect a twofold increase in incidence above the general population (post hoc power analysis suggested a sample size of n=380 to avoid Type II error), which may explain in part our inability to confirm the increased risk of epilepsy after mTBI found in population-based studies. However, compared with population-based studies, one advantage of our cohort study is that the diagnostic classification of mTBI/concussion was strictly defined, eliminating the heterogeneity of TBI classification inherent in previous epidemiologic studies. Population-based studies have not heretofore been able to reliably exclude cases of moderate or severe TBI, which could also explain our divergent findings regarding the risk of epilepsy after mTBI, that is, the different findings may reflect our cohort’s strictly homogeneous mTBI classification. In addition, our methods allowed us to exclude cases of concussive convulsions as incident epilepsy, and also to exclude cases of exacerbation of pre-existing epilepsy by mTBI (although no such cases appeared in our post-concussion cohort).
The prevalence of epilepsy in our post-concussion cohort was lower than in the general population, possibly owing to an over-representation of athletes among our patients. However, there is no evidence that athletes are less likely than non-athletes to suffer from post-traumatic epilepsy, and so for the aim of this study—to analyze the incidence of epilepsy in post-concussion patients in whom concussion was strictly defined—a lower baseline epilepsy prevalence was not important.
Clinical perspectives on the epidemiologic data
In the epilepsy clinic, it is not uncommon for patients to describe one or more past head injuries as the suspected cause of their epilepsy. It is also not uncommon to find in these same patients, upon investigation with neuroimaging and EEG, evidence of a focal epilepsy associated with structural abnormalities such as hippocampal sclerosis or developmental cortical malformations, that is, well-defined epilepsy etiologies predating the recollected head injuries. If medical registry documentation (e.g., upon presentation to an emergency department) existed for any of the head injuries, patients such as these would be imprecisely identified as incident cases of post-traumatic epilepsy by the methods of registry data set-based epidemiological studies.
Similarly, patients with primary generalized epilepsy and a history of mTBI antedating a first seizure (e.g., Case 3 in this paper) would also be subject to capture as incident cases of post-traumatic epilepsy in epidemiologic studies. Given the observation that mTBI can exacerbate pre-existing, clinically manifest epilepsy,Reference Tai and Gross 38 it is conceivable that mTBI could also unmask latent, for example, primary generalized, epilepsy. This might account for much of the increased risk of epilepsy after mTBI that has been described in individuals with a family history of epilepsy. Case 3 may represent an example of a latent primary generalized epilepsy revealed by a concussion, although the possibility that his epilepsy would have manifested spontaneously around the same time, irrespective of the mTBI, cannot be excluded. Either way, whether unmasked by the mTBI or emerging spontaneously, the onset of seizures after a concussion would have classified his case as incident post-traumatic epilepsy in epidemiologic studies, which would be a clear misrepresentation of a genetically predetermined epilepsy. Even more importantly, in the clinical realm, unquestioning acceptance of concussion as a plausible risk factor for epilepsy resulted in misdiagnosis of his generalized epilepsy syndrome, and 30 years of incomplete seizure control, before the correct diagnosis was made and seizure freedom attained after a simple change of medications.
Another case report has been published describing an elite rugby player with new-onset seizures—and the attendant assumption of a focal post-traumatic epilepsy—in whom the diagnosis turned out be an easily treatable 3 Hz polyspike and wave generalized epilepsy, emphasizing the need to avoid a presumption of post-traumatic epilepsy in individuals with a history of concussion/mTBI.Reference McGinty and Costello 43
Limitations
A main limitation of our study is the length of follow-up after initial concussion. Although the largest and longest study of its kind, the mean patient follow-up of 7.6 years leaves open the possibility that concussion/mTBI could be a risk factor for the de novo development of epilepsy long removed from the initial head injury, for example, 10 years or more post-injury. Indeed, some epidemiologic studies have described a small increased risk of epilepsy to persist >10 years after mTBI,Reference Mahler, Carlsson, Andersson, Adelöw, Ahlbom and Tomson 5 , Reference Christensen, Pedersen, Pedersen, Sidenius, Olsen and Vestergaard 7 , Reference Hung, Carroll and Cancelliere 20 although other studies found no increased risk beyond 5 years.Reference Annegers, Grabow, Groover, Laws, Elveback and Kurland 2 , Reference Annegers, Hauser, Coan and Rocca 3 , Reference Annegers and Coan 19 All epidemiologic studies have, however, reported the maximum increase in relative risk to be within the first few years post-injury, particularly within the first 6-12 months,Reference Annegers, Grabow, Groover, Laws, Elveback and Kurland 2 , Reference Annegers, Hauser, Coan and Rocca 3 , Reference Mahler, Carlsson, Andersson, Adelöw, Ahlbom and Tomson 5 , Reference Christensen, Pedersen, Pedersen, Sidenius, Olsen and Vestergaard 7 , Reference Annegers and Coan 19 , Reference Hung, Carroll and Cancelliere 20 and a large majority of our patients had follow-up that covered this period of previously described peak relative risk.
Another limitation is the total number of patients in the cohort. The advantages of the cohort in terms of uniformity of concussion/mTBI diagnosis are clear, however, even a cohort as large as this was slightly underpowered for the detection of small increases in relative risk of epilepsy, when compared with population-based studies. Future investigation of a larger cohort drawn from either a multi-specialist clinic or a multi-center study would be ideal.
Although many of the patients in our predominantly sports-related concussion cohort had sustained multiple concussions (mean 3.3/patient), in the absence of any incident cases of epilepsy we could not specifically address the question of whether multiple concussions confer an increased relative risk. Our findings suggest that a small number of concussions does not increase the risk for epilepsy, however, the shorter mean follow-up after last concussion (1.4 years) limits the strength of this conclusion.
Clinical perspectives on this study’s data
The results of this cohort study pertain specifically to patients with an accurate diagnosis of concussion/mTBI, and the findings cannot be extended to patients with moderate or severe TBI. In the clinic, however, patients referred for concussion may actually have suffered, in some cases, a more severe TBI marked by structural damage visible on CT or MRI. Our retrospective cohort study was not designed to address the question of neuroimaging utilization in patients with TBI. Nevertheless, absence of structural abnormality on brain CT/MRI is part of the diagnostic criteria for concussion/mTBI.Reference McCrory, Meeuwisse and Aubry 14 , Reference McCrory, Meeuwisse and Dvorak 15 In assembling the cohort for this study, 26 patients initially referred for concussion were excluded because their neuroimaging studies showed abnormalities, most commonly hemorrhagic contusions or encephalomalacia in the frontal or temporal regions. For the sake of interest, we did look to see how many of these excluded patients had evidence of post-traumatic seizures or epilepsy and found four patients who did: two with a single early post-traumatic seizure and two with definite post-traumatic focal epilepsy. These four patients all had bilateral contusions and/or encephalomalacia plus, in one case, a subdural hematoma. It is thus easy to understand how clinical misclassification of moderate-severe TBI as concussion can confound an accurate appreciation of the risk of epilepsy after mTBI.
Current recommendations suggest that patients with a clear history of concussive convulsion require no further epilepsy investigation (e.g., neuroimaging or EEG).Reference McCrory and Berkovic 34 , Reference Ellis and Wennberg 36 Notwithstanding, if there is any question as to the exact circumstances of the convulsion—most importantly, whether or not it occurred within 2 seconds of impact—then it would seem reasonable to obtain neuroimaging to exclude more severe TBI.
In the concussion clinic, patients with a history suggestive of epileptic seizures (and particularly those with a family history of epilepsy) should ideally be investigated with high-quality EEG or video-EEG (to confirm an epilepsy diagnosis) and high-quality CT/MRI, with the expectation that either: (a) traumatic structural abnormalities are present (and that the head injury was therefore more severe than concussion/mTBI), or that (b) the patient may have a pre-existing epileptic disorder, for example, primary generalized epilepsy. Normal EEG and MRI investigations in a post-concussion patient effectively exclude the diagnostic possibility of epilepsy.
In the epilepsy and general neurology clinic, a past history of concussion should not easily be accepted as a plausible risk factor for epilepsy. Patients with epilepsy should be investigated with high-quality, high resolution MRI and EEG or video-EEG to arrive at an accurate diagnosis of epilepsy type (focal or generalized) and etiology (structural or genetic/idiopathic). A post-traumatic etiology for epilepsy should not be presumed in a patient with a past history of strictly defined concussion/mTBI.
Conclusion
In this large cohort of post-concussion patients we found no increased incidence of epilepsy. For at least the first 5-10 years post-injury, concussion/mTBI should not be considered a significant risk factor for epilepsy. In patients with epilepsy and a past history of concussion, the epilepsy should not be presumed to be post-traumatic.
Statement of Authorship
RW conceived the study and wrote the manuscript. All authors contributed significantly to data collection and critical review of the manuscript, and all authors approved the final version.
Disclosures
The findings of this study were presented in abstract form at the 5th International Conference on Concussion in Sport, Berlin, Germany, October 2016. The authors have no other conflicts of interest to declare.


