1. Introduction
There is evidence suggesting that bilingualism may help individuals maintain cognition against the consequences of brain aging and consequential dementia neuropathology (Abutalebi et al., Reference Abutalebi, Canini, Della Rosa, Sheung, Green and Weekes2014; Anderson et al., Reference Anderson, Grundy, De Frutos, Barker, Grady and Bialystok2018, Reference Anderson, Grundy, Grady, Craik and Bialystok2021; Berkes et al., Reference Berkes, Calvo, Anderson and Bialystok2021; Costa & Sebastián-Gallés, Reference Costa and Sebastián-Gallés2014; Gallo et al., Reference Gallo, Myachykov, Shtyrov and Abutalebi2020; Gold et al., Reference Gold, Johnson and Powell2013; Grasso et al., Reference Grasso, Clark, Petersen and O'Bryant2023; Li et al., Reference Li, Abutalebi, Emmorey, Gong, Yan, Feng, Zou and Ding2017; Olsen et al., Reference Olsen, Pangelinan, Bogulski, Chakravarty, Luk, Grady and Bialystok2015; Schroeder & Marian, Reference Schroeder and Marian2012). Research has shown that bilingual older adults who progress to impairment reach the clinical threshold for diagnosis of Alzheimer's Disease approximately 4.5 years later than comparable monolinguals (Craik et al., Reference Craik, Bialystok and Freedman2010; Gollan et al., Reference Gollan, Salmon, Montoya and Galasko2011). Several other studies have replicated and extended these results. These studies show that bilinguals with smaller gray matter and white matter volumes consistently perform cognitive tasks similarly to age-matched monolinguals with higher brain volumes (Berkes et al., Reference Berkes, Calvo, Anderson and Bialystok2021; Gold et al., Reference Gold, Johnson and Powell2013; Schweizer et al., Reference Schweizer, Ware, Fischer, Craik and Bialystok2012). But these results are balanced by contrary findings showing little to no effects from bilingualism. One contrary study shows that bilingualism is associated with better performance on memory and executive function tasks cross-sectionally, but improved abilities were not sustained, suggesting that bilingualism may not be a protective factor against cognitive decline (Zahodne et al., Reference Zahodne, Schofield, Farrell, Stern and Manly2014). The lack of evidence for protective effects has been reinforced by other research, whereby bilingualism has been associated with a paradoxical increase in the risk of developing dementia (Crane et al., Reference Crane, Gibbons, Arani, Nguyen, Rhoads, McCurry, Launer, Masaki and White2009; Sanders et al., Reference Sanders, Hall, Katz and Lipton2012). For instance, Sanders et al. (Reference Sanders, Hall, Katz and Lipton2012) investigated the effects of bilingualism on the incidence of dementia in a group of non-native English speakers with varying levels of education. They concluded that non-native English-speaking status did not confer protection against dementia regardless of education status. The study suggests that non-native English speakers with more education may have a greater risk of developing dementia compared to English monolinguals (Sanders et al., Reference Sanders, Hall, Katz and Lipton2012). Crane et al. (Reference Crane, Gibbons, Arani, Nguyen, Rhoads, McCurry, Launer, Masaki and White2009) reported a similar result, indicating that self-reported written Japanese proficiency increases dementia risk among Japanese-American men living in Hawaii. In sum, bilingualism may be a protective factor against cognitive decline, but there remains some controversy about these results.
1.1. Operationalizing Bilingualism
The lack of consistency in the protective effects of bilingualism against cognitive decline may be due to two conceptual and methodological considerations (Watson et al., Reference Watson, Manly and Zahodne2016). First, varying results may reflect the methodological challenges of separating the effects of bilingualism from confounding differences associated with bilinguals’ and monolinguals’ socioeconomic status, age, and educational achievement (Bialystok et al., Reference Bialystok, Craik, Klein and Viswanathan2004, Reference Bialystok, Craik and Luk2012; McNealy et al., Reference McNealy, Mazziotta and Dapretto2011; Mukadam et al., Reference Mukadam, Sommerlad and Livingston2017; Perani & Abutalebi, Reference Perani and Abutalebi2015; Watson et al., Reference Watson, Manly and Zahodne2016). Second, operational definitions of bilingualism lack consistency in previous literature. For example, previous literature has categorized bilingualism solely using a binary indicator, being that the participant either knows more than one language or not (Gold et al., Reference Gold, Johnson and Powell2013; Schroeder & Marian, Reference Schroeder and Marian2012; Schweizer et al., Reference Schweizer, Ware, Fischer, Craik and Bialystok2012; Watson et al., Reference Watson, Manly and Zahodne2016; Zahodne et al., Reference Zahodne, Schofield, Farrell, Stern and Manly2014). In other approaches, bilingualism has been examined via differences in language use frequency, context of use (e.g., at home vs. at work), proficiency level, and ease of switching to the second language (Calabria et al., Reference Calabria, Hernández, Cattaneo, Suades, Serra, Juncadella, Reñé, Sala, Lleó, Ortiz-Gil, Ugas, Ávila, Ruiz, Ávila and Costa2020; DeLuca et al., Reference DeLuca, Rothman, Bialystok and Pliatsikas2020; Green & Abutalebi, Reference Green and Abutalebi2013). Evaluating bilingualism by the frequency of language use has produced further controversy, as bilingualism has been examined within three different domains (Green & Abutalebi, Reference Green and Abutalebi2013). The first domain is single language, in which known languages are spoken in mutually exclusive environments (i.e., Spanish at home and English at work). The second domain is dual language, in which two languages are spoken within the same context (i.e., switching between English and Spanish at work). Finally, bilingualism has been evaluated as dense code-switching, in which words of both languages are mixed within a single semantic unit (i.e., English words for 80% of a sentence and Spanish words for the other 20%) (Green & Abutalebi, Reference Green and Abutalebi2013). In a complementary approach to frequency of language use, DeLuca et al. (Reference DeLuca, Rothman, Bialystok and Pliatsikas2020) analyze bilingualism by levels such as immersion in high second language exposure environments and the extent of second language use in the home versus community settings. Active and passive bilingualism have also been distinguished. Passive bilinguals are individuals who know a second language but do not consistently speak it. Active bilinguals, on the other hand, are individuals who switch between both languages daily (Arce Rentería et al., Reference Arce Rentería, Casalletto, Tom, Pa, Harrati, Armstrong, Rajan, Manly, Mungas and Zahodne2019). Previous research suggests that relative to passive bilinguals, active bilinguals may achieve greater cognitive benefits long-term because they engage in linguistically diverse environments that require significant cognitive effort and active code-switching (Arce Rentería et al., Reference Arce Rentería, Casalletto, Tom, Pa, Harrati, Armstrong, Rajan, Manly, Mungas and Zahodne2019; Kroll et al., Reference Kroll, Dussias, Bogulski and Kroff2012). In sum, bilingualism lacks consistent and nuanced definitions in the literature, which may contribute to mixed results in previous studies.
1.2. The current study
In the current study, we sought to better understand the interrelations of linguistic competence with the brain and cognitive function for verbal episodic memory and executive function at baseline and longitudinally. We aimed to understand how active bilingualism directly impacts cognitive function across two domains, and how bilingualism may moderate the effects of brain variables on verbal episodic memory and executive function performance, gray matter volumes, and for longitudinal analyses, atrophy in the relevant domains.
As a first step, we proposed a model of Spanish monolingualism and Spanish–English bilingualism contributing to variance in cognitive outcome. We considered individual baseline levels (random effect intercepts) and linear change rates (random effect slopes) for two cognitive outcomes: episodic memory and executive function. Our language categories included Spanish monolingualism and active bilingualism, defined as participants with knowledge of both English and Spanish who use both languages daily. We analyze the strengths of brain-cognition associations using brain characteristics of gray matter thickness and longitudinal atrophy rates. The strengths of these associations are measured by the slopes of regression lines separately in monolingual and bilingual groups. We hypothesized that active bilingualism may produce a smaller positive slope of association between cognitive outcome and brain measures than in monolingual speakers, thus partly “decoupling” the association between brain and cognitive outcomes. This could be interpreted as evidence that bilingualism confers protection to episodic memory and executive function both at baseline and longitudinally, by moderating the effects of brain atrophy on cognition.
2. Methods
2.1. Sample
The study population was drawn from participants from the University of California Davis Alzheimer's Disease Research Center (UCD ADRC) who self-identified with Hispanic ancestry, primarily from Mexico and Latin America. Participants in this cohort were recruited from Sacramento and East Bay, California, communities of long-term residents. The UCD ADRC has been described previously (Mungas et al., Reference Mungas, Beckett, Harvey, Farias, Reed, Carmichael, Olichney, Miller and DeCarli2010). Our Hispanic cohort comprised 153 individuals for whom single-time measurements of cognition and brain were available (i.e., our baseline cohort), and a second longitudinal analysis of 84 individuals for whom multiple MRI and cognitive measures were accessible. Details about the relationships between MRI images and cognitive assessments are reported below.
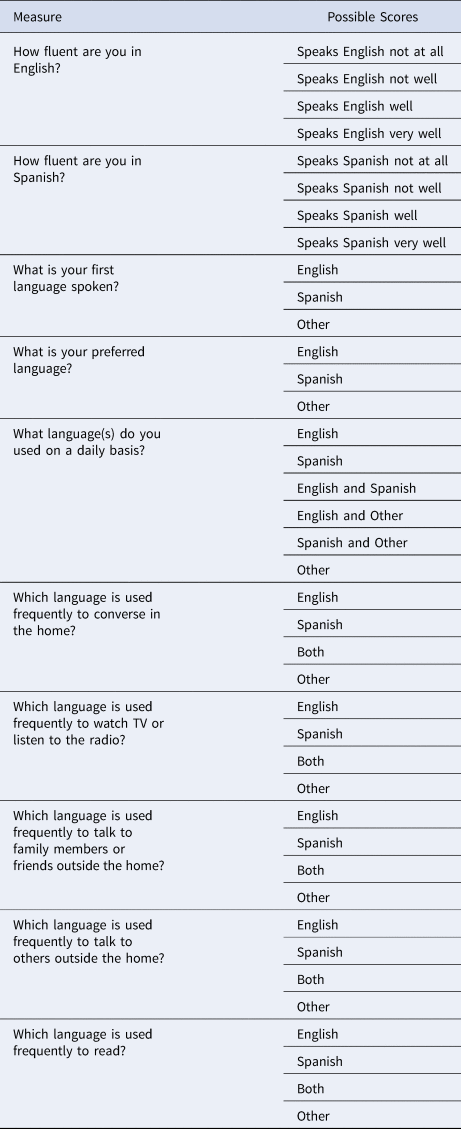
2.2. Language measures
We used two categorical variables designating self-rated bilingual knowledge. Our first measure was the categorization of self-reported bilingual knowledge – meaning knowledge of Spanish and English – or monolingual, meaning knowledge of one language, either Spanish or English. Thus, bilingualism was a dichotomous variable, reflecting self-reported knowledge of one language or two. Then to provide a more nuanced measure of bilingualism, we classified bilingual participants (i.e., those with knowledge of both languages) by habitual daily language use, determined by nine questions (Table 1) from the Spanish and English Neuropsychological Assessment Scales (SENAS) questionnaire regarding the use of language in different settings as well as self-reported language proficiency (Mungas et al., Reference Mungas, Reed, Haan and González2005). Individuals who self-reported using both English and Spanish daily were considered active bilinguals (Arce Rentería et al., Reference Arce Rentería, Casalletto, Tom, Pa, Harrati, Armstrong, Rajan, Manly, Mungas and Zahodne2019).
Table 1. Active Bilingualism Measures
2.3. Cognitive measures
The cognitive outcomes in this study were verbal episodic memory and executive function for baseline and corresponding longitudinal measures. These two cognitive domains have been thoroughly studied, thus providing well-known representations of cognition for our aim of investigating whether bilingualism affects the association of brain with cognitive performance. These cognitive domains were derived from the SENAS. SENAS has been extensively developed and valid across race and ethnic groups in both English and Spanish (Mungas et al., Reference Mungas, Reed, Haan and González2005). The episodic memory score is derived from a multi-trial word list learning test. Component tasks of the executive function composite include category fluency, letter fluency, and working memory (e.g., digit-span backward, visual-span backward, and list sorting). These scores are normally distributed and do not have floor or ceiling effects (Mungas et al., Reference Mungas, Reed, Crane, Haan and González2004). Previous random effects modeling from our group found that separate intercepts for episodic memory and executive function, but a single “global slope” for cognitive change, were best-fitting models of the data (Fletcher et al., Reference Fletcher, Gavett, Harvey, Farias, Olichney, Beckett, DeCarli and Mungas2018). Thus, in the current paper, we will use episodic and executive intercepts as baseline measurements but a single global slope rate of change variable for cognitive change in both domains.
2.4. Brain Measures
MRI scans for all participants were acquired at the University of California, Davis. T1-weighted MRI parameters for the UCD ADRC cohort have been described previously (Fletcher et al., Reference Fletcher, Carmichael, Pasternak, Maier-Hein and DeCarli2014). Images were processed by in-house software at the IDeA Laboratory, Department of Neurology, University of California Davis. The full pipeline has been reported previously (Fletcher et al., Reference Fletcher, Carmichael, Pasternak, Maier-Hein and DeCarli2014, Reference Fletcher, Villeneuve, Maillard, Harvey, Reed, Jagust and DeCarli2016, Reference Fletcher, Decarli, Fan and Knaack2021a). Briefly, we extract the brain and intracranial cavity from the skull, then align T1 and FLAIR modalities linearly, followed by nonlinear warping of the T1 image to an age-appropriate template brain to enable cross-sectional comparisons at the voxel level. Cortical gray matter thickness measures were computed by a diffeomorphic algorithm (Das et al., Reference Das, Avants, Grossman and Gee2009), assigning to each voxel a measure depending on the thickness of the cortical layer and the voxel position within it. In participants with two scans separated by at least a year, we also computed longitudinal change (atrophy for GM or expansion for CSF spaces) using an in-house implementation of tensor-based morphometry (TBM) (Fletcher et al., Reference Fletcher, Knaack, Singh, Lloyd, Wu, Carmichael and DeCarli2013).
We selected MRI scan dates as close as possible to the initial cognitive evaluations. There was an average separation of 8 months between MRI and evaluation dates for all 153 participants, and all but nine of them had separations under one year. For our longitudinal analyses, we used the same baseline scan dates and second MRI scan dates at least one year later than the baseline when more scans were available. Of the 84 participants in our longitudinal sub-cohort, the mean separation of serial scans was 5.73 years. Baseline brain measures consisted of cortical gray matter thickness (Das et al., Reference Das, Avants, Grossman and Gee2009). Longitudinal measures consisted of log-Jacobian atrophy rates derived from tensor-based morphometry (TBM) registrations of serial MRI scans (Fletcher et al., Reference Fletcher, Knaack, Singh, Lloyd, Wu, Carmichael and DeCarli2013). Briefly, TBM performs a high dimensional registration of serial MRI scans whose output is a vector field specifying the movement of each voxel location in the earlier time scan to match its position in the later scan. The 3×3 matrix of partial derivatives at each voxel is used to compute an estimate of local volume change via the Jacobian determinant. The log-transform of the Jacobian then roughly estimates the percentage volume change at each voxel (Fletcher et al., Reference Fletcher, Knaack, Singh, Lloyd, Wu, Carmichael and DeCarli2013). All log-Jacobian maps were normalized to represent scan separations of two years.
2.4.1. The Brain Signature Region Concept
A traditional approach to modeling brain associations with behavioral outcomes has used pre-selected brain structures known or suspected to be associated with the behavior of interest. The association of the hippocampus with episodic memory is perhaps the most well-known example. In this paper, we have relied on a different, data-driven approach. The signature region concept entails computing a set of clusters of contiguous brain voxels whose measures are most strongly associated with the given outcome (Dickerson et al., Reference Dickerson, Bakkour, Salat, Feczko, Pacheco, Greve, Grodstein, Wright, Blacker, Rosas, Sperling, Atri, Growdon, Hyman, Morris, Fischl and Buckner2008). Details of the signature approach for baseline and longitudinal cognitive outcomes are documented in a recent publication from our group (Fletcher et al., Reference Fletcher, Gavett, Crane, Soldan, Hohman, Farias, Widaman, Groot, Renteria, Zahodne, DeCarli and Mungas2021b). In the current project, we used brain signatures previously computed in separate, independent data sets and verified independently in two Alzheimer's Disease Neuroimaging Initiative (ADNI) cohorts to guard against selection bias. Signature regions have been shown to perform robustly in different cohorts – in UC Davis Aging Diversity Cohort (UDC ADC) – with substantial diversity by race, ethnicity, and substantial incidence of concomitant vascular disease, and in ADNI consisting of relatively homogeneous demographic cohorts that are largely free of vascular pathology (Fletcher et al., Reference Fletcher, Gavett, Crane, Soldan, Hohman, Farias, Widaman, Groot, Renteria, Zahodne, DeCarli and Mungas2021b).
Baseline predictors of cognition were signature region mean values of local gray matter (GM) thickness (Das et al., Reference Das, Avants, Grossman and Gee2009). The GM signature for baseline verbal memory is illustrated in Figure 1. Color coding for GM signatures reflect varying strengths of association (t = 2 to 7) of GM thickness with baseline verbal memory outcome. We have found that individual domain signatures for memory and executive are highly similar, supporting our use of a single signature in models of both executive and memory (Fletcher et al., Reference Fletcher, Farias, DeCarli, Gavett, Widaman, De Leon and Mungas2023).
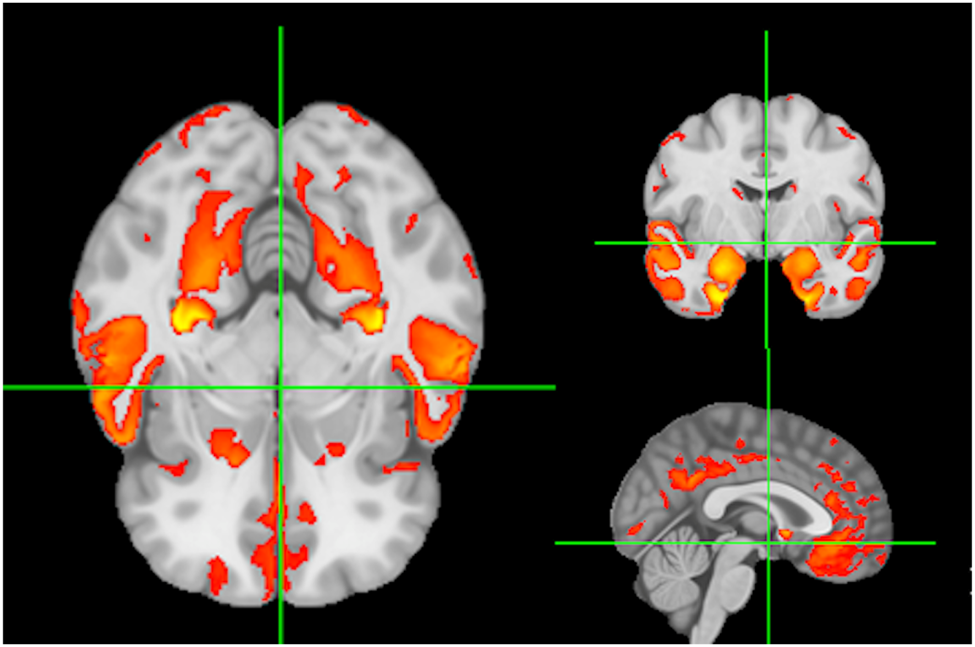
Figure 1. Signature regions associated with verbal memory at baseline. Color coding (red to yellow) reflects strengths of significant associations to baseline memory outcome.
Longitudinal predictors of cognition were signature region means of local atrophy rates generated by tensor-based morphometry over a 2-year period between successive MRI (Fig. 2). For the longitudinal signatures, the regions show strongest association between brain change (tissue atrophy measured by TBM log-Jacobians) and cognitive change.

Figure 2. Signature regions of longitudinal atrophy rates associated with longitudinal change in verbal memory. Color coding indicates strengths of significant associations (t = 7 to 13) between memory change and local volume loss.
All signatures used in this study were generated in a separate cohort (ADNI 2/GO) from our participants, to avoid overfitting within our UCD ADRC cohort. This process has been documented in a previous publication (Fletcher et al., Reference Fletcher, Gavett, Crane, Soldan, Hohman, Farias, Widaman, Groot, Renteria, Zahodne, DeCarli and Mungas2021b). For predictors of separate baseline cognitive intercepts, we used the same baseline signature originally computed for association with episodic memory. For predictor of the common “global slope” change measure of episodic memory and executive function change, we used the longitudinal brain change signature computed for memory change.
2.5. Data analysis
Modeling of Cognition Trajectory Components
SENAS measures of episodic memory and executive function were transformed for the entire dataset using the Blom inverse normal rank order transformation (Blom, Reference Blom1958) to normalize these variables and establish a common scale (mean = 0, SD = 1). Mixed effects and parallel process longitudinal analyses were performed using Mplus version 8.6 multilevel modeling platform (Muthén & Muthén, Reference Muthén and Muthén1998–2017). This approach to modeling longitudinal change in this cohort has been described in detail in previous publications (Fletcher et al., Reference Fletcher, Gavett, Harvey, Farias, Olichney, Beckett, DeCarli and Mungas2018; Gavett et al., Reference Gavett, Fletcher, Harvey, Farias, Olichney, Beckett, DeCarli and Mungas2018). Briefly, the “within” part was a multilevel model estimating intercept and slope random effects for each cognitive domain for each participant. Since performance can improve over time due to practice, we included a term coding for previous exposure to the test. Thus, the within model included a term to account for practice effects and a practice effect by Spanish test administration interaction that has been identified in previous studies with this sample (Brewster et al., Reference Brewster, Melrose, Marquine, Johnson, Napoles, MacKay-Brandt, Farias, Reed and Mungas2014; Early et al., Reference Early, Widaman, Harvey, Beckett, Park, Farias, Reed, DeCarli and Mungas2013; Melrose et al., Reference Melrose, Brewster, Marquine, MacKay-Brandt, Reed, Farias and Mungas2014). We included no other covariates in the within phase. We then estimated second-order latent variables to ascertain whether baseline and cognitive change were best characterized by individual slopes and intercepts or by latent variables to which they were highly correlated. We used a single latent “global slope” best fit for cognitive change in both our domains, but separate intercepts representing baseline data in each domain. Then, in the “between” portion of our model, the random effect intercepts for episodic memory and executive served as dependent variables for the baseline models, and the single “global slope” was used as the dependent variable in the longitudinal model.
Associations of bilingualism with cognition trajectory components
A cross-sectional brain signature of episodic memory (CS Memory Sig) and a longitudinal signature of episodic memory change (CH Memory Sig) were the primary independent variables of interest, and we examined how current language usage moderated the effects of these variables. Current language use was categorized as monolingual Spanish, monolingual English, and active use of both English and Spanish. Our analyses focused on two of these categories, monolingual Spanish and active bilingual in Spanish and English, hereafter designated as our bilingual category. Covariates included age at baseline assessment, sex, education, and recruitment source (clinic versus community). Dichotomous variables were sex and recruitment source. Age was a continuous variable centered at 73 years. Education was a continuous variable centered at 9 years.
We utilized multiple group models to evaluate similarities and differences between monolingual Spanish speakers and active bilinguals in the effects of brain signatures and covariates on baseline episodic memory and executive function, and their change components (e.g., the Between model). In multiple group analyses, models are estimated for each group and specific parameters. Brain signature effects, for example, can either be constrained to be equal across groups or can be freely estimated within groups. Less constrained models are compared to nested, more constrained models to determine if the fit is significantly better when the parameters of interest are allowed to differ across groups. The likelihood ratio test for nested models (Satorra & Bentler, Reference Satorra and Bentler2001, Reference Satorra and Bentler2009) was used to determine if freely estimating specific parameters across groups resulted in a significantly better model fit to the data.
3. Results
3.1. Sample characteristics
Sample characteristics are presented in Table 2, stratified by language usage group: Spanish Monolingual (N = 57) and Active Bilingual (N = 96). Monolingual English speakers were excluded because of the small number of these individuals in this sample (N = 23). As such, the sample characteristics described represent a total of 153 participants from the Spanish Monolingual and Active Bilingual groups. About 61% of the sample identified as female. 88% of the sample were recruited from the community. Average age is relatively uniform and centered around 73 years. Average education was 9.3 years and differed across groups (F [2,165] = 61.843, p = 0.001), with monolingual Spanish speakers having substantially less education with an average of 4.5 years. Average number of cognitive assessments was 6.8, with a standard deviation of 3.40 and range of 2–15 years. Broken down by bilingual category, for monolinguals the corresponding values were mean of 6.49, standard deviation 3.35, range 2–15; for bilinguals, mean 7.51, standard deviation 3.39, range 2–14. The average time between cognitive assessment and MRI was 0.17 years, with standard deviation of 0.81 years, and range of 0 to 5.36 years for the whole cohort. By bilingual category, for monolinguals these were mean 0.18, standard deviation 0.80, range 0–4.38. For bilinguals, these were mean of 0.16, standard deviation of 0.86, and range of 0–5.35. Mean difference of total number of visits was significant (p = 0.009) but the difference in mean MRI vs. cognitive assessments was not. 69% of all participants were cognitively normal, 15% had mild cognitive impairment (MCI), and 16% were diagnosed with dementia at the first assessment. The incidence of MCI (25.9%) and dementia (18.5%) was most prevalent among the Spanish monolingual group. Participants who were not cognitively normal were included in the study to increase statistical power. Average baseline SENAS scores differed across groups (p's < .001).
Table 2. Sample characteristics.

3.2. Brain signature effects on cognitive trajectory components in monolingual Spanish speakers and bilinguals
Effects of baseline cross-sectional verbal episodic memory signature (Mem CS Sig) on cognitive intercepts and global change in Spanish monolingual speakers and active bilinguals are presented in Table 3. Global cognitive change was positively related to Mem CS Sig in Spanish monolinguals but was not significantly related to Mem CS Sig in bilinguals. When the Mem CS Sig effect was constrained to be equal in the two groups, the difference in model fit approached statistical significance (χ2[1] = 2.976, p = 0.085), indicating that there was a trend for different effects across groups. Mem CS Sig was related to episodic memory intercept in both language usage groups (effects did not differ across groups – χ2[1] = 0.016, p = 0.899), and was related to executive function in Spanish monolinguals, but Mem CS Sig effects did not differ across groups for this variable (χ2[1] = 0.549, p = 0.459).
Table 3. Effects of baseline cross-sectional verbal episodic memory signature (Mem CS Sig) and verbal episodic memory change signature (Mem CH Sig) on cognitive intercepts and global change in Spanish monolingual speakers and active bilinguals.
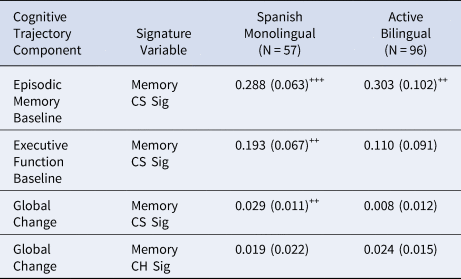
Note. Tabled values are unstandardized regression weights (βs) with standard errors in parentheses. (o=p < .10, +=p < .05, ++=p < .01, +++=p < .001)
To test for the effects of bilingual category and cognitive impairment in models of global slope, we ran two supplemental models, in which we added each of binary category (active bilingual or monolingual) or cognitive impairment (i.e., normal or impaired) and their interactions with each Mem CS Sig. Bilingual category was not significant on its own (p = 0.098), but its interaction with Mem CS Sig was significant (p = 0.04), consistent with the results reported above. Cognitive impairment and its interaction with Mem CS Sig were both significant (p = 0.02, 0.002, respectively).
Mem CH Sig in our longitudinal sub-cohort was positively but not significantly related to cognitive slope in both bilinguals (β = 0.024, SE = 0.015, p = 0.105) and Spanish monolinguals (β = 0.019, SE = 0.022, p = 0.376). This effect approached statistical significance when constrained to be equal across groups (β=0.022, SE = 0.012, p = 0.063). Model fit did not differ when the Mem CH Sig effect was constrained to be equal as opposed to freely estimated (χ2[1] = 0.026, p = 0.871).
In sum, results indicate there is a weaker connection between cross-sectional, baseline brain signature cortical thickness and global slope among active bilinguals, but no such moderation occurs for longitudinal brain signature. This pattern of results is demonstrated in Figure 3. In Spanish monolinguals, the rate of decline in the two cognitive domains is faster in those with lower brain signature values, but in bilinguals, the rate of cognitive decline is not related to brain signature values. Results for Mem CH Sig are presented in Figure 4. Active bilingualism is associated with faster decline at all levels of Mem CH Sig, but the effect of Mem CH Sig on cognitive decline is the same in both groups (the association plots are parallel but that for bilinguals has a lower intercept).
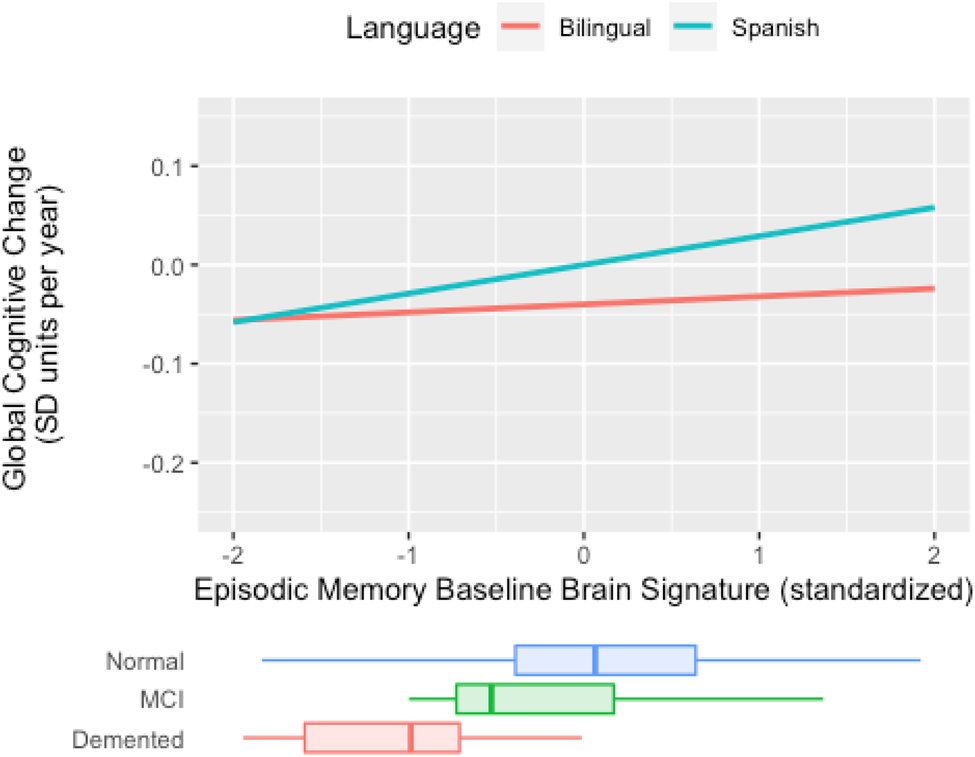
Figure 3. Expected rate of cognitive decline by episodic memory baseline brain signature for bilinguals and Spanish monolinguals (Spanish) in the full cohort (N = 153). The figure plots expected cognitive rate of change (“global slope”) against mean GM thickness in the signature region. In monolinguals (blue line), cognitive rate of change is more strongly associated with GM thickness (e.g., thicker GM is associated to less negative change) than in bilinguals (red line). Baseline brain signature measures are mean cortical gray matter thickness within the signature region. Cognitive decline is the annual decline in standard deviation units of baseline cognitive scores. Distributions of episodic memory baseline brain signature in diagnostic groups are superimposed at the bottom. The language usage by episodic memory baseline brain signature interaction effect on the rate of cognitive decline approached statistical significance (χ2[1] = 2.976, p = 0.085).
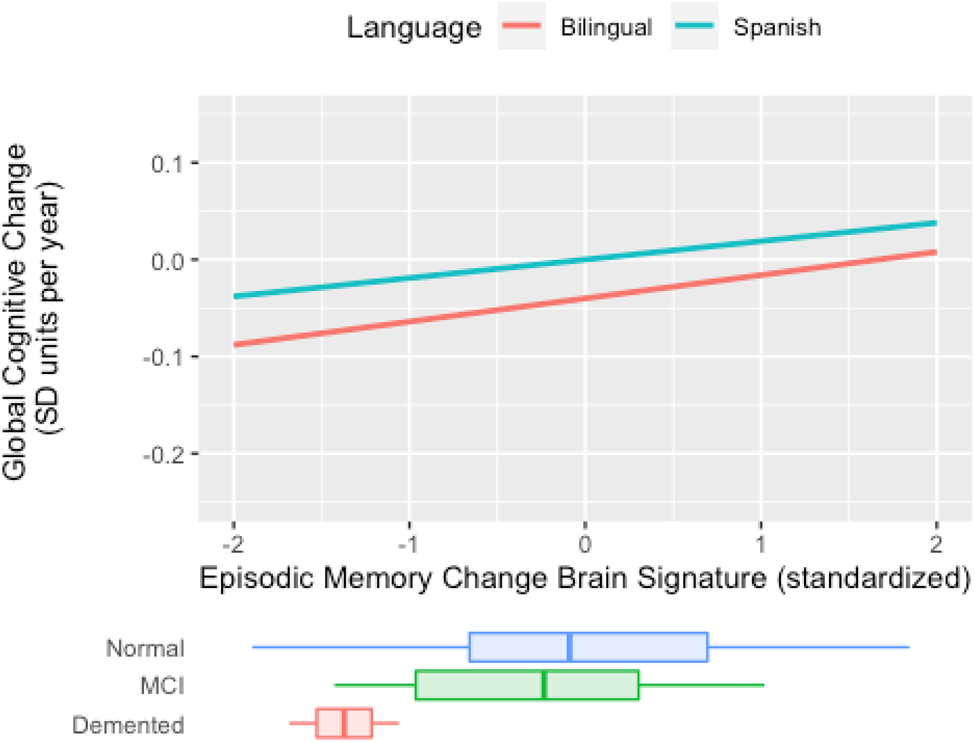
Figure 4. Expected rate of cognitive decline by episodic memory change brain signature for bilinguals and Spanish monolinguals (Spanish) in the longitudinal sub cohort (N = 84). The figure plots cognitive rate of change (“global slope”) vs. brain signature 2-year rate of change. For both groups, less negative atrophy rates (toward the right of the x-axis) are associated with less negative cognitive change. The nearly parallel plots suggest that bilingual or monolingual status has little interaction with cognitive change vs. brain atrophy rate associations. Brain signature units are tissue atrophy rates (2-year percentage volume change) measured by mean log-Jacobians within the signature region. Cognitive decline is the annual decline in standard deviation units of baseline cognitive scores. Distributions of episodic memory change brain signature in diagnostic groups are superimposed at the bottom.
4. Discussion
The current study investigates the relationships between Spanish monolingualism and active bilingualism with cognition and the brain. We operationalized bilingualism as active bilingualism, or daily use of both languages. Active bilingualism was determined by self-reported language use in different settings as well as self-reported language proficiency. Therefore, the current study may be better able to uncover the effects of bilingualism on cognition and the brain among active bilinguals (e.g., individuals who switch between both languages daily), a task believed to result in greater long-term cognitive benefits (Kroll et al., Reference Kroll, Dussias, Bogulski and Kroff2012).
Global cognitive change, represented in our results by “global slope”, was decoupled from cortical gray matter thickness in the baseline brain signature region among active bilinguals (Table 3 and Figure 3). Baseline executive function was similarly decoupled (Table 3). No protective effect from bilingualism was observed longitudinally (Figure 4). Although the Spanish monolinguals show more positive decline rates (i.e., lesser rates of cognitive loss) at all levels of the brain change signature, the parallel association plots suggested that monolinguals and bilinguals had roughly the same strength of association between decline rates and the change signature (Figure 4). Results from this study suggest that active Spanish–English bilingualism may decouple, or moderate, the effects of cross-sectional brain GM volume on global cognitive change (Table 3 and Fig. 3) and baseline domains of executive cognitive function. However, bilingualism may not confer protection against the effects of brain change on longitudinal change in executive function and episodic memory. This last observation must be taken cautiously given the much smaller size of our longitudinal cohort. It is an interesting question whether active bilingualism directly influences rates of cognitive change, in addition to its moderating effects on the associations with baseline brain signature. The answer is no (data not shown). However, with a larger sample size it is possible that there would be a significant direct effect.
Our results contribute to ongoing discussions about the relations between bilingualism, brain reserve, and cognitive reserve. Brain reserve refers to the brain resources that underlie cognition, such that individuals with greater brain resources may be able to tolerate more structural damage before their cognitive abilities are compromised (Stern et al., Reference Stern, Barnes, Grady, Jones and Raz2019). Cognitive reserve is the ability to cope with or compensate for insults to the brain, thereby maintaining cognition in the face of brain loss (Stern, Reference Stern2009). Several studies suggest that bilingualism may serve as a source of cognitive reserve by compensating for the effects of brain atrophy on cognitive ability. (Craik et al., Reference Craik, Bialystok and Freedman2010; Gold, Reference Gold2015; Perani & Abutalebi, Reference Perani and Abutalebi2015; Schweizer et al., Reference Schweizer, Ware, Fischer, Craik and Bialystok2012). Our results support previous findings, that active bilingualism decouples the effects of baseline brain cortical gray matter thickness on global cognitive change. On the other hand, bilingualism did not decouple brain change from cognitive change. Crane et al. (Reference Crane, Gibbons, Arani, Nguyen, Rhoads, McCurry, Launer, Masaki and White2009) found that self-reported Japanese written proficiency did not provide any protection against dementia in late life among English-speaking Japanese American men. Zahodne et al. (Reference Zahodne, Schofield, Farrell, Stern and Manly2014) report a bilingual advantage in memory tasks and executive function at baseline but report no association between bilingualism and cognitive decline over time. Neither of these studies examined the role of the brain. One contribution of the current study may be to suggest that bilingualism failed to alter cognitive trajectories because it does not decouple or moderate the effects of brain change on cognitive change. Our findings thus appear to reflect both the presence and the absence of benefits from bilingualism, depending on baseline or longitudinal brain measures. Our study extends this previous research by suggesting ways to resolve contradictions arising from different operational definitions of bilingualism. By using an operationalized definition of bilingualism-based frequency of language use among Spanish–English bilinguals, we propose that active bilingualism may provide evidence of cognitive reserve against cross-sectional brain atrophy, but not against longitudinal brain change.
Our study has certain limitations. First, our population size is relatively small, decreasing from 153 participants at baseline to 84 participants longitudinally. This small population may be reflected by the small magnitude of associations. Thus, our findings need to be confirmed in larger cohorts. Furthermore, due to our small sample size, we were unable to analyze the effects of bilingualism separately for normal and cognitively impaired participants. This is an interesting topic, because bilingualism may interact differently with brain associations to cognition depending on the degree of cognitive impairment (Calabria et al., Reference Calabria, Hernández, Cattaneo, Suades, Serra, Juncadella, Reñé, Sala, Lleó, Ortiz-Gil, Ugas, Ávila, Ruiz, Ávila and Costa2020; Duncan et al., Reference Duncan, Nikelski, Pilon, Steffener, Chertkow and Phillips2018; Perani et al., Reference Perani, Farsad, Ballarini, Lubian, Malpetti, Fracchetti, Magnani, March and Abutalebi2017; Schweizer et al., Reference Schweizer, Ware, Fischer, Craik and Bialystok2012). Such an investigation is important for investigating if the interaction between cognitive impairment and Mem CS Sig could explain the differences in our results, instead of bilingualism alone, and may be a crucial limitation of our study. As such, this investigation should be pursued in a larger cohort. Second, relying on self-report questionnaire data from individuals with MCI or dementia makes our data less reliable than data collected from cognitively normal individuals. Third, it is difficult to rule out the effects of factors other than bilingualism that may contribute to variation in cognitive outcomes. We note that our Spanish monolingual group had lower education and a lower percentage of cognitively normal participants. As such, centering education at 9 years may have over-estimated education as a factor in our models. While we controlled for education, it is still possible that this discrepancy could be a factor for our findings such that higher education contributes to better brain and cognitive outcomes. Education may also be associated with active bilingualism. These are beyond the scope of the current paper but pose interesting questions for further investigation. Our current results are consistent with the hypothesis that bilingualism can support cognitive ability in the face of brain GM atrophy, but findings only support a cross-sectional effect. As such, a further, more nuanced study is necessary to explore the factors related to this result.
The current study also has many important strengths. By examining the effects of English-Spanish bilingualism vs. Spanish monolingualism, we were able to apply a precise definition of bilingualism as active bilingualism (Arce Rentería et al., Reference Arce Rentería, Casalletto, Tom, Pa, Harrati, Armstrong, Rajan, Manly, Mungas and Zahodne2019) thus sharpening the focus of the study and reducing ambiguities in research associated with inconsistent definitions of bilingualism. Definitions of bilingualism focusing solely on the knowledge of two languages ignore potential variations in language exposure and competence among participants, as well as how often such participants actively engage in the second language (Abutalebi, Reference Abutalebi2008; Abutalebi & Green, Reference Abutalebi and Green2007; Arce Rentería et al., Reference Arce Rentería, Casalletto, Tom, Pa, Harrati, Armstrong, Rajan, Manly, Mungas and Zahodne2019; Luk et al., Reference Luk, De Sa and Bialystok2011; Perani et al., Reference Perani, Abutalebi, Paulesu, Brambati, Scifo, Cappa and Fazio2003). By analyzing the consistency of language use among self-reported bilinguals, we achieve a clear differentiation regarding the interrelationships between bilingualism, the brain, and cognition. Our second principal strength was the use of robust brain signature regions previously shown to be strongly associated with cognitive outcomes (Fletcher et al., Reference Fletcher, Gavett, Crane, Soldan, Hohman, Farias, Widaman, Groot, Renteria, Zahodne, DeCarli and Mungas2021b), both for baseline and high-resolution TBM measures of longitudinal brain change. Third, to our knowledge, we are among the first to test the effects of active bilingualism on the brain and cognition for both baseline and longitudinal executive function and episodic memory domains. Finally, our contradictory results contribute to ongoing discussions about bilingualism and cognitive reserve by addressing previous limitations in definitions of bilingualism and analyses that occur at baseline and longitudinal scales.
In conclusion, our findings suggest a nuanced pattern of moderation of brain atrophy effects on cognitive decline in self-reported bilinguals depending on whether participants were Spanish monolinguals or active Spanish and English bilinguals. Active Spanish–English bilinguals showed reduced associations between cognition and brain signature at baseline, but these results dissipated longitudinally. These results offer insights into the relationships between bilingualism, cognition, and the brain, and may help clarify our understanding of how bilingualism relates to cognitive reserve but require further studies with different and larger populations.
Data availability statement
Data generated during this study are available from the corresponding author upon request.
Acknowledgements
We have no known conflict of interest to disclose.









