Currently, one of the most alarming threats to human health is obesity. In the western world where high energetic food is highly available and ever present, the maintenance of a healthy eating style is very important. Especially during stress and/or negative affect, eating behaviours might change. Although most people tend to eat less when experiencing stress, around 40 % of people increase their food intake( Reference Gibson 1 ) particularly for high sweet and/or fatty foods( Reference Schepers and Markus 2 ). This stress or negative-affect-induced (emotional) eating might be a contributing factor to the growing epidemic of obesity, as an association between emotional eating and BMI has often been reported( Reference Anglé, Engblom and Eriksson 3 – Reference van Strien, Rookus and Bergers 6 ). The heterogeneity in eating behaviour during stress has not been fully explained yet, but has extensively been explored over the past years in different scientific fields. Numerous influencing factors for emotional eating have been found in the form of behavioural disinhibition( Reference Guerrieri, Nederkoorn and Stankiewicz 7 ), attention bias( Reference Shafran, Lee and Cooper 8 ), restrained eating styles( Reference Greeno and Wing 9 ), genetics( Reference Keskitalo, Tuorila and Spector 10 – Reference Tholin, Rasmussen and Tynelius 12 ) and heightened brain reward responses to food( Reference Bohon, Stice and Spoor 13 ). However, as emotional eating specifically occurs after stress experiences, it might be important to investigate the influence of individual difference in stress vulnerability, its underlying bio-psychological mechanisms and hence how this relates to the risk for emotional eating and hence weight gain.
A system that is related to stress vulnerability and thus eating behaviour is the brain serotonergic (5-HT) system. Dysfunction of the brain serotonergic system is associated with vulnerability towards stress and an increase in energetic intake specifically for palatable carbohydrate-rich foods( Reference Markus 14 ). Dysfunction of the 5-HT system is found to be promoted by a genetic factor. This commonly recognised genotype involves a functional polymorphism in the length of the 5-HT transporter-linked transcriptional promoter region (5-HTTLPR), resulting in a short (S) and a long (L) repeat sequence. The S-allele is associated with less mRNA expression, less 5-HT binding and lower 5-HT availability( Reference Heils, Teufel and Petri 15 ). Ample evidence from numerous studies and/or meta-analyses now clearly reveals that the S-allele 5-HTTLPR gene significantly increases stress responsiveness and/or the negative affective consequences of stress exposure. For instance, people carrying the S-allele 5-HTTLPR are shown to (1) have greater activation of the emotional brain network to fearful stimuli( Reference Hariri and Holmes 16 , Reference Murphy, Norbury and Godlewska 17 ), (2) increased behavioural and neuroendocrine stress responses( Reference Gotlib, Joormann and Minor 18 – Reference Markus and De Raedt 21 ) and (3) an increased risk for depressive symptoms in response to stressful life events( Reference Karg, Burmeister and Shedden 22 , Reference Miller, Wankerl and Stalder 23 ). We should state a note of caution that there is still some debate about the interaction between 5-HTTLPR genotype and stress on the development of depression, as some meta-analyses were not able to show this effect( Reference Culverhouse, Saccone and Horton 24 , Reference Risch, Herrell and Lehner 25 ), whereas other meta-analyses were successful in finding this interaction( Reference Karg, Burmeister and Shedden 22 , Reference Clarke, Flint and Attwood 26 , Reference Sharpley, Palanisamy and Glyde 27 ).
Apart from a greater risk to experience stress and therefore (potentially) depression, S-allele carriers also show increased vulnerabilities for obesity( Reference Erritzoe, Frokjaer and Haahr 28 , Reference Sookoian, Gemma and García 29 ), anxiety( Reference Graeff, Guimarães and De Andrade 30 ) and eating disorders( Reference Di Bella, Catalano and Cavallini 31 – Reference Rozenblat, Ong and Fuller-Tyszkiewicz 33 ). Consequently, such vulnerabilities may make these genotype carriers also more vulnerable to stress-induced emotional eating. However, direct body weight or eating disorders by gene associations are not always found( Reference Hinney, Barth and Ziegler 34 – Reference Sundaramurthy, Pieri and Gape 36 ), suggesting that the role of 5-HTTLPR in emotional eating is more contributing than deterministic.
The evaluation of changes in one’s environment requires appraisal, indicating that the level of stress perceived is determined by the meaning and level of importance a person assigns to it( Reference Folkman and Lazarus 37 ). This appraisal therefore might play an important part in emotional eating behaviour, especially in individuals with a genetic stress vulnerability. Recently, several studies showed support for this hypothesis, whereas neuroticism, a personality trait that promotes the intensity and frequency of stressful events( Reference Affleck, Tennen and Urrows 38 – Reference Larsen and Ketelaar 43 ), moderated the effect of 5-HTTLPR on body weight( Reference Markus and Capello 44 ). However, in the following studies, neuroticism and genotype did not interact on emotional eating behaviour after academic stress( Reference Capello and Markus 45 ) or an experimental stressor( Reference Capello and Markus 46 ). Having a neurotic personality does not directly cause stress; however, it does promote ruminative thinking( Reference Lam, Smith and Checkley 47 , Reference Watkins 48 ). Ruminative thinking is usually defined as uncontrollable perseverative thinking about past or present events. Different definitions exist, but most refer to rumination as a negative aspect (brooding), whereas some also propose positive forms of rumination (reflective)( Reference Watkins 48 ). In the current study, unless stated otherwise, rumination will be defined as a negative trait (brooding) as it is known to put the body in a state of long-lasting cortisol levels even after the stressor disappeared( Reference Zoccola and Dickerson 49 ). Complimentary evidence for the cognitive stress vulnerability that ruminative thinking poses can be found in the fact that it plays an important role in depression( Reference Rood, Roelofs and Bögels 50 ) and eating disorders( Reference Birmingham and Firoz 51 ) and is a solid predictor for negative affect in healthy subjects( Reference Roelofs, Huibers and Peeters 52 ). One of the few studies concerning rumination’s relation to emotional eating even found that rumination was a mediator for the effect of daily stress on food intake among obese adults( Reference Kubiak, Vögele and Siering 53 ).
In the current study, a model is proposed in which biological and cognitive stress vulnerabilities interact on emotional eating behaviour( Reference Schepers and Markus 2 ). When a stressor is perceived, this is expected to lead to an increased 5-HT and hypothalamic–pituitary–adrenal (HPA) axis activation as a form of stress adaptation( Reference Dickerson and Kemeny 54 ). However, if a person is inclined to ruminate about negative events, the stress response will be prolonged; these long periods of 5-HT and HPA activation can lead to desensitisation of these systems, thereby increasing the risk of developing stress-related and/or affective disorders( Reference Zoccola and Dickerson 49 , Reference Jans, Riedel and Markus 55 , Reference van Praag 56 ). In particular, in combination with a genetic stress vulnerability (5-HTTLPR), this effect might intensify because this genotype already causes greater brain 5-HT sensitisation( Reference David, Murthy and Rabiner 57 ) and HPA stress responsiveness( Reference Miller, Wankerl and Stalder 23 ). On the basis of these findings, it is highly likely that individuals with a combination of these cognitive and biological stress vulnerabilities will be more susceptible to develop stress-related disturbances, such as emotional eating. Therefore, in the current study, we expected the S-allele to contribute to weight gain exclusively among individuals with a tendency to ruminate about negative events.
Methods
Participants
Through email and flyers, students from different faculties of University Maastricht were invited to participate in the study; they were told that the experiment entailed filling in an online questionnaire package at home (once) and afterwards visiting the lab to provide a buccal sample for 5-HTTLPR genotyping. The digital questionnaire package was offered as an online survey set on the digital research platform ‘EMIUM’ and contained questions about general information (age, weight, height, health, family history of health, eating habits and so on) and standardised questionnaires regarding depression, emotional eating and rumination (see below). The sample size was based on feasibility of recruitment (see ‘Statistical analysis’ section for a sensitivity analysis); in total, 827 students responded, of whom 602 were female, and the mean age of this sample was 21·28 years (sd 2·99). The majority of our participants were female because the pool of potential participants for this study consisted mostly out of students from social sciences faculties, which have a preponderance of female students. A total of thirty-two participants were removed from analysis because of failed genotyping (n 6), incomplete questionnaire responses (n 10) or being diagnosed with an affective or eating disorder (n 16). The study was approved by the ethical committee of the faculty of Psychology and Neuroscience of Maastricht University and all participants were paid for participation.
Measurements
BMI
When participants visited the lab for buccal sample extraction, their weight and height were measured to calculate their BMI as weight/height2 (kg/m2) as a relative measure of body weight.
Rumination
To assess the tendency to ruminate about negative events, we used the Event Related Rumination Inventory (ERRI)( Reference Cann, Calhoun and Tedeschi 58 ). This inventory contains twenty questions concerning rumination after experiencing negative life events, and most importantly discerns deliberate rumination (reflective) from intrusive rumination (brooding). The psychometric properties of this questionnaire have been found to be solid( Reference Cann, Calhoun and Tedeschi 58 ). Both scales (deliberate and intrusive) contain ten items on a four-point scale ranging from 0 (never) to 3 (often), resulting in a total score per scale ranging from 0 to 40. For the analysis of this study, the Intrusive Rumination Scale was used as a measure of Rumination (unless stated otherwise).
Emotional eating behaviour
To measure emotional eating, the Three Factor Eating Questionnaire (TFEQ) R-18 was used. This questionnaire includes eighteen items for measuring different eating behaviour styles across three scales (cognitive restrained, uncontrolled eating and emotional eating). The validity and reliability of this questionnaire are sound( Reference Karlsson, Persson and Sjöström 59 ). The Emotional Eating Scale contains three items, which can be answered on a four-point scale ranging from ‘mostly true’ to ‘definitely false’. These raw scores are transformed to a total score for Emotional Eating ranging from 0 to 100( Reference Karlsson, Persson and Sjöström 59 ).
Depressive symptoms
The Beck Depression Inventory (BDI) was used to measure symptoms of depression through twenty-one standardised questions( Reference Beck, Ward and Menselson 60 ). The BDI has been studied extensively and has been shown as a reliable and valid measure for the severity of depressive symptoms( Reference Beck, Steer and Carbin 61 ).
Genotyping
Participants provided a buccal sample for genotyping triallelic variants of 5-HTTLPR ( Reference Wendland, Martin and Kruse 62 ). The triallelic variants were classified as S'-carrier (S/S, S/Lg, Lg/Lg, S/L, S/La and La/Lg) or L'/L'-carrier (La/La). Exclusively for the calculation of the Hardy–Weinberg equilibrium (HWE), the triallelic variants were classified with a separate heterogeneous category: S'/S' (S/S, S/Lg and Lg/Lg), S/L (S/La and La/Lg) and L'/L' (La/La).
Statistical analysis
Data were first examined for accuracy of data entry, missing values and normal distributions. BMI values were log transformed to create a more normal distribution, before transformation (skewness=1·43, kurtosis=4·952) and after transformation (skewness=0·759, kurtosis=1·741).
HWE was determined on the DNA database (n 821) using χ 2-tests, revealing that the genotype frequencies of L'/L' (n 214), S'/L' (n 413) and S'/S'(n 194) did not significantly differ from the HWE (χ 2=0·037, P=0·85).
To test for between-group differences of demographics, separate independent samples t test were run with a Bonferroni correction; all statistics were conducted at a two-tailed significance level.
The main analyses were conducted by means of Hierarchical Multiple Regression Analyses (using IBM SPSS 24 for Windows). Analyses were conducted with Genotype (S'-carriers v. L'/L') and Rumination as between-subjects factors on BMI and Depressive Symptoms (BDI). Three blocks were used: block 1 contained Genotype, block 2 contained Genotype and Rumination and block 3 contained Genotype, Rumination and their Interaction. To check whether Intrusive and Deliberate Rumination related differently to 5-HTTLPR, BMI and depressive symptoms, the same three-step model of the main analysis was repeated, except (Intrusive) Rumination was changed to Deliberate Rumination. In addition, these analyses were once rerun controlling for sex differences in a traditional manner by adding sex as a covariate in the model. Second, based on recent criticism on this method of controlling for confounders in Gene×Environment research( Reference Keller 63 ), we also reran the analysis using the method described by Keller, adding sex to the model, as well as all possible two-way interactions with sex. 5-HTTLPR was coded as 0=L'/L', 1=S'-carrier, and Sex was coded as 0=male, 1=female. Multicollinearity was no concern (average variation inflation factor=1·48, average tolerance=0·74) and the errors appeared to be independent (Durbin–Watson value=2·119). Sensitivity analysis (using G*Power 3.1.9.2 for Windows) showed that this test could detect effect sizes of minimally Cohen’s F 2=0·015 (α=0·05, power=0·80, n 795, predictors=4).
To test whether the hypothesised interaction between Genotype and Rumination on BMI were mediated by Emotional Eating, we conducted a Moderated Mediation Analysis as described by Hayes( Reference Hayes 64 ). The computational tool PROCESS( Reference Hayes 64 )was used testing Hayes’ model 7 (see Fig. 1). Because we used a 5000-sample bootstrapping technique, BMI was not logarithmised in this analysis. In this model, Sex was added as a covariate to control for any potential sex-related confounding effects.

Fig. 1 Path diagram for the hypothesised model tested with the Moderated Mediation Analysis.
Ethical standards
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the ethical committee of the faculty of Psychology and Neuroscience of Maastricht University. Written informed consent was obtained from all subjects/patients.
Results
Demographics
For demographics see Table 1; among these variables there were no a priori Genotype differences. There were Sex-related differences on four variables as Males showed higher BMI scores (P=0·002) and showed lower scores on Depressive Symptoms (P=0·005), Rumination (P<0·001) and Emotional Eating (P<0·001).
Table 1 Demographics and clinical characteristicsFootnote † (Mean values and standard deviations; n 795)
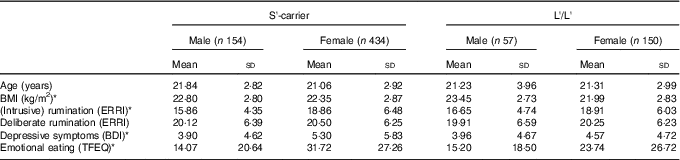
S, short; L, long; ERRI, Event Related Rumination Inventory; BDI, Beck Depression Inventory; TFEQ, Three Factor Eating Questionnaire.
* P<0·008 (Bonferroni correction of 0·05/6) for Sex differences; there were no significant effects of Genotype.
† Scores are grouped by 5-HTTLPR genotype and sex.
5-HTTLPR and Rumination on BMI
Table 2 reports the non-standardised (B) and standardised (β) regression coefficients for the three blocks. Each block proved to be a better model over the preceding one by significant increases of R 2 (in block 3 R 2=0·016). In the last block, an interaction between Genotype and Rumination significantly predicted variance in BMI (β=0·280, P=0·033) as the association of Rumination with higher BMI scores was greater among S'-carriers than among L'/L'-carriers.
Table 2 Summary of hierarchical regression analysis for (logarithmised) BMI (n 795)Footnote † (Non-standardised (B) and standardised (β) regression coefficients with their standard errors)
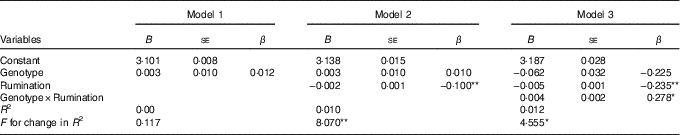
S, short; L, long.
* P<0·05, ** P<0·01.
† Genotype was represented as: L'/L'=0, S'-carrier=1.
Moderated Mediation Analysis on BMI
Table 3 reports the non-standardised (B) regression coefficients of the Moderated Mediation Analysis. According to our additional hypothesis, the size of the indirect effect of Genotype on BMI through Emotional Eating depends on Rumination. As described by Hayes( Reference Hayes 64 ), in such a model, interest is on estimation of conditional indirect effects, which is the value of the indirect effect conditioned on one or more values of the moderator. To test for statistical significance of this conditional indirect effect, we used a 95 % bootstrap CI (BCI) as can be seen in panel B. For the three different levels of Rumination all BCI straddled 0, respectively, for those low in Rumination ((−1 sd), BCI=−0·156–0·108), for those scoring around the mean of Rumination (BCI=−0·050, 0·1286) and for those high in Rumination ((+1 sd) BCI=−0·0438, 0·244). As the indirect effect is equal and insignificant on all three levels of Rumination, it is not justified to assume that an indirect effect of Genotype on BMI through Emotional Eating is moderated by Rumination.
Table 3 Moderated Mediation Analysis (Non-standardised regression coefficients (B) with their standard errors)

S, short; L, long.
* n 795. Genotype was represented as L'/L'=0, S'-carrier=1, R 2=0·051, P<0·001.
† n 795. Bootstrap=5000, controlling for sex; unstandardised coefficients are shown.
5-HTTLPR and rumination on depressive symptoms
Only among the first three blocks, each block proved to be a better model over the preceding by significant increases of R 2 (in block 3 R 2=0·206). Block 3 showed a significant interaction between Genotype and Rumination (β=0·330, P<0·01), as the association of Rumination with higher BDI scores was greater among S'-carriers than among L'/L'-carriers.
5-HTTLPR and Deliberate Rumination on BMI
To check whether Intrusive and Deliberate Rumination related differently to 5-HTTLPR, Sex and BMI, the same three-step model of the main analysis was repeated, except (Intrusive) Rumination was changed with Deliberate Rumination. In block 1, Genotype could not significantly predict variance in BMI (β=0·012, P=0·733), in block 2 both Genotype (β=−0·012, P=0·741) and Deliberate Rumination (β=−0·023, P=0·513) could not significantly predict variance in BMI and this block did not show a significant increase in R 2 (F 1,792=0·428, P=0·513) compared with block 1. In block 3, Genotype (β=−0·180, P=0·132), Deliberate Rumination (β=−0·077, P=0·268) and a Genotype×Deliberate Rumination–Interaction (β=0·226, P=0·093) could not significantly predict variance in BMI, and this block did not show a significant increase in R 2 (F 1,791=2·825, P=0·093) compared with block 2.
5-HTTLPR and Deliberate Rumination on Depressive Symptoms
To check whether Intrusive and Deliberate Rumination related differently to 5-HTTLPR, Sex and Depressive Symptoms, the same 3-step model of the main analysis was repeated, except (Intrusive) Rumination was changed with Deliberate Rumination. Each block proved to be a better model over the preceding by significant increases of R 2 (in block 4 R 2=0·127). Block 3 showed a significant interaction between Genotype and Deliberate Rumination (β=0·357, P<0·01) as the association of Deliberate Rumination with higher BDI scores was greater among S'-carriers than among L'/L'-carriers.
Controlling for Sex differences
The four previously mentioned hierarchical regression analyses were rerun with Sex as an added covariate. In all cases, this did not change the earlier conclusions. (See Table 4 for the results of controlling for Sex in the model of 5-HTTLPR and Rumination on BMI.)
Table 4 Regression results on BMI while controlling for SexFootnote * (Non-standardised regression coefficients (B) with their standard errors)
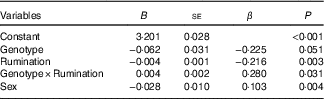
S, short; L, long.
* n 795. Genotype was represented as L'/L'= 0, S'-carrier=1; Sex was represented as male=0, female=1.
Controlling for Sex differences as proposed by Keller
In Addition, these four hierarchical regression analyses were rerun controlling for Sex using the method as proposed by Keller( Reference Keller 63 ). This only changed conclusions for the model concerning 5-HTTLPR and Rumination on BMI (see Table 5).
Table 5 Regression results on BMI while controlling for Sex as suggested by KellerFootnote * (Non-standardised regression coefficients (B) with their standard errors)

S, short; L, long.
* n 795. Genotype was represented as L'/L'= 0, S'-carrier=1; Sex was represented as male=0, female=1.
Discussion
The aim of the current study was to explore whether the combined possession of a genetic (S-allele 5-HTTLPR) and a cognitive (Rumination) vulnerability for stress may increase the tendency for emotional eating and thereby promote weight gain. In support of the hypothesis, a high ruminative thinking style significantly increased BMI scores more in S'-carriers than in L'/L' genotypes.
As described in the introduction, stress experiences are found to increase the risk for overeating, probably by way of ‘self-medicating’ from negative affect. Among the different mechanisms involved, both cognitive (rumination) and genetic (S-allele 5-HTTLPR) vulnerabilities are separately found to be involved. A cognitive ruminative thinking style is found to prolong stress experiences and responsiveness, which thereby may further increase the risk for destabilising 5-HT and HPA systems involved in stress( Reference Zoccola and Dickerson 49 , Reference Jans, Riedel and Markus 55 , Reference van Praag 56 ). In addition, the S-allele of 5-HTTLPR is commonly found to enhance stress vulnerability most likely by decreasing 5-HT binding and availability, eventually leading to sensitised HPA and 5-HT responsiveness( Reference Schepers and Markus 2 , Reference Karg, Burmeister and Shedden 22 , Reference Miller, Wankerl and Stalder 23 ). Both these factors not only appear to promote the experience of stress and/or the development of stress-related affective disorders, they also are both found to increase susceptibility for weight gain and/or reduced control of eating behaviour. For instance, 5-HTTLPR genotype has been linked to increased body weight( Reference Sookoian, Gemma and García 29 ) and eating disorders( Reference Di Bella, Catalano and Cavallini 31 ), whereas high scores on rumination seemed to be a mediator for the effect of daily stress on the urge to eat( Reference Kubiak, Vögele and Siering 53 ). The focus of the current study was to explore whether the possession of both vulnerability factors in combination may profoundly enhance the risk of emotional eating and thereby weight gain.
In line with the hypothesis, current findings revealed an interaction between rumination and 5-HTTLPR on BMI as the positive association between rumination and BMI was greater among S'-carriers than among L'/L'-carriers. These findings elaborate on the former suggestions of a direct effect of 5-HTTLPR on body weight. Although multiple studies found an increased body weight by direct effect of carrying an S-allele( Reference Sookoian, Gemma and García 29 , Reference Fuemmeler, Agurs‐Collins and Mcclernon 65 – Reference Sookoian, Gianotti and Gemma 68 ), these results are not consistent( Reference Hinney, Barth and Ziegler 34 , Reference Iordanidou, Tavridou and Petridis 69 ). Data from the present study suggest that 5-HTTLPR genotype has an effect on body mass but that this is conditional on the presence of ruminative thinking patterns. Comparable results have been found before where an effect of S-allele 5-HTTLPR on BMI was dependent on having a neurotic personality( Reference Markus and Capello 44 ) or emotional eating prevalence was highest among S-allele carriers with high depressive symptoms( Reference van Strien, van der Zwaluw and Engels 70 ). These findings underline the importance of cognitive stress vulnerabilities as moderators on 5-HTTTLPR-related eating disturbances. We should, however, be cautious interpreting these results, while although the traditional method of controlling for sex by adding it as a covariate in the model did not change the conclusion of our analysis, using the recently suggested method of Keller( Reference Keller 63 ), however (adding sex and all of its possible interactions), caused the initial found interaction of genotype and rumination to lose statistical significance. Whether this change in significance is caused by a confounding effect of sex or by overfitting the model (as the initial effect already was of modest size f 2=0·022) cannot be discerned in this study. If in time Keller’s proposed method of controlling for confounding effects in Gene×Environment studies becomes the new standard, larger samples sizes should be used to prevent overfitting and give definitive conclusions about the potential confounding effect of sex in 5-HTTLPR×Rumination interactions on BMI.
We expected that a combination of biological (S-allele 5-HTTLPR) and cognitive (rumination) stress vulnerabilities would increase the risk for weight gain through emotional eating behaviour. Surprisingly, our Mediated Moderation analysis could not prove that the moderating effect of rumination on 5-HTTLPR effect on body mass was mediated by scores on the emotional eating scale of the TFEQ. These findings correspond with earlier studies where an increased BMI among high neurotic S-allele carriers was found( Reference Markus and Capello 44 ), although this population did not show increased (self-reported) energetic intake during a stressful examination period( Reference Capello and Markus 45 ) or after an experimental stressor( Reference Capello and Markus 46 ). Contradictorily, mediating effects of emotional eating on BMI have been reported in the past as the effect of depressive symptoms on increased BMI was mediated by scores on an emotional eating questionnaire (Dutch Eating Behavior Questionnaire)( Reference van Strien, Konttinen and Homberg 71 , Reference van Strien, Winkens and Toft 72 ). Conceivably, the discrepancy between these results arises out of the heterogeneity of measuring emotional eating behaviour/tendency. Maybe the Three Factor Eating Questionnaire shows different sensitivities to measuring affect-related changes in BMI caused by emotional eating, compared with the also commonly used Dutch Eating Behavior Questionnaire, or observations of affect-related food intake.
In addition to exploring the effect of 5-HTTLPR and rumination on BMI, the current study also explored whether 5-HTTLPR and rumination have an interacting effect on depression. As both these factors are associated with clinical depression and depressive symptoms in healthy subjects( Reference Karg, Burmeister and Shedden 22 , Reference Sharpley, Palanisamy and Glyde 27 , Reference Rood, Roelofs and Bögels 50 , Reference Papageorgiou and Wells 73 ), we expected and confirmed in our database that high ruminating S'-carriers showed a greater incidence of depressive symptoms. We propose that, in combination, the stress vulnerability caused by carrying an S'-allele will be further exacerbated by the prolonging of stress responses caused by ruminative thinking( Reference Schepers and Markus 2 ). Although ruminative thinking occurs mostly in relation to the experience of stress( Reference Allbaugh 74 , Reference Smith and Alloy 75 ), this corresponds well with findings that that although a few studies found a direct effect of 5-HTTLPR on depression( Reference Collier, Stöber and Li 76 – Reference Joiner, Johnson and Soderstrom 78 ) a great number of associations between 5-HTTLPR and depression relied on the occurrence of stressful life events( Reference Sharpley, Palanisamy and Glyde 27 ). These findings once again underline the importance of cognitive stress vulnerabilities in the associations between 5-HTTLPR, stress and the development of depression.
As validation we further analysed the distinction of the two scales of the ERRI. Although our main hypothesis was focused on rumination as a cognitive stress vulnerability, we expected that exclusively the intrusive (brooding) scale of the ERRI as opposed to the deliberate (reflective) scale would show an effect on depressive symptoms and body mass, as the intrusive scales focuses on the purely negative aspect of rumination, whereas the deliberate scale is aimed at reflection. In our sample, scores on the two scales showed a large overlap (r 0·5) and showed no different effects with regard to 5-HTTLPR, and depressive symptoms. These data correspond with previous reports of high correlations between the two ERRI scales( Reference Cann, Calhoun and Tedeschi 58 , Reference Allbaugh 74 ) and their similar associations with depressive symptoms( Reference Allbaugh 74 ). Interestingly, there were differences in their effects on BMI and 5-HTTLPR genotype. As expected, S'-carriers with a high intrusive ruminative thinking style showed the highest BMI scores, whereas deliberate rumination showed no main or interaction effects on BMI. This finding validates the distinction of the two scales, whereas deliberate rumination shows no effect on body mass; intrusive rumination probably exacerbates the biological stress vulnerability effect of carrying an S'-allele thereby increasing the tendency to cope with stress by overeating and thereby weight gain. In support, high scores on specifically the intrusive rumination scale of the ERRI have been linked to other negative coping styles in the form of behavioural disengagement and substance use( Reference Cann, Calhoun and Tedeschi 58 ) and a lower life satisfaction and loss of meaning in life( Reference Triplett, Tedeschi and Cann 79 ).
Strengths, limitations and future directions
Strengths of the current study were the inclusion of a sample of 5-HTTLPR genotyped individuals (n 827) meeting HWE and taking in account the triallelic Lg’s functional equivalency to the S-allele( Reference Wendland, Martin and Kruse 62 ). A limitation was the absence of insight in the amount of stress our participants had perceived, as described this might be a crucial factor in research related to 5-HTTLPR genotype and stress-related affect and/or behaviour. A second limitation is the inability to discern cause from effect within the found associations. Both limitations could be controlled for in future (experimental) studies. Finally, we would like to note that although we had a large sample (n 827) compared with a lot of similar studies in the field, recently there is still some debate on the ideal sample size for Gene×Environment research, some researchers even vowing for samples of thousands of subjects( Reference Dick, Agrawal and Keller 80 ). Reproducibility of our findings is of great importance to draw irrefutable conclusions on 5-HTTLPR and ruminative-thinking-related body weight associations.
Conclusion
The present study is the first to show that the combined possession of biological (S-allele 5-HTTLPR) and cognitive (ruminative thinking) stress vulnerabilities increases the risk for weight gain. These findings elaborate on theories describing the influence of genes on eating behaviour by incorporating the moderating effect of ruminative thinking on the association between 5-HTTLPR and body mass. The present study thereby underlines the importance of accounting for cognitive factors when exploring genotypical influences on body mass and eating-related disturbances.
Acknowledgements
This research was funded by The Netherlands Organization for Scientific Research (NWO) as part of the NWO Food, Cognition and Behavior Project awarded to Professor C. R. M. (FCB 2014; dossier 057-13-004). NWO had no role in the design, analysis or writing of this article.
R. S. conducted the research and analysed the data under guidance of C. R. M. R. S. and C. R. M. equally contributed to the writing of the manuscript. The theoretical model/hypothesis described in the manuscript represents the core statement as first laid down in the research proposal of C. R. M., which was granted by NWO.
Neither of the authors has any conflicts of interest to declare.








