A relative consensus exists regarding the consequences of childhood maltreatment on emotional, behavioral, and social functioning during childhood and adolescence (Ferrara et al., Reference Ferrara, Guadagno, Sbordone, Amato, Spina, Perrone and Corsello2016; Hunt, Slack, & Berger, Reference Hunt, Slack and Berger2016). Longitudinal studies have also shown that the negative impact of maltreatment may persist into adulthood and affect multiple domains of life, such as physical and mental health, intimate relationships, employment, and criminal offending (Herrenkohl, Hong, Klika, Herrenkohl, & Russo, Reference Herrenkohl, Hong, Klika, Herrenkohl and Russo2013; Mersky & Topitzes, Reference Mersky and Topitzes2010; Smith, Ireland, & Thornberry, Reference Smith, Ireland and Thornberry2005). Embracing a developmental psychopathology framework could help to understand the negative (and potentially positive) adaptations unfolding over time (Toth & Cicchetti, Reference Toth and Cicchetti2013). While longitudinal study designs for which measures of functioning and hypothesized causal factors have been collected prospectively remain ideally positioned to make a contribution to the field, additional objectives are pursued. Among them, the identification of the pathways by which maltreatment jeopardizes health and behavioral functioning, through the adoption of multiple levels of analysis and a transactional approach for which the biological, emotional, and psychological processes transcend divisions between normality and abnormality (Beauchaine & McNulty, Reference Beauchaine and McNulty2013; Cicchetti, Reference Cicchetti2016). To date, though, the mechanisms underlying the long-lasting impact of maltreatment on functioning remain elusive, and are often examined in isolation and according to artificially truncated sources of influences.
The hypothalamic–pituitary–adrenal (HPA) axis and the sympathetic nervous system (SNS) are hypothesized to play a central role in the association between early adversity and health (Doom & Gunnar, Reference Doom and Gunnar2013; Lupien, McEwen, Gunnar, & Heim, Reference Lupien, McEwen, Gunnar and Heim2009). While a flattened pattern of diurnal cortisol secretion has been reported in maltreated children with some consistency (Bernard, Frost, Bennett, & Lindhiem, Reference Bernard, Frost, Bennett and Lindhiem2017; Cicchetti, Rogosch, Gunnar, & Toth, Reference Cicchetti, Rogosch, Gunnar and Toth2010; Power, Thomas, Li, & Hertzman, Reference Power, Thomas, Li and Hertzman2012), both lower (Bernard et al., Reference Bernard, Frost, Bennett and Lindhiem2017; Bruce, Fisher, Pears, & Levine, Reference Bruce, Fisher, Pears and Levine2009; Power et al., Reference Power, Thomas, Li and Hertzman2012) and higher (Bruce et al., Reference Bruce, Fisher, Pears and Levine2009; Bugental, Martorell, & Barraza, Reference Bugental, Martorell and Barraza2003; Engert, Efanov, Dedovic, Dagher, & Pruessner, Reference Engert, Efanov, Dedovic, Dagher and Pruessner2011; Fries, Shirtcliff, & Pollak, Reference Fries, Shirtcliff and Pollak2008; Saridjan et al., Reference Saridjan, Huizink, Koetsier, Jaddoe, Mackenbach, Hofman and Tiemeier2010) basal cortisol levels are noted. Inconsistent findings have also been reported regarding the directionality of the association between maltreatment and cortisol responses to psychosocial stress. Adolescents and adults who had been maltreated as children showed either higher (Harkness, Stewart, & Wynne-Edwards, Reference Harkness, Stewart and Wynne-Edwards2011; Heim et al., Reference Heim, Newport, Heit, Graham, Wilcox, Bonsall and Nemeroff2000; Sullivan, Bennett, & Lewis, Reference Sullivan, Bennett and Lewis2013), no difference (Hagan, Roubinov, Mistler, & Luecken, Reference Hagan, Roubinov, Mistler and Luecken2014; Suzuki, Poon, Papadopoulos, Kumari, & Cleare, Reference Suzuki, Poon, Papadopoulos, Kumari and Cleare2014) or lower cortisol responses to stress than controls (Carpenter et al., Reference Carpenter, Carvalho, Tyrka, Wier, Mello, Mello and Price2007; Carpenter, Shattuck, Tyrka, Geracioti, & Price, Reference Carpenter, Shattuck, Tyrka, Geracioti and Price2011; Cook, Chaplin, Sinha, Tebes, & Mayes, Reference Cook, Chaplin, Sinha, Tebes and Mayes2012; Elzinga et al., Reference Elzinga, Roelofs, Tollenaar, Bakvis, van Pelt and Spinhoven2008; Lovallo, Farag, Sorocco, Cohoon, & Vincent, Reference Lovallo, Farag, Sorocco, Cohoon and Vincent2012; MacMillan et al., Reference MacMillan, Georgiades, Duku, Shea, Steiner, Niec and Schmidt2009; Ouellet-Morin et al., Reference Ouellet-Morin, Odgers, Danese, Bowes, Shakoor, Papadopoulos and Arseneault2011; Peckins, Dockray, Eckenrode, Heaton, & Susman, Reference Peckins, Dockray, Eckenrode, Heaton and Susman2012; Trickett, Gordis, Peckins, & Susman, Reference Trickett, Gordis, Peckins and Susman2014; Voellmin et al., Reference Voellmin, Winzeler, Hug, Wilhelm, Schaefer, Gaab and Bader2015). Mixed findings also exist in regard to the SNS response to stress (Cook et al., Reference Cook, Chaplin, Sinha, Tebes and Mayes2012; De Bellis, Lefter, Trickett, & Putnam, Reference De Bellis, Lefter, Trickett and Putnam1994; Gordis, Feres, Olezeski, Rabkin, & Trickett, Reference Gordis, Feres, Olezeski, Rabkin and Trickett2010; MacMillan et al., Reference MacMillan, Georgiades, Duku, Shea, Steiner, Niec and Schmidt2009; Voellmin et al., Reference Voellmin, Winzeler, Hug, Wilhelm, Schaefer, Gaab and Bader2015).
A common source of inconsistency among studies examining the association between maltreatment and these stress response systems is the selected methodology. Besides the confounding effects of factors, such as the time of day, medications, and current symptomatology, another, more concealed source of discrepancy may lie in the measure of maltreatment. Most studies relied on data collected in participants drawn from the general population, including individuals who were maltreated (with or without official records) and others who were not. Without the necessary precautions (e.g., overrepresentation of participants who report maltreatment), this strategy may reduce the heterogeneity of the maltreatment experiences captured in these samples, in terms of frequency and severity. Moreover, the use of a dichotomous index of maltreatment (presence vs. absence) may be problematic if the association with the stress response systems changes direction (i.e., is not linear) as maltreatment becomes more severe. The presence of a point of inflexion, a shift, in cortisol response to stress along the continuum of maltreatment severity echoes in part the stress inoculation model, which proposes that moderate stress dampens cortisol response to future stress (Parker, Buckmaster, Schatzberg, & Lyons, Reference Parker, Buckmaster, Schatzberg and Lyons2004). However, exposure to more severe stress may not be “inoculating” per se, but could sensitize the HPA axis to subsequent stress (Obradovic, Reference Obradovic2012). Consequently, the restricted range of maltreatment captured in some studies, because of the participants’ selection procedures or due to how maltreatment was measured or analyzed, may not adequately depict the association between maltreatment and the stress response systems. Further tests are warranted.
The impact of these methodological concerns depends, among other things, on whether or not the association linking maltreatment and the stress response systems is expected to be linear. If maltreatment is linearly associated with cortisol (i.e., lower or higher cortisol response as maltreatment severity increases), the constrained range of maltreatment experiences or the use of a dichotomous index bear no (or fewer) consequences in understanding this association. In contrast, if a shift in the physiological stress response exists somewhere along the maltreatment distribution, only a partial representation of the maltreatment–cortisol association would be revealed. Such a possibility cannot be easily assessed from existing studies. For example, higher levels of adverse childhood experiences (ACEs) were associated with lower cortisol responses to stress in a sample of young women (Voellmin et al., Reference Voellmin, Winzeler, Hug, Wilhelm, Schaefer, Gaab and Bader2015). However, it is not clear whether, in a sample of participants who reported an average of 2.8 ACEs, those who reported the highest level of adversity (i.e., 4 or more ACEs) were confronted by moderate or higher levels of adversity so that lower or higher patterns of cortisol response could be present along the ACE distribution. As both lower and higher cortisol responses to stress have been reported following maltreatment, a closer look at the directionality of this association, according to the severity of maltreatment experiences, is warranted.
Another source of inconsistency may be that studies generally examine one stress response system at a time, precluding a more systematic, integrated view of how maltreatment may have long-lasting effects on these systems (Bauer, Quas, & Boyce, Reference Bauer, Quas and Boyce2002). The locus coeruleus-norepinephrine/SNS and the HPA axis do not always present symmetrical (lagged) responses to stress (Allwood, Handwerger, Kivlighan, Granger, & Stroud, Reference Allwood, Handwerger, Kivlighan, Granger and Stroud2011; Bae et al., Reference Bae, Stadelmann, Klein, Jaeger, Hiemisch, Kiess and Dohnert2015; Cook et al., Reference Cook, Chaplin, Sinha, Tebes and Mayes2012; El-Sheikh, Erath, Buckhalt, Granger, & Mize, Reference El-Sheikh, Erath, Buckhalt, Granger and Mize2008; Gordis, Granger, Susman, & Trickett, Reference Gordis, Granger, Susman and Trickett2006; van Goozen et al., Reference van Goozen, Matthys, Cohen-Kettenis, Gispen-de Wied, Wiegant and van Engeland1998). Moreover, despite their neural connections, these stress systems have distinct secretion time course, effects on targeted tissues, and regulatory mechanisms (Sapolsky, Romero, & Munck, Reference Sapolsky, Romero and Munck2000). Important individual variability in the HPA axis and SNS responses to stress have been reported (Kudielka, Hellhammer, & Wust, Reference Kudielka, Hellhammer and Wust2009), so that while some individuals show marked responses in both systems when confronted by stress, only one stress system may be activated in others (Bauer et al., Reference Bauer, Quas and Boyce2002). According to the biological sensitivity to context model (Boyce & Ellis, Reference Boyce and Ellis2005), genetically and environmentally mediating factors have the potential to calibrate the stress systems. It is thus possible that the specific genetic and environmental etiology of the HPA axis and SNS may prompt asymmetric responses to stress. On a related point, the biological sensitivity to context model underlines how the integrated actions and connections of the stress systems should be understood in their ecological contexts, past or present (Ellis & Boyce, Reference Ellis and Boyce2008). However, the possibility that the symmetry of the HPA and SNS responses to stress vary according to childhood maltreatment has rarely been investigated (for an exception, see Gordis, Granger, Susman, & Trickett, Reference Gordis, Granger, Susman and Trickett2008). In summary, the restricted range of maltreatment captured in some studies, the overlook of potential nonlinear association between these constructs as well as the omission of testing the possibility that maltreatment severity affects the symmetry of HPA and SNS responses to stress, may obscure how they jointly affect later vulnerability to psychopathology.
The present study had three objectives. We examined whether young adults exposed to childhood maltreatment, as defined using the standardized Childhood Trauma Questionnaire (CTQ) guidelines (Bernstein & Fink, Reference Bernstein and Fink1998), had a disrupted pattern of stress responses in comparison to those who did not reach that threshold, using cortisol and heart rate (HR) as markers of HPA axis and SNS reactivity to stress. This dichotomous index of maltreatment experiences, however, would not allow uncovering the nonlinear patterns of associations, if present. To this end, we investigated if the cortisol and HR responses to stress varied according to maltreatment severity. Here, we operationalized maltreatment experiences so that individual variation within the CTQ-based nonmaltreated participants could be depicted along with their differential patterns of associations with the stress response systems. Finally, we explored whether the symmetry between cortisol and HR responses to stress also varied as a function of maltreatment severity.
Method
Sample
Because the study's general objective was to understand the biosocial roots of general aggression, including verbal and physical aggression, and that these behaviors are manifested more frequently by men than by women (Archer, Reference Archer2004), the sample only comprised men. Moreover, uncertainty remains about the existence of sex differences in cortisol response to stress (Carpenter et al., Reference Carpenter, Tyrka, Ross, Khoury, Anderson and Price2009; Kudielka, Buske-Kirschbaum, Hellhammer, & Kirschbaum, Reference Kudielka, Buske-Kirschbaum, Hellhammer and Kirschbaum2004) and regarding the maltreatment-cortisol associations (Carpenter et al., Reference Carpenter, Tyrka, Ross, Khoury, Anderson and Price2009; Negriff, Saxbe, & Trickett, Reference Negriff, Saxbe and Trickett2015). Because we could not reach the statistical power required to adequately test these possible sexually dimorphic associations, only men were initially investigated. Participants were recruited using ads posted online and on public billboards inviting them to participate in a study about early life experiences. Trained research assistants conducted a phone interview with interested individuals, screening for health and about experiences of childhood maltreatment using the short form of the CTQ (Bernstein & Fink, Reference Bernstein and Fink1998). The sample included 155 participants aged from 18 to 35 years (M = 24.10, SD = 3.70).
Procedure
We invited the participants to take part in our study, lasting about 3 hr 30 min. During that time, the participants took part in the Trier Social Stress Test (TSST), a well-established, standardized stress paradigm that induces social–evaluative threat by subjecting participants to a 5-min mock job interview in front of a “panel of behavioral experts,” and followed by 5 min of mental arithmetic. Participants communicated with the panel using an intercom and were filmed with a video camera in a stand-up position in front of a one-way window (Andrews et al., Reference Andrews, Wadiwalla, Juster, Lord, Lupien and Pruessner2007). Both the “panel-in” and “panel-out” methods have been shown to elicit reliable cortisol responses in laboratory settings (Andrews et al., Reference Andrews, Wadiwalla, Juster, Lord, Lupien and Pruessner2007). The TSST took place in the early afternoon for all participants (M = 13:41, SD = 0:53). Ethical approval was granted by the Research Center of the Montreal Mental Health University Institute Ethics Committee (Montréal, Canada).
Measures
CTQ-SF
The CTQ-SF inquiries information about emotional, physical, and sexual abuse and neglect that have occurred before age 18 and have been selected because of its demonstrated validity in community samples (Bernstein et al., Reference Bernstein, Stein, Newcomb, Walker, Pogge, Ahluvalia and Zule2003). Participants responded to each of the 28 items in the context of “when you were growing up” and answered according to a 5-point Likert scale ranging from 1 = never true to 5 = very often true, leading to total scores of 5 to 25 on each subscale (plus 3 items of validity). The CTQ-SF included items such as “People in my family said hurtful or insulting things to me” (emotional abuse) and “I was punished with a belt, a board, a cord, or some other hard object” (physical abuse). To test the first objective of the study, we identified 56 men (35.9%) who reported experiences suggesting the occurrence of at least one type of maltreatment (i.e., CTQ-based maltreatment) using the manual's recommended classification scores (Bernstein & Fink, Reference Bernstein and Fink1998). The experiences of the remaining participants (n = 99; controls) did not reach that threshold (i.e., CTQ-based nonmaltreatment). Being identified as positive for a category corresponds with endorsing a number of experiences as “often true.” The CTQ has good internal consistency and criterion validity in clinical and community samples and convergent reliability with clinical assessments of abuse is high (Bernstein et al., Reference Bernstein, Stein, Newcomb, Walker, Pogge, Ahluvalia and Zule2003). Acceptable internal consistency was also found in this sample (Cronbach α = 0.78). To test the second objective of the study, we calculated the total scale of maltreatment by summing up all 25 items, as an index of maltreatment severity (total sample: M = 37.43, SD = 11.04), and derived three equal groups of participants who reported increasing levels of maltreatment experiences, thereafter referred to as no (few) maltreatment experiences (CTQ total score ≤ 31); some experiences of maltreatment (CTQ total score 32–40); and more experiences of maltreatment (CTQ total score ≥ 41). The groups based on the CTQ's guidelines for maltreatment, M = 30.85, SD = 3.96 and M = 49.18, SD = 9.81, respectively; F (1, 154) = 271.15, p < .001, as well as the groups derived according to the total CTQ score, significantly differed in the levels of maltreatment reported, M = 28.12, SD = 2.00, M = 34.91, SD = 2.63, and M = 50.78, SD = 9.07, respectively; F (2, 153) = 239.02, p < .001, with post hoc analyses showed significant differences between each group (ps < .001).
Stress biomarkers
Cortisol was measured through the collection of five saliva samples via passive drool. The first two samples were collected 2 and 20 min before the TSST. The third, fourth, and fifth samples were collected 15, 25, and 35 min after the beginning of the TSST. Saliva samples were stored in a –20 °C freezer and analyzed in a single batch with a high sensitivity enzyme immune assay kit (Salimetrics State College, PA, Catalogue No. 1-3102). The range of detection for this assay is between 0.012 and 3 ug/dl and the intra- and interassay coefficients of variation were 4.1% and 8.3%, respectively. All samples were assayed in duplicates, Winsorized, and log-transformed prior to statistical analyses.
HR was measured using an Omron 7 Series Plus BP765 Automatic Blood Pressure Monitor, which records blood pressures in a noninvasive manner using a cuff placed on the upper arm. HR was measured, at a 2-min interval, three times prior to and six times during the TSST.
Risk factors and covariates
Information about the sociodemographic, health, and lifestyle factors, including age, occupation, and cigarette, alcohol, and other drugs consumption were enquired during the initial phone interview. We also asked the participants to complete the Beck Depression Inventory during the laboratory visit, which is a 21-question multiple-choice self-report inventory measuring depressive symptoms and severity (Beck, Steer, & Brown, Reference Beck, Steer and Brown1996).
Statistical analyses
Before conducting the main analyses, we identified, from a wide range of health-related factors (e.g., medication, cigarettes, alcohol and drug use, and allergies), those uniquely associated with cortisol and HR. Two were identified for cortisol: being a smoker and having had a flu in the past month and one for HR: anti-inflammatory medication. These variables were statistically controlled for in all analyses. We tested the study hypotheses in three steps. First, we tested whether the participants showed a distinct pattern of stress response according to the CTQ-based maltreatment status using a repeated-measures analysis of variance (ANOVA; cortisol) and growth curve analyses (HR). A repeated-measures ANOVA was preferred for cortisol because we had no missing data and the saliva samples were all collected at predetermined, constant times in relation to the beginning of the TSST. Greenhouse–Geisser corrections for repeated measures were reported to correct for the violated sphericity assumption. Because missing data occurred for HR due to technical difficulties, we conducted growth curve analyses using full information maximum likelihood estimation in MPlus (Version 6.11; Muthén & Muthén, Reference Muthén and Muthén1998–2011). Second, we wanted to expand on the previous analysis and test if distinct, nonlinear patterns of cortisol and HR responses to stress could be detected according to the severity of maltreatment. As described in the Method, we split the total sample in three groups according to the continuously distributed total CTQ score of maltreatment, yielding to participants who reported no (few) maltreatment experiences; some experiences of maltreatment; or more experiences of maltreatment. Tertiles were chosen to allow a similar number of participants in each group and sufficient statistical power to test the study's hypotheses. Third, we explored whether the symmetry between cortisol and HR responses to stress was moderated by maltreatment severity using the regression model included in the SPSS's macro PROCESS (Hayes, Reference Hayes2013).
Results
Did the TSST elicit a significant cortisol stress response, and could distinct patterns of cortisol responses to stress be detected according to the CTQ-based maltreatment status?
A repeated measures ANOVA showed that the TSST elicited a robust and significant increase in cortisol, time: F (2.05, 311.56) = 57.99, p < .001. Figure 1 illustrates the patterns of secretion for the participants for whom the experiences reached the CTQ's threshold for maltreatment (solid line) and those for whom it did not (dashed line). The CTQ-based maltreated participants had higher cortisol responses to the TSST, Time × CTQ-Based Maltreatment: F (2.08, 313.27) = 2.89, p = .05, and a trend for significantly higher cortisol levels throughout the TSST, CTQ-based maltreatment: F (1, 151) = 3.69, p = .06, in comparison to the CTQ-based nonmaltreatment group. Specifically, while the two groups did not differ prior to the TSST, –20 min: F (1, 154) = 1.01, p = .32; –2 min: F (1, 154) = 0.88, p = .35, distinct responses emerged subsequently, F (1, 151) = 4.60, p = .03.
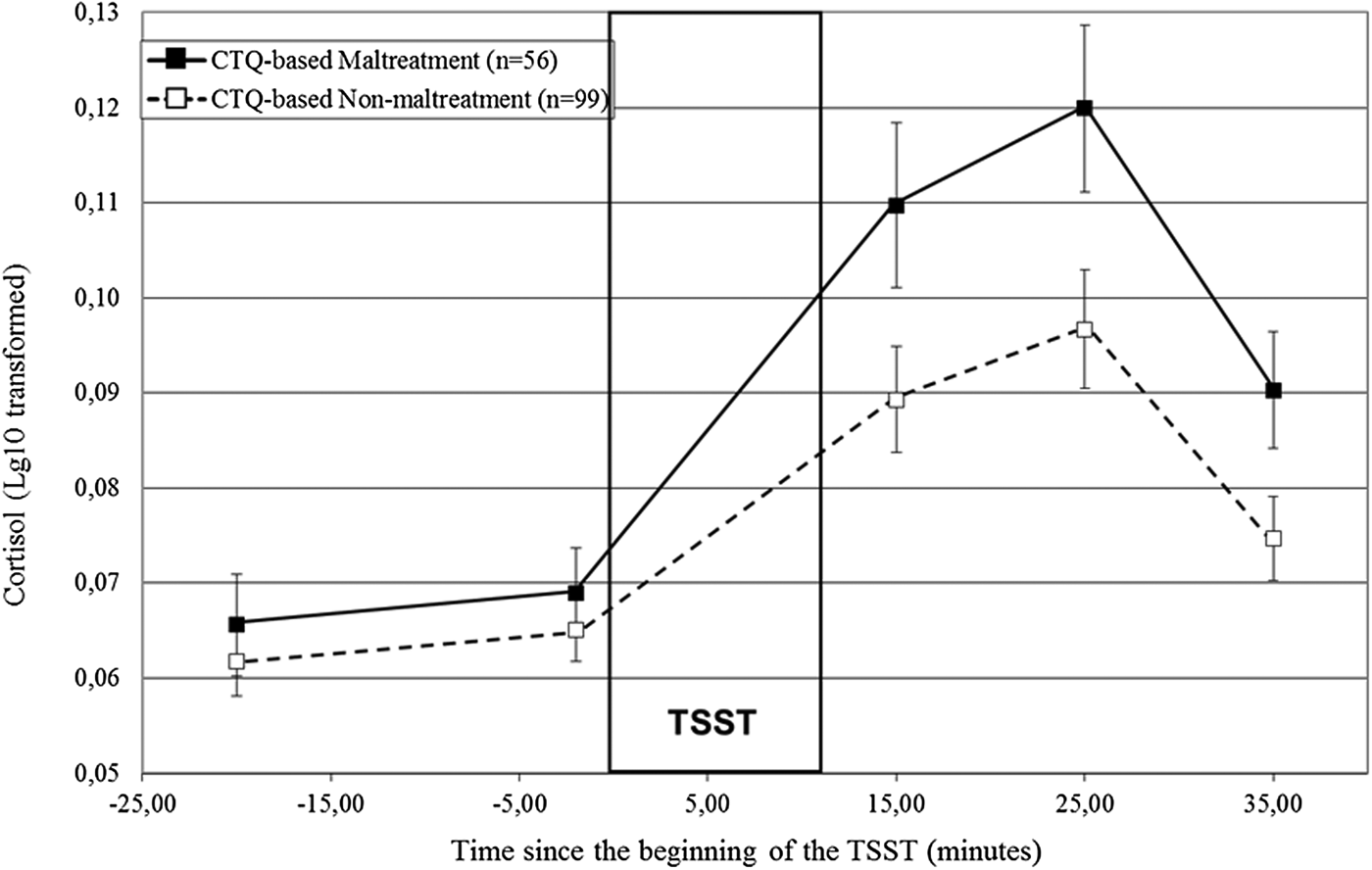
Figure 1. Cortisol responses (± SEM) to the TSST according to CTQ-based maltreatment status. SEM, standard mean error; n, number of participants; TSST, Trier Social Stress Test; CTQ, Childhood Trauma Questionnaire.
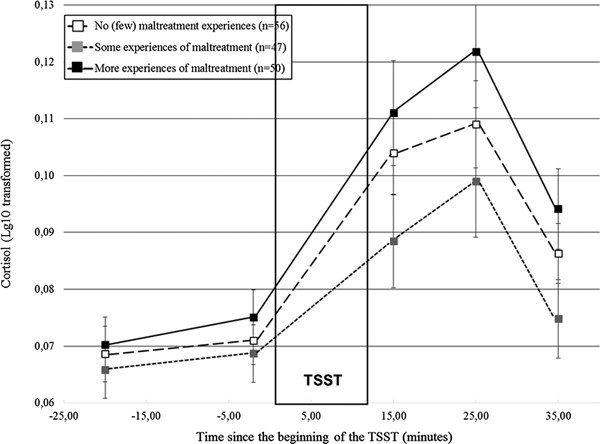
Did distinct patterns of cortisol responses to stress emerge according to maltreatment severity?
Distinct cortisol responses were noted according to maltreatment severity, F (4.17, 312.89) = 2.84, p = .02. Figure 2 shows that while the three groups had similar initial cortisol levels, participants who reported more experiences of maltreatment had higher cortisol responses to the TSST in comparison to the remaining groups. We formally tested this observation by contrasting the groups two by two. Participants who reported more experiences of maltreatment had higher cortisol responses than those who reported some experiences, F (2.00, 186.16) = 5.18, p = .006, but did not differ significantly from the participants with no or few experiences, F (2.10, 217.95) = 1.45, p = .24. Moreover, participants who reported some experiences of maltreatment had lower responses than controls, F (1, 101) = 3.89, p = .05. Altogether, these findings suggest lower responses when maltreatment departs from the no (or few) experiences, followed by higher cortisol levels in participants confronted to more experiences of maltreatment, a finding hereafter referred to as a shift from moderate to lower to higher responses as maltreatment severity increased.
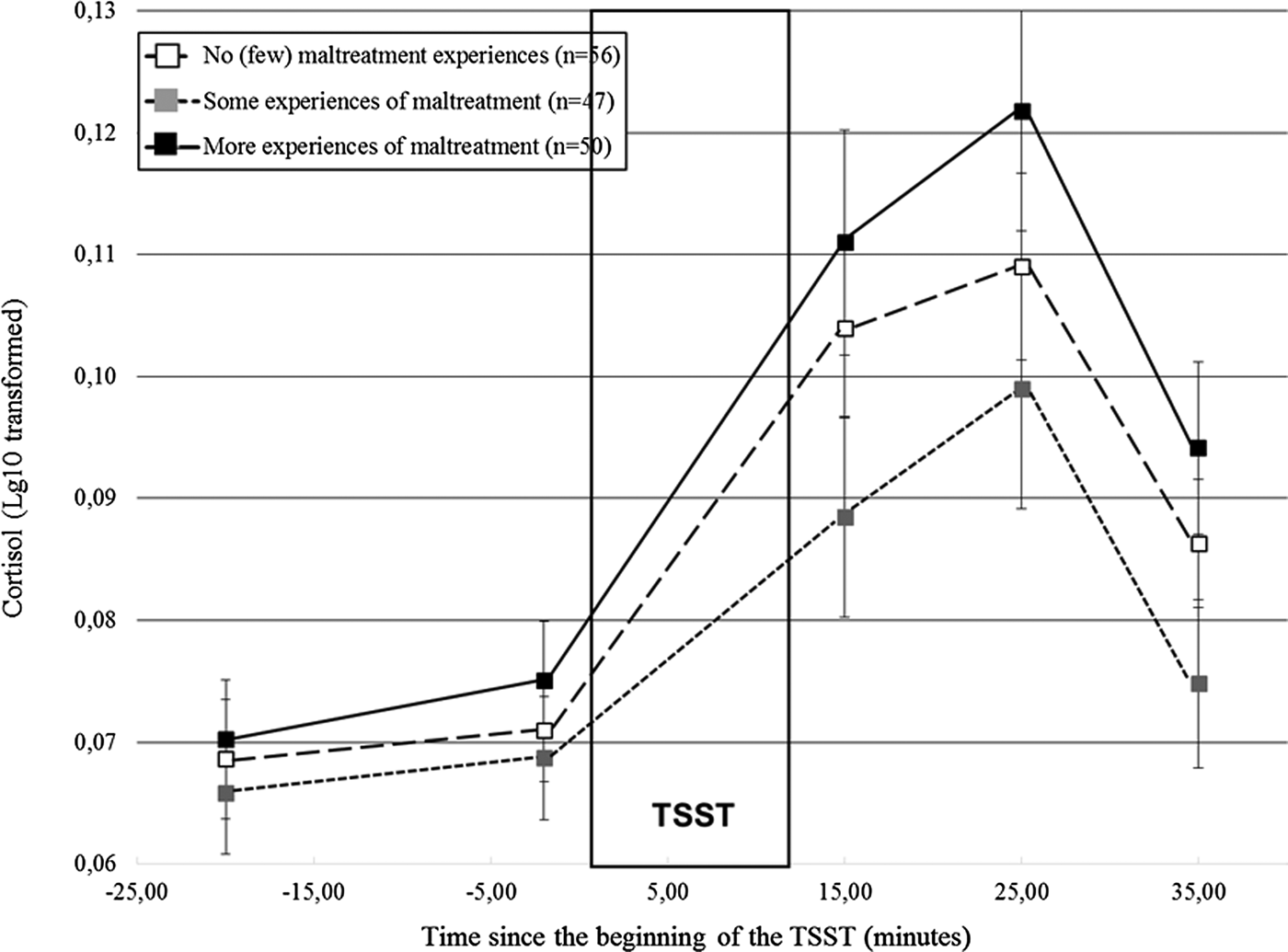
Figure 2. Cortisol responses (± SEM) to the TSST according to the severity of maltreatment. SEM, standard mean error; n, number of participants; TSST, Trier Social Stress Test.
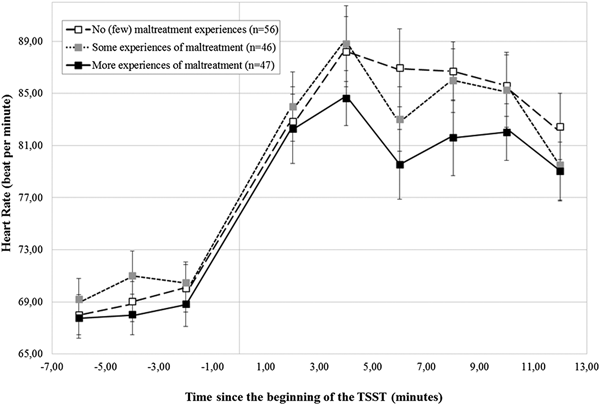
Were similar patterns of findings observed for HR response to stress?
As reported in the online-only Supplementary Table S.1, participants showed, on average, a significant HR increase during the TSST, B = 6.95 (0.41), critical ratio = 16.96, p < .001, which did not vary according to the CTQ-based maltreatment groups, B = –0.31 (0.85), critical ratio = –0.36, p = .72 (Figure 3). In addition, no differences in HR could be detected according to maltreatment severity, main level: B = –0.31 (1.01), critical ratio = –0.31, p = .76; stress response: B = –0.61 (0.48), critical ratio = –1.26, p = .21 (Figure 4).

Figure 3. Heart rate responses (± SEM) to the TSST according to CTQ-based maltreatment status. SEM, standard mean error; n, number of participants; TSST, Trier Social Stress Test; CTQ, Childhood Trauma Questionnaire.
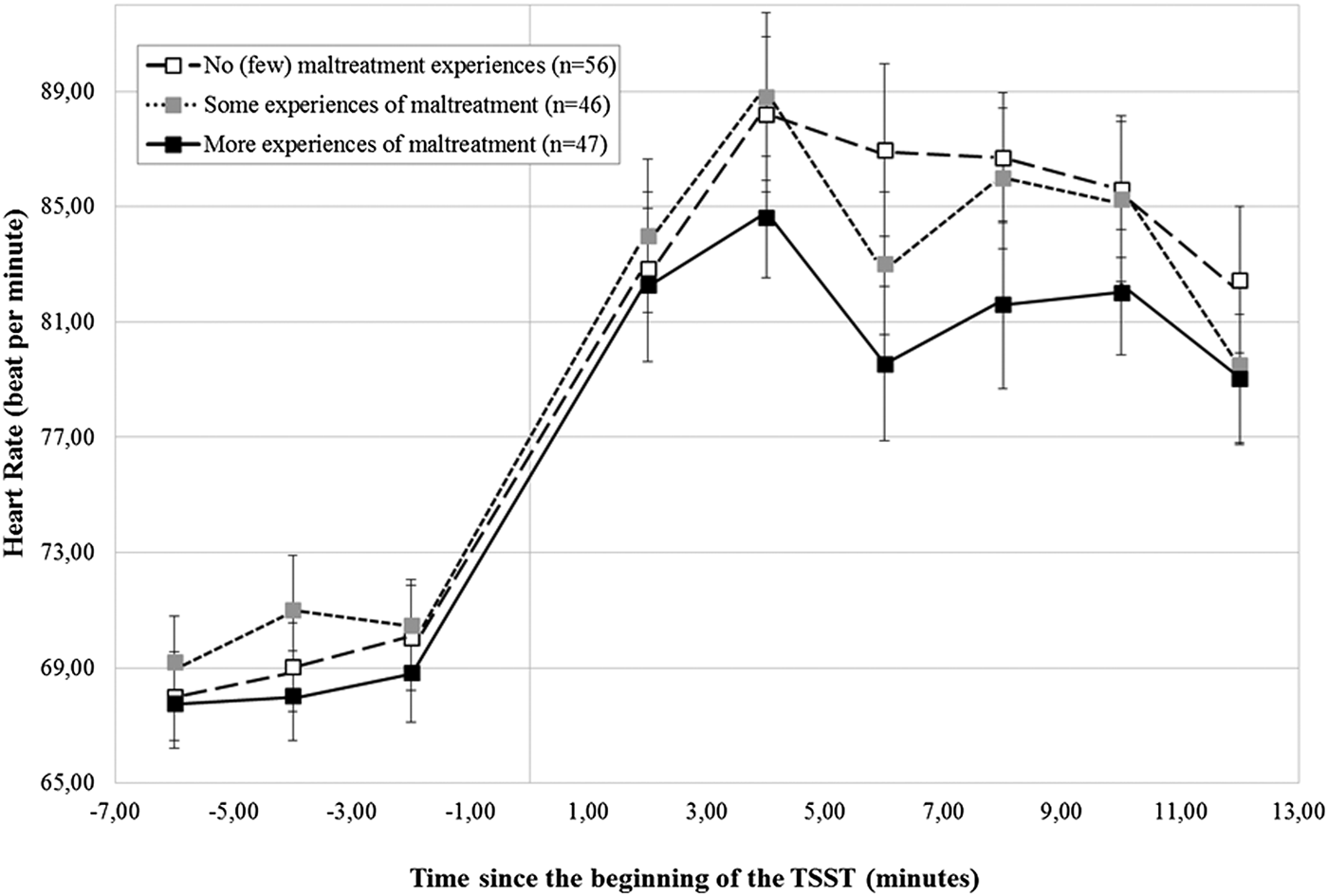
Figure 4. Heart rate (± SEM) to the TSST according to the severity of maltreatment. SEM, standard mean error; n, number of participants; TSST, Trier Social Stress Test.
Other potential confounders
In addition to the factors affecting cortisol and HR, we explored whether other factors differed between participants who reported CTQ-based maltreatment and those who did not (Table 1). Participants assigned to the CTQ-based maltreatment group have had more flu in the previous month and more depressive symptoms than those who did not. While the first factor was already accounted for in the previous analyses, we reran all the analyses to ensure that the depressive symptomatology did not explain away the findings. Participants who reported maltreatment that reached the CTQ-based threshold still showed higher cortisol responses to the TSST in comparison to those who did not, F (1.86, 276.14) = 4.07, p = .02. The distinct patterns of cortisol responses to stress also remained according to increasing levels of maltreatment severity, F (3.75, 275.94) = 3.71, p = .007, for which lower responses were noted for participants who reported some experiences of maltreatment in comparison to those who reported more experiences, F (1.80, 162.24) = 6.32, p = .003. Findings also remained unchanged for HR.
Table 1. Participant characteristics in the total sample and according reported experiences of maltreatment
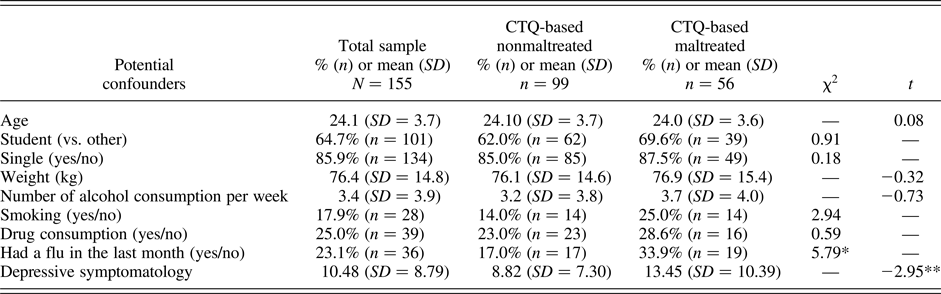
Note: SD, standard deviation; n, number of participants; kg, kilogram. *p < .05; **p < .01; ***p < .001.
Did the symmetry between cortisol and HR vary according to maltreatment severity?
Maltreatment severity moderated the association between cortisol and HR responses to stress, F (1, 146) = 5.30, p = .02. To explore the direction and relative magnitude of these associations according to maltreatment severity, we conduct post hoc Pearson correlations. As illustrated in Figure 5, no significant associations were observed for participants exposed to no (or few; r = –.17, p = .22) or some experiences of maltreatment (r = .20, p = .18), whereas a significant association emerged for those who reported more experiences of maltreatment (r = .34, p = .02).
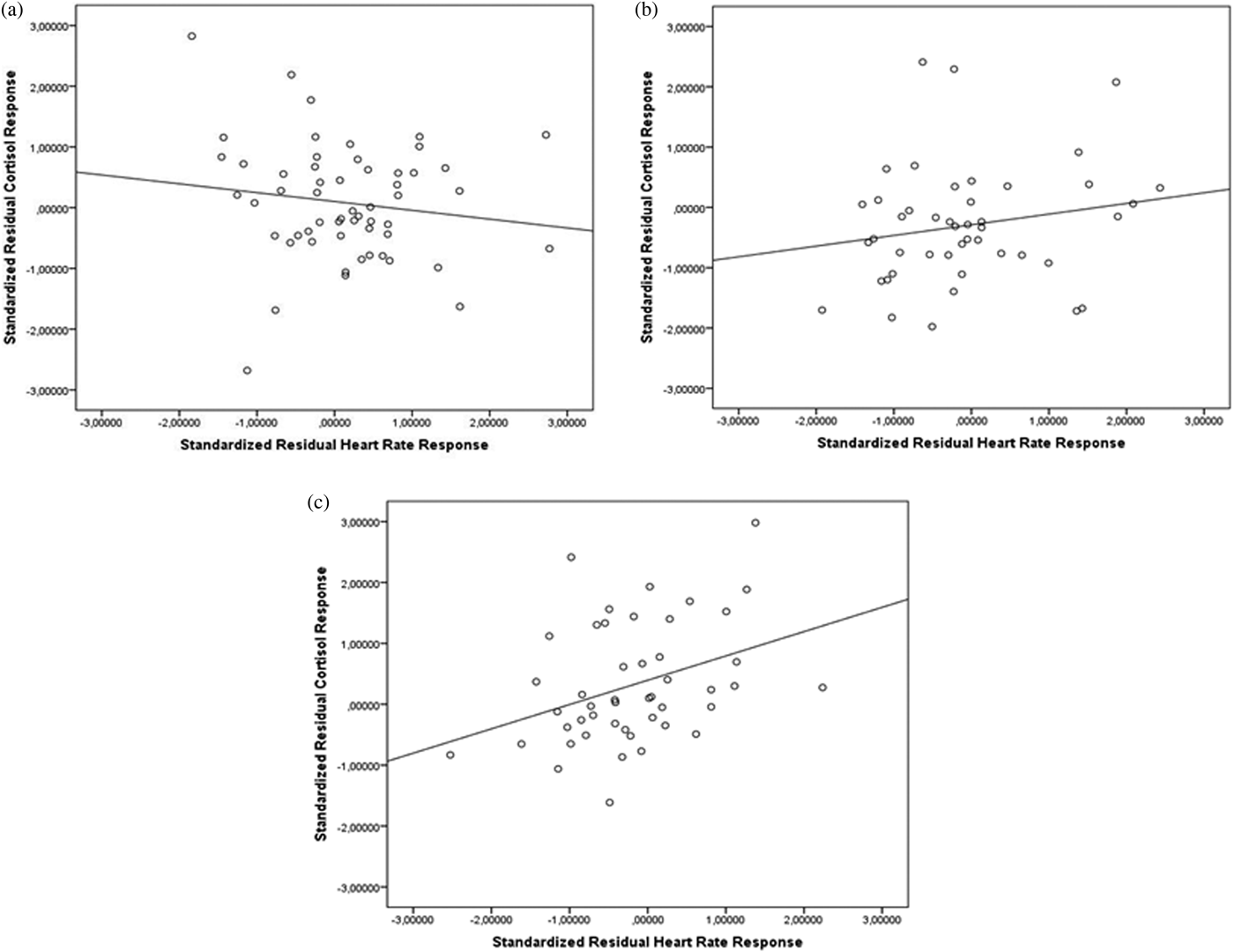
Figure 5. Associations between cortisol and heart rate responses to the TSST according to the severity of maltreatment. (a) No (few) maltreatment experiences, (b) some experiences of maltreatment, and (c) more experiences of maltreatment. TSST, Trier Social Stress Test.
Discussion
The present study examined the association between childhood maltreatment, cortisol, and HR responses to a psychosocial stress in early adulthood. Consistent with previous findings, we found indications of both lower and higher cortisol responses to stress in the context of maltreatment. As previously hypothesized (Del Giudice, Ellis, & Shirtcliff, Reference Del Giudice, Ellis and Shirtcliff2011; Obradovic, Reference Obradovic2012), these patterns of secretion may embody different expressions of changes made by the HPA axis in an attempt to adapt to the perceived environmental demands according to past experiences, such as childhood maltreatment.
Three features of these findings are noteworthy. First, the lower cortisol response noted in participants who reported only some experiences of maltreatment falls in line with the findings from studies that have recruited participants from the general population (Carpenter et al., Reference Carpenter, Carvalho, Tyrka, Wier, Mello, Mello and Price2007, Reference Carpenter, Shattuck, Tyrka, Geracioti and Price2011; Elzinga et al., Reference Elzinga, Roelofs, Tollenaar, Bakvis, van Pelt and Spinhoven2008; Lovallo et al., Reference Lovallo, Farag, Sorocco, Cohoon and Vincent2012; Ouellet-Morin et al., Reference Ouellet-Morin, Odgers, Danese, Bowes, Shakoor, Papadopoulos and Arseneault2011; Peckins et al., Reference Peckins, Dockray, Eckenrode, Heaton and Susman2012; Peckins, Susman, Negriff, Noll, & Trickett, Reference Peckins, Susman, Negriff, Noll and Trickett2015) and Child Protective Services (Trickett et al., Reference Trickett, Gordis, Peckins and Susman2014). A direct comparison of the relative severity of maltreatment captured in these studies is difficult to make, however. More promising is the consideration that the association between maltreatment and cortisol reactivity may not be linear. Many biological and psychological processes are expected to be nonlinear because of the regulating actions of several intertwined structures and systems. Still, linear models remain the analytical strategy of choice (Mattei, Reference Mattei2014; Young & Benton, Reference Young and Benton2015). HPA axis reactivity to stress is similarly modulated by several brain structures processing the cognitive and emotional information according to present and past contexts (e.g., frontal cortex, the hippocampus, and the amygdala), which jointly regulate cortisol secretion through excitatory and negative-feedback pathways at the level of the hypothalamus, the pituitary, and the adrenal glands (Gunnar & Vazquez, Reference Gunnar, Vazquez, Cicchetti and Donald2006). Multiple embedded systems also interact at a molecular level to regulate the initiation, amplitude, and termination of the stress response, including genetic and epigenetic processes, glucocorticoid and mineralocorticoid receptors, endogenous sex steroids, and oral contraceptives (de Kloet, Reference de Kloet2014; Hamstra, de Kloet, van Hemert, de Rijk, & Van der Does, Reference Hamstra, de Kloet, van Hemert, de Rijk and Van der Does2015; Houtepen et al., Reference Houtepen, Vinkers, Carrillo-Roa, Hiemstra, van Lier, Meeus and Boks2016; Kudielka et al., Reference Kudielka, Hellhammer and Wust2009; Meaney, Reference Meaney2010; Ouellet-Morin et al., Reference Ouellet-Morin, Wong, Danese, Pariante, Papadopoulos, Mill and Arseneault2013). The shift from moderate to lower to higher cortisol responses as maltreatment severity increased suggests we should revisit the assumption of linearity when investigating the association between maltreatment and HPA axis reactivity. Nonlinear models may help to depict more precisely the changes (e.g., directionality and strength) of the association between maltreatment and cortisol response to stress according to the severity of the experiences, to better document individual variation in this change and the determinants affecting it (e.g., relative severity and chronicity of exposure, timing; for an in-depth discussion on how nonlinear growth models may be helpful in developmental research, see Grimm, Ram, & Hamagami, Reference Grimm, Ram and Hamagami2011).
Second, the lower cortisol responses in participants who reported some experiences of maltreatment are also consistent with the stress inoculation model, according to which dampened activations of the HPA axis befall milder to moderate exposure to stress (Crofton, Zhang, & Green, Reference Crofton, Zhang and Green2015; Parker et al., Reference Parker, Buckmaster, Schatzberg and Lyons2004). Nevertheless, while lower cortisol response to stress may protect the body against excessive exposure over time, it may not be synonymous to resilience, which refers to better than anticipated adaptation to detrimental environmental influences (Luthar & Zelazo, Reference Luthar, Zelazo and Luthar2003; Shirtcliff, Peres, Dismukes, Lee, & Phan, Reference Shirtcliff, Peres, Dismukes, Lee and Phan2014). Caution may also be warranted given that lower basal cortisol levels and response to stress have been associated with antisocial behaviors and posttraumatic stress disorder (Boks et al., Reference Boks, Rutten, Geuze, Houtepen, Vermetten, Kaminsky and Vinkers2016; Lupien et al., Reference Lupien, Ouellet-Morin, Hupbacha, Tu, Buss, Walker, McEwen, Cicchetti and Donald2006; Susman, Reference Susman2006; van Goozen, Fairchild, Snoek, & Harold, Reference van Goozen, Fairchild, Snoek and Harold2007). Future studies need to identify the mechanisms by which maltreatment confers vulnerability (or resilience) to mental health or behavioral problems (Bowes & Jaffee, Reference Bowes and Jaffee2013).
Third, the shift from moderate to lower to higher cortisol responses to the TSST as maltreatment increased in severity may be better understood, in all its complexity, as diverse expressions of the HPA axis attempting to adapt to distinct maltreatment experiences. This is consistent with the allostatic load (Juster, McEwen, & Lupien, Reference Juster, McEwen and Lupien2010; McEwen, Reference McEwen1998) and the adaptive calibration models (Del Giudice et al., Reference Del Giudice, Ellis and Shirtcliff2011), proposing that the HPA axis’ responses reflect long-term individual trajectories that can be recalibrated according to environmental circumstances and for which adaptive and detrimental costs may ensue. Our findings also parallel Del Giudice et al.’s (2012) model predicting a succession of moderate, lower, and higher stress responses in safe, moderately stressful and risky environments. One difference lies at the very end of the continuum where severe and traumatic environments are hypothesized to result in even lower (males) and higher (females) responses (Del Giudice et al., Reference Del Giudice, Ellis and Shirtcliff2011). However, others have not found evidence for that fourth cortisol profile either (Peckins et al., Reference Peckins, Susman, Negriff, Noll and Trickett2015).
More generally, other studies have also reported a shift for basal cortisol activity, where higher morning levels were noted in moderate adversity while lower levels were detected in more severe conditions (Gustafsson, Anckarsater, Lichtenstein, Nelson, & Gustafsson, Reference Gustafsson, Anckarsater, Lichtenstein, Nelson and Gustafsson2010; van der Vegt, van der Ende, Kirschbaum, Verhulst, & Tiemeier, Reference van der Vegt, van der Ende, Kirschbaum, Verhulst and Tiemeier2009). Higher to lower morning basal cortisol levels were also observed in abused females, from adolescence to adulthood (Trickett, Noll, Susman, Shenk, & Putnam, Reference Trickett, Noll, Susman, Shenk and Putnam2010; see also Trickett, Noll, & Putnam, Reference Trickett, Noll and Putnam2011)). Likewise, Doom, Cicchetti, and Rogosch (Reference Doom, Cicchetti and Rogosch2014) reported higher to lower basal cortisol levels over time in maltreated school-aged children. This findings may also depend, in addition to maltreatment severity, on factors such as the age, duration and type of maltreatment, as well as time passed since the end of maltreatment, and the presence of co-occurring factors enhancing vulnerability (e.g., puberty) or promoting resilience (e.g., social support; Gunnar & Vazquez, Reference Gunnar, Vazquez, Cicchetti and Donald2006; Lupien et al., Reference Lupien, McEwen, Gunnar and Heim2009; Miller, Chen, & Zhou, Reference Miller, Chen and Zhou2007). Future studies should also investigate further whether maltreated individuals with lower or higher patterns of response to stress are more vulnerable to mental health problems and behavioral difficulties. Drawing from the adaptive calibration model, the recalibration of the stress systems may be adaptive by amplifying, or filtering the information present in the environment, in order to avoid further aggression or to develop a sense of normality, for instance. Trade-offs may, however, be present, such as increased sensitivity to social judgment, taxing even further the individuals’ already solicited stress systems and coping mechanisms. The higher risks of internalizing (e.g., depression) and externalizing (e.g., antisocial behavior) problems in individuals with higher and lower cortisol activity, respectively, suggest the possibility that distinct costs may arise depending of the recalibration of the stress systems (Struber, Struber, & Roth, Reference Struber, Struber and Roth2014; Susman, Reference Susman2006). Longitudinal studies investigating the recalibration of the stress response systems as they unfold and according to the numerous intervening factors would be uniquely suited to depict the time-varying trade-offs following these adaptations (Mead, Beauchaine, & Shannon, Reference Mead, Beauchaine and Shannon2010).
Nevertheless, it is only among participants who reported more experiences of maltreatment that a significant degree of symmetry emerged between cortisol and HR responses to stress. This finding, albeit preliminary and exploratory in nature, echoes in part the permissive or preparative actions of glucocorticoids on the SNS, previously suggested by Sapolsky, Romero, and Munck (Reference Sapolsky, Romero and Munck2000). Both the permissive and the preparative actions of glucocorticoids theoretically enhance the body's arousal to a new or repeated appearance of a stressor. In this cross-sectional study for which no measure of diurnal secretion was made, we can only speculate about the possibility that the exposure to higher levels of maltreatment could have also induced persistent changes in basal secretion, so that an enhancing action of glucocorticoids on HR and cortisol responses to the TSST may have been taken place (i.e., permissive actions). Alternatively, the higher cortisol responses to stress observed in participants who reported more maltreatment experiences may reflect a stable pattern of responsivity (i.e., preparative actions). The time-varying and reciprocal nature of these influences complicates the understanding of the symmetry between cortisol and HR responses to stress and its adaptive value (or cost) on health and functioning. Future studies should measure both basal and reactive HPA axis and SNS to disentangle the potential permissive and/or preparative effects of glucocorticoids on the stress systems in increasing levels of maltreatment.
The present findings should be considered in light of some limitations. First, childhood maltreatment was assessed using the CTQ-SF, a well-validated and widely used questionnaire, for which the recall of experiences may, however, have been tainted by memory loss and bias. Nevertheless, other findings suggested that recall bias emerging as a result of directed forgetting and relabeling (Epstein & Bottoms, Reference Epstein and Bottoms2002), for instance, accounted for less than 1% of the maltreatment's variance and that the use of retrospective reports has little effect on the investigation of its long-term impact on functioning (Fergusson, Horwood, & Boden, Reference Fergusson, Horwood and Boden2011). This strategy may even be preferable to the sole consideration of recent exposure to stress that may not have lasted long enough to induce persisting changes in cortisol response to stress (Peckins et al., Reference Peckins, Susman, Negriff, Noll and Trickett2015). Second, we did not examine the possibility that distinct associations may have emerged as a function of maltreatment subtypes (e.g., emotional abuse and neglect) nor did we take into account the fact that the items included in the CTQ are not equally severe in their impact on children (Barnett, Manly, & Cicchetti, Reference Barnett, Manly, Cicchetti and Cicchetti1993). On a related point, our measure could not untangle the impact of frequency from the severity of maltreatment, two concepts shown to be differentially associated with behavioral outcomes (Jackson, Gabrielli, Fleming, Tunno, & Makanui, Reference Jackson, Gabrielli, Fleming, Tunno and Makanui2014). Future research with more participants who were confronted with maltreatment and for whom the experiences could be operationalized more homogenously based on the information available in the official records holds the promise of better describing the impact of this complex, multifaceted concept on the stress response systems and functioning (see Mennen, Kim, Sang, & Trickett, Reference Mennen, Kim, Sang and Trickett2010). Third, since our measure of maltreatment encompassed all experiences that have occurred before 18 years of age, we could not test whether the nature or timing further affected cortisol responses in a nonlinear manner. More generally, the absence of prospectively and repeatedly collected measures in this study impeded us from investigating the developmental processes and time-varying individual and environmental influences that may also have affected the course of stress responses systems. Fourth, our sample was only composed of adult males. Because sex differences in cortisol response to stress (Carpenter et al., Reference Carpenter, Tyrka, Ross, Khoury, Anderson and Price2009) and sexually dimorphic associations with maltreatment have been reported (Negriff et al., Reference Negriff, Saxbe and Trickett2015), although inconsistently so (Kudielka et al., Reference Kudielka, Buske-Kirschbaum, Hellhammer and Kirschbaum2004), our findings may not be generalizable to female participants. Fifth and finally, although HR is a fairly common measure of SNS activity, it remains highly variable and could easily be modulated by factors such as position of the participant (Vogel, Wolpert, & Wehling, Reference Vogel, Wolpert and Wehling2004), in spite of specific instructions asking participants to stay still at a preidentified, marked place during the TSST.
In conclusion, this study extends prior findings regarding the impact of childhood maltreatment on HPA axis and SNS reactivity to stress by reporting distinct expressions of cortisol dysregulated patterns of stress response as maltreatment increased in severity. This study calls into question the linearity assumed to exist in the association between maltreatment and the stress response systems, as well as in regard to its impact on health and behavioral functioning.
Supplementary Material
To view the supplementary material for this article, please visit https://doi.org/10.1017/S0954579418000123.