The prevalence of autism spectrum disorder (ASD) is rising and was estimated to be around 1·5 % in developed countries in 2016(Reference Lyall, Croen and Daniels1). ASD is characterised by impairments in social interaction and repetitive behaviour and is associated with executive dysfunction such as impaired working memory, inhibition and flexibility(Reference Lai, Lau and Lui2). Furthermore, ASD is often associated with multiple comorbidities such as attention-deficit/hyperactivity disorder (ADHD) and depression(Reference Murphy, Wilson and Robertson3). The condition prevails into adulthood, and current treatment options are scarce and come with significant side effects(Reference Murphy, Wilson and Robertson3). Safe alternatives are therefore needed, e.g. essential fatty acids, which over the past decades have attracted attention in the management of neurodevelopmental disorders.
Long-chain n-3 PUFA (n-3 LCPUFA), specifically DHA (22:6 n-3), accumulate in the central nervous system and are essential for optimal brain development. Deficiency in n-3 fatty acids is associated with impaired cognitive function in rodents and monkeys, among other stereotypic behaviour, hyperactivity, inattention and loss of memory as well as deficits in executive functions and changes in monoaminergic neurotransmitters(Reference Healy-Stoffel and Levant4,Reference Hashimoto, Hossain and Al Mamun5) . Blood indices of high n-3 LCPUFA status have been associated with improved function in a wide array of neurocognitive and psychological measures in children and adolescents(Reference Van Der Wurff, Von Schacky and Berge6–Reference Lassek and Gaulin9). Studies in schoolchildren have reported potential benefits of n-3 LCPUFA on neurocognitive functions, e.g. attention, which appear to be modified by gender(Reference Lauritzen, Harsløf, Sørensen, Moran and Lowe10). The most recent meta-analysis indicated that n-3 LCPUFA supplementation improves attention, impulsivity and hyperactivity in children with ADHD(Reference Pei-Chen Chang, Su and Mondelli11), and randomised clinical trials (RCT) indicate beneficial effects in adults with depression(Reference Luo, Feng and Yang12).
A number of studies have observed that children with ASD have low levels of n-3 LCPUFA in different blood fractions(Reference Mazahery, Stonehouse and Delshad13), which have been shown to be associated with impaired cognitive function in children with ASD(Reference Martins, Bandarra and Figueiredo-Braga14). A number of RCT in children with ASD have investigated effects of n-3 LCPUFA, some of which have shown improvements in selected executive functions, but results from meta-analysis are inconsistent(Reference Mazahery, Stonehouse and Delshad13,Reference De Crescenzo, Loreto D’alò and Morgano15–Reference Horvath, Łukasik and Szajewska17) . Effects of n-3 LCPUFA in adults with ASD have only been investigated in one small uncontrolled study that examined changes in the severity of problematic behaviours that had a very high variability(Reference Politi, Cena and Comelli18). Most of the aforementioned studies in children had a low number of participants, used n-3 LCPUFA doses < 1·5 g/d and examined only a few neurocognitive outcomes – mainly assessed using questionnaires and no objective tests. None of them have examined whether the effects are influenced by comorbid ADHD or depression.
The present randomised crossover study aims to explore the effect of fish oil (FO) on attention and spatial working memory as well as cognitive flexibility, general executive functions and core symptoms of ASD and ADHD in adults with ASD. In light of the shared and additive cognitive impairments in individuals with combined ASD and ADHD, we hypothesise that individuals with comorbid ADHD will show the most pronounced effects, but we will also examine potential interactions with depression and gender.
Methods
In order to investigate the cognitive effects of n-3 LCPUFA in adults with ASD, we performed a 2 × 4 week randomised double-blind crossover study with FO and safflower oil (SO) supplementation. The study was conducted at the Department of Nutrition, Exercise, and Sports at University of Copenhagen in December 2019 – February 2020.
The study was conducted in accordance with the Helsinki Declaration and registered at ClinicalTrials.org in March 2021 (NCT04779632). According to the Danish National Ethics Committee, the project is exempt from formal approval, as the intervention only consisted of dietary supplements and the finger-prick blood samples were used only as an indicator of compliance. The collection of data was performed in accordance with the General Data Protection Regulation and the Danish Act on Processing of Personal Data. Written informed consent was collected from participants before randomisation.
Participants
Participants were recruited from the vicinity of Copenhagen through advertisements on autism-related institutions and Facebook, as well as via personal networking. The inclusion criteria were 18–40 years of age and a self-reported clinical diagnosis of ASD, either Asperger’s syndrome, autism disorder or pervasive developmental disorder – not otherwise specified. Subjects were excluded, if they reported substance abuse, major psychopharmacological adjustments or n-3 LCPUFA supplementation during ≤ 28 d before the beginning of the study.
Intervention and randomisation
The intervention composed of two 1-month periods of supplementation with FO and SO, respectively, without a washout period. The participants were randomised to intervention sequences: FO → SO or SO → FO, using an online-available, random number generator (http://www.randomization.com) with a blocks size of six. A person, who was not involved in the data collection, generated the randomisation list and labelled the oil capsule containers with ID and period. Participants were allotted ID numbers based on date and time of their first visit and supplied with the relevant capsule container in the beginning of each period. The participants were instructed to take four capsules two times per day until the next scheduled visit – corresponding to approximately 5·2 g/d of n-3 PUFA (hereof 2·4 g EPA (20:5 n-3) and 1·6 g DHA) from the FO or 2·8 g/d of linoleic acid from the SO. The FO and SO capsules were donated in kind by two different companies, 1 ml capsules of Eskimo-3 High 65 % from Midsona (Malmö, Sweden) and 0·75 m capsules of SO from Natur-Drogeriet A/S (Hørning, Denmark), and they differed in colour as well as in smell and taste. Both types of capsules were supplied in white containers of similar appearance and in the same known excess amount of what was required for four weeks. The investigator tried to prevent unblinding due to differences in rattling sound and weight of the containers by use of noise cancelling in ear headphones and by asking colleagues and participants to handle the containers. Formal unblinding did not occur before the primary statistical analysis was completed.
Outcome assessment
Participants were tested at baseline and at the end of each intervention period, and the three visits were scheduled four weeks apart (±2 d). The test visits took place at the department or in the participant’s home based on personal preferences of the participants. All three test visits took place at the same time of the day and in the same location, except for two of the participants. The same investigator collected all data, and the order of the individual elements was kept constant across all visits. The participants were instructed to eat one to four hours before the visit.
Background information about diagnoses and medication, as well as living conditions and lifestyle, was collected in an interview at the baseline visit. At the follow-up visits after the two periods, they were asked about potential events that could affect the outcomes (e.g. changes in medication) in the preceding intervention period. We also asked about possible sources of performance-interfering variables (e.g. lack of sleep or food, etc.) in the last two days before the visit.
The primary outcomes were short-term spatial working memory assessed by the Corsi block-tapping test and sustained attention by the d2-test of attention. We performed a Stroop colour and word test and asked the participants to complete three neurocognitive questionnaires as additional secondary outcomes. The selection of the d2 attention and Corsi memory scores as the primary outcomes was based on the assumption that these tests would be more sensitive than the scores from the questionnaires. We did not make any sample size calculation because recruitment was limited by a lack of funding and time.
Neurocognitive tests
The d2-test of attention(Reference Bates and Lemay19) is a paper and pencil test that consists of fourteen lines with the characters ‘d’ and ‘p’ that have one to four dashes above and/or below the character. Participants were given 20 s per line to mark all d’s with two dashes (d2’s) and none of the distracting characters. Omission errors, i.e. missed d2’s, were used as a measure of inattention and the number of commission errors, i.e. marked distractors, as an indicator of lack of inhibition. Total errors were expressed as percent of all processed characters to avoid the influence of processing speed. We calculated processing variability as the difference between the maximum and minimum number of processed characters per line as an indicator of persistence control(Reference Steinborn, Langner and Flehmig20).
The Stroop test(Reference Scarpina and Tagini21) tests the ability to process two conflicting stimuli simultaneously and is used to assess inhibition and cognitive flexibility. We used the colour and word version, which consists of a word card, a colour card and a word-colour card. The last card presents a conflict, as the words are written in a colour that differs from the colour that the word spells. The participants were asked to read the cards aloud and correct, i.e. correct mistakes before moving on, and to state the colour of the letters and ignore the meaning of the word on the word-colour card. The reading speed of word and colour cards were used as measures of processing speed, whereas the time for the word-colour card was used as an indicator of the ability to inhibit reactions to distractors. The measure of cognitive flexibility, i.e. the relative Stroop effect, was calculated as time used for the word-colour card relative to the average time for the two simple cards.
The Corsi block-tapping test of short-term visuo-spatial working memory(Reference Berch, Krikorian and Huha22) (downloaded in a trial version from millisecond.com) consists of ten yellow blocks that are scattered in an undiscernible pattern on a blue screen on a 15·4” laptop. In the beginning of each run, a number (two at level 1 and nine at level 8) of the yellow blocks flash in a sequence that the test subject has to memorise and subsequently repeat by mouse clicks on the squares in the correct order (with the possibility to correct accidental clicks). Each level consists of two rounds or more until the test subject manages to perform two sequential correct test runs and thus moves to the next level. The test ends when the test subject makes three consecutive incorrect runs. The block span score indicates the last correctly completed level, and the total score is based on the total number of blocks in correct runs.
The investigator ensured optimal lighting and lack of sensory disturbances during the testing.
Neurocognitive questionnaires
The participants were provided with three questionnaires: Conners Adult ADHD Rating Scale (CAARS short version), the adult version of the Behavioural Rating Inventory of Executive Function (BRIEF-A) and the Social Responsiveness Scale (SRS-2 s edition of the self-reported adult version), which they had to answer in the given order. High scores indicate dysfunction in all of the questionnaires scales.
The Conners scale (23) measures ADHD-related symptoms based on thirty questions each with four choices (never – often), which generate four scores: a total symptom score (range 0–54) with subscores for inattention and hyperactivity and impulsivity (nine questions each) and an ADHD index score (range 0–36) based on the last twelve questions, which assess key items that differ between subjects with and without ADHD. The ADHD symptom score was converted to T-scores based on age- and gender-specific norms.
BRIEF-A is designed for use in a wide variety of psychiatric disorders, including ADHD, ASD, and depression(Reference Roth, Lance and Isquith23). It contains seventy-five items with three options (never – always a problem) that sums up in an overall global executive function composite (GEC) score (range 75–225), which has two broad subscales: the behavioural regulation index (BRI) (range 30–90) and the meta-cognition index (MI) (range 45–135). The BRI score includes impulse inhibition, flexibility, emotional control and self-monitoring scales, and the MI score includes subscales for cognitive initiation, working memory, planning, organisation of materials and task monitoring. The BRI sub-score for impulse inhibition and MI sub-score for working memory can be used to differentiate between the hyperactive-impulsive ADHD subtypes and the predominantly inattentive subtype(Reference McCandless and O’Laughlin24).
SRS-2 assesses core ASD symptoms, i.e. social communication and interaction, and repetitive behaviours and restricted interests(Reference Bruni25). The questionnaire has sixty-five questions with four answer categories (not true – usually true) that sum up to a total SRS score (range 0–195) with age and T-scores were generated based on gender-specific norms. There are two sub-scores, the repetitive behaviours and restricted interests score (range 0–36) and the social communication and interaction score (range 0–159), and the latter is subdivided into four sub-scores: awareness, cognition, communication and motivation.
Compliance assessment
Compliance was assessed in three ways: (1) participant reported compliance as a crude estimate of percentage of the targeted number of capsules they had taken, (2) counting of returned unused capsules and (3) analysis of EPA + DHA in whole-blood samples.
Blood was collected from the tip of the fourth finger and applied on approximately 2·5 cm2 of antioxidant-prepared chromatography paper (Grade 3MM, Whatman Ltd; supplied by Frederiksen Scientific A/S), which was prepared within 6 h before the visit. Samples were dried at room temperature and stored in regular postal envelopes at −25°C for a maximum of 3 months before they were shipped to Department of Kinesiology, University of Waterloo, Canada. Fatty acid composition was determined by fast gas chromatography as previously described(Reference Metherel, Hogg and Buzikievich26). In brief, trans-methylated fatty acids were separated on a Varian 3900 gas chromatograph equipped with a DB-FFA 15 m × 0·10 mm capillary column coated with 0·10 μmnitroterephthalic-acid-modified polyethylene glycol (J & W Scientific; Agilent Technologies). Peaks were identified by retention time comparisons with an external standard (GLC-246; Nu Chek Prep Inc.), and fatty acids were expressed as a percentage of all fatty acids (FA%). All samples, except two baseline samples, were successfully analysed.
Statistical analysis
The assumption of normality was verified by inspection of histograms and Shapiro–Wilk’s test. Variables in the continuous scale are presented as mean ± sd, count variables as median (25th; 75th percentile) and categorical variables as n (%). Statistical significance was established at P < 0·05, and trends at P < 0·10.
Baseline characteristics in subgroups were compared by t test for continuous variables with equal and unequal variance as determined by Barlett’s test. Count variables were compared by Mann–Whitney U-test, and Barnard’s CSM test was used for comparisons of categorical variables. Correlations between the three measures of compliance (reported, capsule counts and whole-blood DHA + EPA) were examined by linear regressions.
Treatment effects of the oil intervention were analysed as complete-case, excluding participants with missing data at baseline or one of the follow-up visits. The primary analysis of differences between the oils in variables on the ordinal and continuous scale was performed by linear mixed models with participant ID as random effect and treatment, baseline, sequence and period as fixed effects. Potential differences in count variables were analysed by generalised linear mixed models (Poisson or binomial) with participant ID as random effects and the same fixed effect variables as in the models for the continuous variables. Models were verified by inspection of normal residual and Q-Q plots. Estimated differences between the oil supplements are presented as mean (95 % CI) for continuous variables and incidence ratio or odds ratio for the count variables. Due to the presence of some extreme outliers, sensitivity analysis was performed in two steps by removal of the most extreme outliers based on the calculation of Cook’s D, excluding the most influential outlier in the secondary analysis and the two most influential in the tertiary analysis. Both levels of sensitivity analysis were performed exactly as the primary analysis. Potential carryover effects were examined in linear mixed models with the inclusion of a period × sequence–interaction term in addition to the fixed and random effects. Potential differences in the oil treatment effects in subgroups (ADHD diagnosis yes/no, depression yes/no, and man/woman) were examined by stratified analyses similar to the primary analysis and by analysis in the combined group with the inclusion of a subgroup × treatment interaction term.
Dose–response relationships between whole-blood DHA + EPA at follow-up and means of effects on outcomes were examined by linear mixed models with participant ID as random effect and baseline, sequence and period as fixed effects, and the results are given as r 2.
Results
We recruited twenty-six participants with an even distribution of men and women (Table 1). Most of the participants had 3 years of education after primary school, ten were still in education, but for those, who had finished their education, the ratio between employed/unemployed was almost 50:50. Their smoking and alcohol habits were very moderate (i.e. only three non-heavy smokers and alcohol mainly at parties), but the habitual intake of fish and the level of physical activity were low. They were generally unmarried and living alone, hereof ∼50 % in housing facilities for people with autism. The mean total SRS-2 T-score was 63 ± 11, and the most common ASD diagnosis was Asperger’s syndrome. The frequency of comorbidities was high, primarily ADHD and affective disorders, and they typically received more than one type of medication, psychostimulants against ADHD and various types of drugs for depression and anxiety (antidepressants and anticonvulsants) or sleep problems (hypnotics and antipsychotics).
Table 1. Baseline characteristics in all participants and stratified by gender and comorbidities
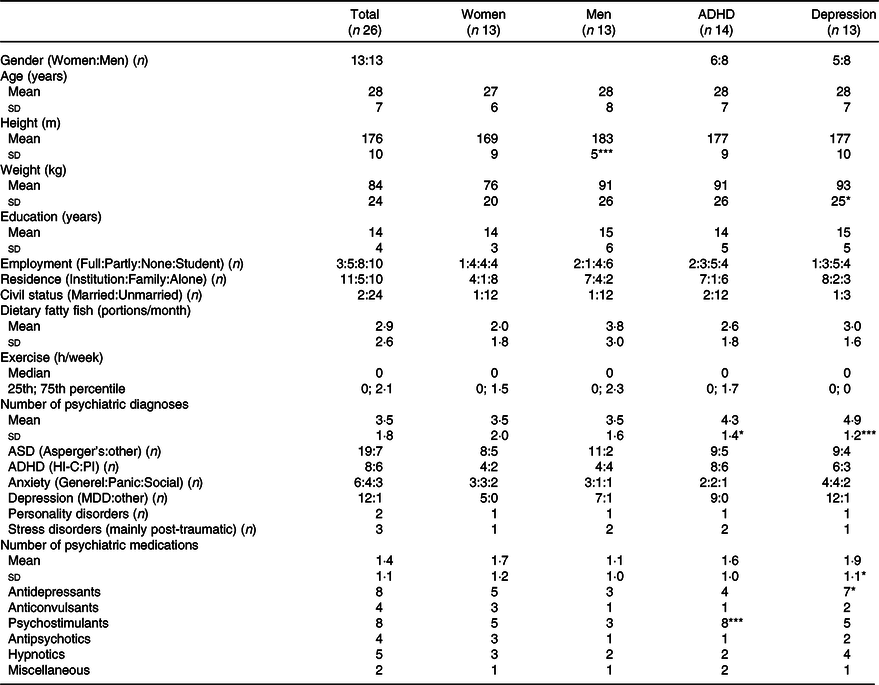
Data are given as mean ± sd, median (25th; 75th percentile) or n. Differences from the remaining participants are marked by *P < 0·05; **P < 0·01 and ***P < 0·001.
ADHD, attention-deficit/hyperactivity disorder including the predominantly inattentive (PI) and predominantly hyperactive-impulsive and combined (HI-C) subtypes; MDD, major depression disorder (most with additional seasonal depression); employment (full time: part time or flex job: unemployed: student).
Four participants (two from each allocation group) dropped out between baseline and the first follow-up visit, three due to depression and one due to disc herniation (Fig. 1). Dropouts were characterised by more psychiatric diagnoses, more psychopharmacological treatments, reduced executive function scores and higher scores on Conners ADHD rating scale compared with the completers (online Supplemental Table 1). One of the completing participants did not want to answer the questionnaires at the last visit, leaving twenty-one participants for analysis of effects on the questionnaire scores, twenty-two for the tests, and twenty for dose–response analysis due to two missing baseline values for EPA + DHA.

Fig. 1. Flow diagram of the crossover trial with fish oil (FO) v. safflower oil (SO).
Seventeen participants (77 %) guessed correctly about the allocated oil sequence, and the investigator guessed 68 % correct. The participants reported that they had taken 96 % (range 41–105 %) of the requested capsules with no difference between the two oils, and this was supported by counts of returned capsules (94 %) (r 2 = 0·49, n 22, P = 0·011). Furthermore, the capsule counts correlated with whole-blood EPA + DHA after the FO period (r 2 = 0·50, n 22, P < 0·001). The difference in EPA + DHA after the oil interventions was 2·7 ± 0·3 FA%, mainly caused by an increase in EPA after FO (Table 2 and the full fatty acid composition in online Supplemental Table 2). The FO-induced increase in n-3 LCPUFA replaced a similar decrease in linoleic acid, thus, no difference in total PUFA, but a 50 % lower n-6 to n-3 fatty acid ratio after FO compared with SO. The total whole-blood content of SFA was higher, and MUFA was lower after FO compared with SO.
Table 2. Effect of the oil intervention on whole-blood fatty acid composition
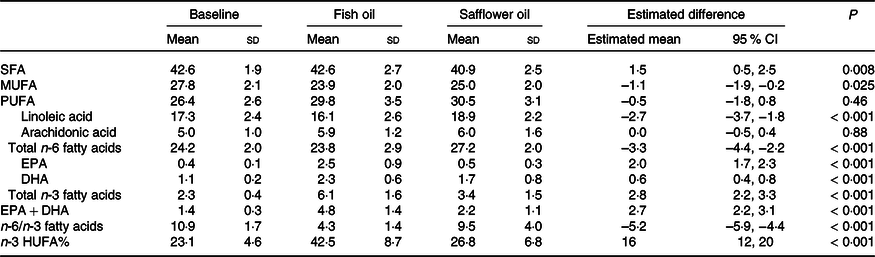
Data are given as percentage of all fatty acids and presented as mean ± sd (n 24 at baseline and n 20 at the end of the intervention periods). The effect of fish oil is presented as the estimated mean (95 % CI) differences relative to safflower oil based on linear mixed models with participant ID as random effect and treatment, baseline, sequence, and period as fixed effects.
n-3 HUFA%, long-chain n-3 fatty acids (≥ C20 : ≥ 3 n-3) of all long-chain PUFA.
FO supplementation gave rise to a 30 % decrease in the likelihood of errors in the d2 test mainly due to a reduction in omission errors (Table 3). The processing speed in the d2 test showed no difference, but the reading speed for both the word card and the conflicting word-colour card was faster with no change in the relative Stroop effect. In addition, we observed improved performance in the Corsi test, both block span and total score, after FO compared with SO. No carryover effects (i.e. main effects of sequence) were observed for any of the outcomes (online Supplemental Table 3). All these differences persisted after exclusion of the one or two most influential outliers (online Supplemental Table 4). Furthermore, the effects on the Stroop cards and Corsi test scores were supported by dose–response analysis with whole-blood EPA + DHA (with r 2 in the range 0·15–0·20, n 20, and P-values of 0·029–0·040, data not shown).
Table 3. Effect of the oil intervention on neurocognitive test performance
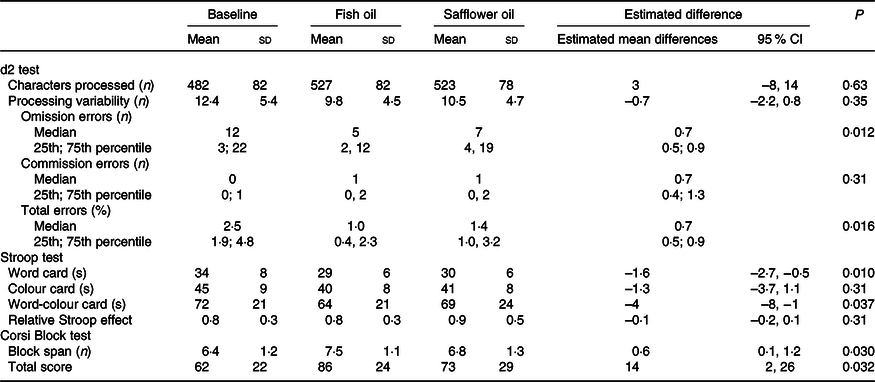
Data are given as mean ± sd or median (25th; 75th percentile) at baseline (n 26) and at the end of each oil intervention period (n 22). Estimated mean differences (95 % CI) between the oils (fish oil v. safflower oil) are based on linear mixed models with participant ID as random effect and treatment, baseline, sequence and period as fixed effects. Count variables were analysed by generalised linear mixed models (Poisson or binomial) adjusting for the same factors and covariates, and the results are shown as incidence ratio (omission and commission errors) or odds ratio (total error%).
The only significant and sensitivity analysis persistent difference between the treatments in the questionnaires (Table 4 and Supplemental Table 5) was in the Conners score for inattention, which like the effect on d2 errors, improved by around 0·3 × sd after FO supplementation. This was supported by a near-significant correlation with the increase in whole-blood EPA + DHA (r 2 = 0·15, n 20, P = 0·073). The decrease in the inattention score was reflected in a tendency for improvement in the combined score for ADHD symptoms, which became significant after exclusion of outliers (online Supplemental Table 5).
Table 4. Effect of the oil intervention on neurocognitive scale scores

ADHD, attention-deficit/hyperactivity disorder; RI&RB, restricted interests and repetitive behaviour; BRI, behavioural regulatory index; GEC, global executive function composite; MI, meta-cognition index.
Data are given as mean ± sd (n 26 at baseline and n 21 at the end of the two oil intervention periods). Estimated mean differences (95 % CI) between the fish oil and safflower oil treatments are based on linear mixed models with participant ID as random effect and treatment, baseline, sequence and period as fixed effects.
As expected, participants with ADHD had higher inattention and total ADHD symptom scores than participants, who did not have an ADHD diagnosis (online Supplemental Table 6), and this translated into a difference in the ADHD symptom T-score of 60 ± 12 v. 47 ± 10 (P = 0·014). They did not differ in BRIEF scores or test performance (online Supplementary Table 6), but responded differently to the FO intervention. Participants without ADHD exhibited the most pronounced benefits on performance in the neurocognitive tests (Table 5). Their odds for d2 errors after FO were 0·4 (0·2; 0·6) of that after SO (P = 0·004), while the change in those with the diagnosis was negligible (0·9 (0·6; 1·3), P interaction = 0·002). Furthermore, the relative Stroop effect was only improved among participants without ADHD (P interaction = 0·058) due to a specific improvement in word-colour card speed, whereas participants with ADHD appeared to get ∼2 s faster on all cards, although only significant for the word card (Table 5).
Table 5. Effect of the oil intervention on test performance in participants with and without attention-deficit/hyperactivity disorder (ADHD)

Data are given as mean ± sd and estimated mean difference (95 % CI) between fish oil and safflower oil based on eleven participants in each strata. Analyses were performed by linear mixed models with participant ID as random effect and treatment, baseline, sequence and period as fixed effects. Omission and commission errors were analysed by generalised linear mixed models (Poisson) with the same adjustments, and results are presented as incidence ratio, except for commission errors in participants with ADHD due to lack of fit with the model. Potential differences in oil effects between the strata were also examined by inclusion of a subgroups × treatment–interaction term in models for the combined group and effect modification by ADHD diagnosis is indicated by *P = 0·058; **P = 0·007.
Contrary to the effects of FO on test performance, the improvements in ADHD symptom ratings after FO were confined to participants with ADHD, who tended to report reductions in both the inattention and hyperactivity and impulsivity scales (Fig. 2). This summed up to a 3·5 point reduction in the total ADHD symptom score after FO compared with SO, which is equivalent to approximately 40 % of the score difference between participants with and without ADHD at baseline (P interaction = 0·096). This was supported by an improved BRI score (mainly impulse inhibition and self-monitoring). Interestingly, FO appeared to have the opposite effect in those without ADHD (Fig. 2), who showed a near-significant exacerbation of GEC, while those with the ADHD diagnosis had a non-significant score reduction (P interaction = 0·039). The main contributor to the adverse effect on GEC in participants without ADHD was MI (P = 0·064) (driven by a decrease in working memory), but the pattern was similar for most of the individual BRIEF scores (online Supplemental Table 7).
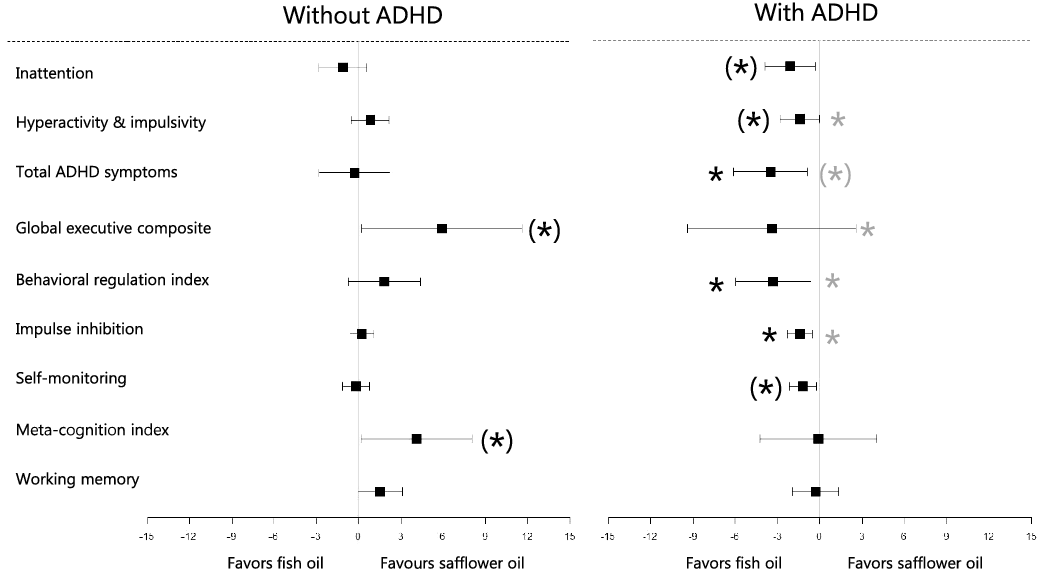
Fig. 2. Estimated difference in neurocognitive questionnaires scores after fish oil v. safflower oil supplementation in participants with and without attention-deficit/hyperactivity disorder (ADHD). Estimates are given as mean (95 % CI, n 10 with ADHD and n 11 without ADHD) from stratified linear mixed model analysis with participant ID as random effect and treatment, baseline, sequence and period as fixed effects. Effect modification by ADHD diagnosis was examined by a similar analysis in the combined group with inclusion of a subgroup × treatment–interaction term. Black asterisks indicate the level of significance within the subgroups, and grey asterisks indicate ADHD status interaction ((*) P < 0·10 and * P < 0·05).
There was no effect of the intervention on any of the SRS-2 scores regardless of ADHD diagnosis status, and no signs of effect modification by depression or gender (online Supplemental Table 8). The scores in neurocognitive tests and questionnaires did not differ between participants with and without depression (data not shown).
Discussion
The results showed improvement in both primary outcomes, attention in the d2 test, and working memory, after FO v. SO, and this was supported by better Conners scores of inattention and dose–response relationships with whole-blood DHA + EPA. We observed amelioration of ADHD symptoms among participants with ADHD, who also reported better BRI and GEC scores. Those without ADHD appeared to have adverse effects of FO on BRIEF scores, mainly MI, but they achieved the most pronounced benefit on d2 and faster completion of the Stroop word-colour card.
The effect of n-3 LCPUFA in adults with ASD has only been investigated in one small uncontrolled study, which, based on an assessment with a high variability, suggested a potential decrease in the severity of problematic behaviour(Reference Politi, Cena and Comelli18). A recent meta-analysis of nine RCT (n 405 children) did not find strong evidence for an effect of 0·2–2·2 g/d n-3 LCPUFA on ASD symptoms(Reference De Crescenzo, Loreto D’alò and Morgano15). Three meta-analyses from 2017 came to different conclusions – two showed benefits on lethargy(Reference Cheng, Tseng and Chen16,Reference Horvath, Łukasik and Szajewska17) and restricted stereotyped behaviours(Reference Mazahery, Stonehouse and Delshad13,Reference Cheng, Tseng and Chen16) , one found improved social interaction(Reference Mazahery, Stonehouse and Delshad13), but two of the meta-analyses indicated a potential adverse effect on total SRS score(Reference Cheng, Tseng and Chen16,Reference Horvath, Łukasik and Szajewska17) . A subsequent randomised controlled trial (RCT) providing 0·7 g/d of DHA to seventy-three children indicated improved total SRS(Reference Mazahery, Conlon and Beck27), whereas a crossover study with sixty-eight children found neither adverse nor beneficial effects of 1 g/d of n-3 LCPUFA on core ASD symptoms(Reference Parellada, Llorente and Calvo28). The RCT have varied in duration (6–52 weeks), age (2–17 years), and exclusion based on medication or comorbidities. None of the meta-analysis made stratified analysis, but there are no apparent pattern in effects based on these variables. Overall, the observed null effect on SRS scales in our medicated adult participants with comorbidities is in line with previous results in children with ASD.
As for ASD, there are no previous studies on the effect of n-3 LCPUFA on ADHD symptoms in adults, and RCT in children vary in results and design. The latest meta-analysis of seven RCTs reported that n-3 LCPUFA supplementation improved clinical symptoms in children with ADHD(Reference Pei-Chen Chang, Su and Mondelli11). An older meta-analysis based on 10 RCT, of which 50 % included children with other psychiatric diagnoses, showed a reduction in ADHD symptoms of around 0·3 × sd (Reference Bloch and Qawasmi29). Notably, this is similar to the observed effect size in the recent meta-analysis and the estimated treatment difference in the present study. Both meta-analysis indicate dose-dependency and most pronounced effects in studies with ≥ 0·5 g/d of EPA, but the number of trials and variation in DHA does not allow for differentiation between the two n-3 LCPUFA. A recent RCT found adverse effects on Conners scores after an intervention with EPA-rich FO (∼0·6 g/d of EPA) in children with ADHD(Reference Cornu, Mercier and Ginhoux30). Moreover, two new RCT in children with ADHD used DHA supplements, one saw no effects of 0·5 g/d of DHA(Reference Crippa, Tesei and Sangiorgio31), while the other found a reduction in ADHD symptoms (mainly attention) after 1 g/d(Reference Rodríguez, García and Areces32).
Contrary to the effects on clinical outcomes, we found that participants with ASD without comorbid ADHD exhibited pronounced benefits of FO in the neurocognitive tests, especially on d2 errors and the conflicting Stroop card. None of the studies in children with ASD used objective cognitive tests. A meta-analysis of RCT in children with ADHD reported that n-3 LCPUFA supplements improve performance in attention tests(Reference Pei-Chen Chang, Su and Mondelli11), and this was supported by two(Reference Crippa, Tesei and Sangiorgio31,Reference Pei-Chen Chang, Su and Mondelli33) of four trials that were published after the meta-analysis(Reference Cornu, Mercier and Ginhoux30,Reference Rodríguez, García and Areces32) . Two large meta-analyses of RCT in both children and adults with ADHD (some RCT also included subjects with other neurodevelopmental disorders or typical development) do not provide strong support for cognitive effects of n-3 LCPUFA(Reference Cooper, Tye and Kuntsi34,Reference Emery, Häberling and Berger35) . However, both indicated an effect on working memory mainly in a clinical setting after provision of an adequate dose(Reference Cooper, Tye and Kuntsi34,Reference Emery, Häberling and Berger35) . Studies in schoolchildren have shown benefits of n-3 LCPUFA supplements on cognitive functions, including attention(Reference Lauritzen, Harsløf, Sørensen, Moran and Lowe10), and we recently found a dose-dependent improvement of attention, impulsivity, and cognitive flexibility as well as reduced behavioural problems in children, who received fatty fish compared with poultry(Reference Teisen, Vuholm and Niclasen36).
The FO-induced amelioration of ADHD symptoms among participants with ADHD might be expected, as they would have more room for improvement. Moreover, the effect seems consistent as the decrease in the hyperactivity and impulsivity score was accompanied by better BRI scores, but the alleviation of attention was not supported by fewer d2 errors. It is also puzzling that participants without ADHD showed benefits in d2 and Stroop that were not supported by the Conners and BRI scores and that they experienced an adverse effect on MI based on an exacerbation of working memory, while their Corsi test performance was better after FO. We expected that the tests would be more sensitive than the neurocognitive questionnaires, but we saw some ceiling effects, e.g. in the d2 test, where ∼15 % of the participants processed characters faster than the allotted time and 10 % did not make any errors. Furthermore, learning effects were indicated in the measures from d2, Stroop, and some of the scales, including GEC and BRI, and this could blur effects of FO. However, it is unlikely that learning or test sensitivity could give rise to ADHD comorbidity-dependent differences in effects of FO.
Our study is strengthened by the crossover design, which is a specific advantage in trials with cognitive outcomes and subjective assessments, and furthermore, accommodates heterogeneity among the participants. Furthermore, the study excels by the high dose of n-3 LCPUFA, which is within safety limits(37), but not easily achieved by intake of fish. The reported compliance was good and verified by whole-blood EPA + DHA, although the observed EPA + DHA levels were lower than expected with 5 g/d(Reference Patterson, Chalil and Henao38). This might be ascribed to the very low baseline and lack of saturation after 4 wk. Incorporation of n-3 LCPUFA could have been increased, if the study was extended, but several potential mechanisms of action may exert short-term effects, e.g. via signalling molecules and transcription factors(Reference Hashimoto, Hossain and Al Mamun5). Studies in rodents have shown that n-3 fatty acid deficiency and supplementation can affect monoaminergic neurotransmitters(Reference Healy-Stoffel and Levant4), which are involved in regulation of cognitive function and ADHD pathology. Blinding is an inevitable problem in RCT with FO due to the smell and taste and a concern due to the difference in capsule size. The proportion of correct allocation guesses was 50 % higher than expected by chance. Whole-blood DHA + EPA did not indicate that the participants took FO during the SO intervention period, but we cannot exclude that the participant’s or investigator’s beliefs about the benefits of FO could affect the results, which is probably less likely for the neurocognitive tests than for the self-reported questionnaire answers.
Our primary outcomes were the psychometric measures of attention and working memory, but we used more than one measure from each test, and these cognitive domains were also targeted by the clinical questionnaires. The large number of statistical tests increases the risk of type I errors, but a standard Bonferroni correction would over-adjust due to correlations between the outcomes. Furthermore, seven significant differences in the main analysis are more than expected by chance with thirty-four outcomes, and the differences were generally persistent in sensitivity analysis and supported by dose–response relationships. Unfortunately, the sample size was limited by a lack of funding and time. The small sample size may have restricted our ability to detect differences, most likely in the clinical scales, but power was increased by the crossover design, and we based our interpretation on consistency in observed effects and tendencies. Low power is a particular concern for the stratified analyses, which were limited to subgroups with even representation. We kept our inclusion criteria to a minimum, in order to achieve a heterogeneous group of participants in terms of medication and comorbidities, thereby enabling exploratory analyses of potential influences of comorbidities, which is clinically relevant and advantageous for generalisability.
Although we did not find any effects of FO on the core symptoms of ASD, the results support a benefit on ADHD and changes in the BRI scores suggest effects on behavioural regulation problems. The SRS-2 and ADHD T-scores of the completers were moderate, but dropouts were more dysfunctional, specifically with respect to ADHD severity and they could potentially have benefitted more from the FO supplement. One might also suspect that the effect on ADHD could be larger in people with comorbid ASD, but the observed effect on ADHD symptoms was similar to that previously seen in studies in children with ADHD(Reference Pei-Chen Chang, Su and Mondelli11). The observed effect size in the participants with ADHD is equivalent to ∼0·4 × sd or a 5-point reduction in T-score, which is considered to be of clinical relevance as it amounts to 30–50 % of reported effect sizes of commonly prescribed pharmacological ADHD medications(Reference Cortese, Adamo and Del Giovane39). It is noteworthy that the observed effects occur on top of the effects of pharmacological treatments. The employed neuropsychological test measures are considered relevant in the diagnosis of ADHD in preschool children(Reference Merkt, Siniatchkin and Petermann40), and scores from the d2 test have been shown to correlate with improvements in ADHD symptom after methylphenidate treatment(Reference Wienbruch, Paul and Bauer41). Furthermore, visuospatial working memory tests have been shown to predict reading(Reference Pham and Hasson42) and mathematical performance in children(Reference Allen, Higgins and Adams43).
Conclusion
Our results did not show any effect of FO on the core symptoms in adults with ASD, but indicated improvements in attention and working memory, which appeared most pronounced in subjects without ADHD. Furthermore, the study supports a benefit of n-3 LCPUFA on ADHD symptoms and behavioural regulation in subjects with comorbid ADHD, but the results indicated potential adverse effects on meta-cognition in subjects without ADHD. These results warrant further studies of the effects of a high dose of FO and potential differences in effects in individuals with ADHD, ASD or both diagnoses.
Acknowledgement
We thank all the participants for their time and interest and lab technician Inge Rasmussen for her support and help with the blood samples.
The study did not receive any financial support; apart from a small student allowance from the department and in kind supply of the supplement oils from Midsona and Natur-Drogeriet.
B. L. and L. L. designed the study and drafted the manuscript; B. L. collected the data and performed statistical analysis; A. S. E. and L. L. supervised the interpretation of the clinical data; C. R. supervised the statistical analysis and K. D. S. was responsible for the fatty acid analysis. All authors contributed to the manuscript and approved of the final version.
None of the authors have any conflicts of interest to declare.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114522000393









