Article contents
The photocathodic behavior of hierarchical ZnO/hematite hetero nanoarchitectures
Published online by Cambridge University Press: 07 April 2016
Abstract
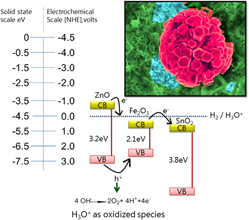
The photocathodic current density of ZnO/hematite hetero nanoarchitectures electrode has been reported in the current investigation. The electrode was obtained with a cheap and two-step hydrothermal functionalization of pristine silicon doped hematite film. The optical, structural, and morphological properties of the electrodes have been studied in detail and it is found that the ZnO functionalization of hematite changes its crystallographic properties by decreasing the Bragg peak intensity ratio for (104) planes. The morphology obtained in this case is unique in the sense that it does not cover the original hematite film and is formed in an isolated manner. Finally, employing a qualitative energy band gap model for mixed metal oxides energy levels has identified the photocathodic properties of the electrode. Here it is found that the photocathodic properties of the electrode are much higher when an electron transfer takes place from the conduction band of ZnO into the electrolyte while hole generated in ZnO is transferred back to hematite but it also degrades the structures after running photoanodic current density sweep. That points to the decrease of water oxidation behavior of hematite in conjunction with ZnO.
- Type
- Articles
- Information
- Journal of Materials Research , Volume 31 , Issue 11: Focus Issue: Advanced Materials and Structures for Solar Fuels , 14 June 2016 , pp. 1554 - 1564
- Copyright
- Copyright © Materials Research Society 2016
References
REFERENCES
- 5
- Cited by




