Implications
Although the role of the hormone prolactin (PRL) in dairy ruminants has been controversial for many years, this review presents recent evidence that PRL is galactopoietic (induces the formation and secretion of milk) in ruminant lactation. We also discuss how PRL modulation could be used as a management tool to improve energy balance during the postpartum period and during acute nutritional stress as well as to facilitate drying-off.
Introduction
Ninety years ago, Stricker and Grueter (Reference Stricker and Grueter1929) reported that mammary growth and lactation could be induced in rabbits by injecting aqueous pituitary extracts. Subsequently, Riddle et al. (Reference Riddle, Bates and Dykshorn1933) determined that a hormone produced by the anterior pituitary, which they named PRL, was responsible for these effects. Although several substances can act as PRL-releasing factors, the secretion of PRL appears to be regulated primarily by the inhibitory action of dopamine released by the tuberoinfundibular neurons of the hypothalamus (Freeman et al., Reference Freeman, Kanyicska, Lerant and Nagy2000). Diverse stimuli from the environment and the internal milieu can affect the secretion of PRL. Parturition is associated with a major peak in PRL concentration, and the suppression of PRL prevents lactogenesis (Johke, Reference Johke1986). Prolactin release is also induced by suckling and milking, but this response decreases as lactation progresses (Selmanoff and Selmanoff, Reference Selmanoff and Selmanoff1983).
Prolactin is known to be mammogenic and lactogenic in both monogastric and ruminant mammals. Although the galactopoietic role of PRL in monogastric lactation is well recognised, only recently has that role been demonstrated in ruminant lactation. In this review, we will present recent evidence of the galactopoietic role of PRL in ruminants. For a more comprehensive review, readers are invited to consult Lacasse et al. (Reference Lacasse, Ollier, Lollivier and Boutinaud2016).
The galactopoietic role of PRL in lactating dairy cows was demonstrated using PRL inhibitors. The earlier studies used bromocriptine, an ergot alkaloid which acts as a dopamine agonist and inhibits PRL secretion by lactotrophs. However, bromocriptine has affinity for serotonin and adrenergic receptors (Brownell, Reference Brownell1998). Quinagolide is a non-ergot dopamine agonist that binds specifically to the dopamine D2 receptor of lactotrophs and has little affinity for serotonin and α-adrenergic binding sites (Brownell, Reference Brownell1998). In animal models, quinagolide has a longer half-life, has fewer side effects, and is 200 times more potent than bromocriptine in terms of inhibiting rodent lactation (Brownell, Reference Brownell1998). Cabergoline is also a potent dopamine D2 receptor agonist. However, it also possesses significant affinity for D3 and D4 dopamine receptors and several serotonin receptors (Sharif et al., Reference Sharif, McLaughlin, Kelly, Katoli, Drace, Husain, Crosson, Toris, Zhan and Camras2009).
Lacasse et al. (Reference Lacasse, Lollivier, Bruckmaier, Boisclair, Wagner and Boutinaud2011) inhibited PRL release in lactating dairy cows by injecting them with quinagolide daily for 9 weeks. At the dose used in that study (1 mg/day), quinagolide reduced but did not totally prevent PRL release at milking. Nevertheless, milk production declined faster in the quinagolide-treated cows than in the control cows. Milk production was correlated with the amounts (area under the curve) and peak values of milking-induced PRL release, and the correlation coefficients were similar in the control cows and the quinagolide-treated cows (Lacasse et al., Reference Lacasse, Lollivier, Bruckmaier, Boisclair, Wagner and Boutinaud2011). In another study, Knight et al. (Reference Knight, Foran and Wilde1990) reported a 21% decrease in milk production in dairy goats treated with bromocriptine for 8 days. Moreover, a single injection of cabergoline caused a 28% decrease in milk yield on the day after the injection (Figure 1). In addition, Knight (Reference Knight1993) showed that the effect of bromocriptine on milk production in goats was attenuated when PRL was injected with bromocriptine. Similarly, Lollivier et al. (Reference Lollivier, Lacasse, Angulo Arizala, Lamberton, Wiart, Portanguen, Bruckmaier and Boutinaud2015) reported that the effect of quinagolide on milk production in dairy cows was attenuated by twice-daily intravenous injections of PRL (2 μg/kg BW) at milking time. These last experiments indicate that the effect on milk production of these dopamine agonists is due to the inhibition of PRL release.
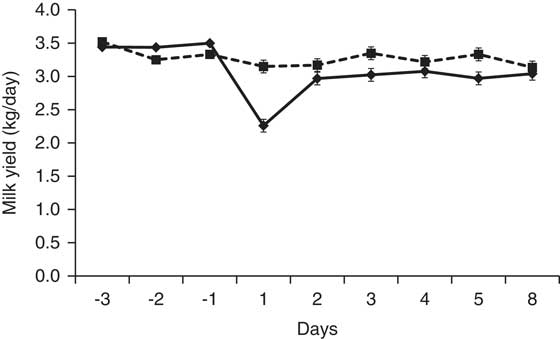
Figure 1 Milk production of dairy goats at 60 days in milk injected intramuscularly with 1 mg of cabergoline (♦, solid line; n = 5) or water (control; ■, dashed line; n = 5). Milk production on the day of the injection was lower (P < 0.01) in the goats injected with cabergoline. This figure is adapted from Lacasse et al. (Reference Lacasse, Ollier, Lollivier and Boutinaud2016).
A complete demonstration of the galactopoietic function of PRL in ruminants requires showing that enhancing the PRL concentration has a positive effect on lactation. In dairy cows, although Plaut et al. (Reference Plaut, Bauman, Agergaard and Akers1987) observed no effect of injecting a high dose of recombinant PRL (120 mg/day for 14 days), Wall et al. (Reference Wall, Crawford, Ellis, Dahl and McFadden2006) found that injections of a much smaller dose (1 µg/kg of BW) twice a day for the first 3 weeks of lactation tended to increased milk production. Recombinant PRL injections in goats increased milk yield by over 10%, an increase that was comparable and additive to the increase elicited by growth hormone (Flint and Knight, Reference Flint and Knight1997). In recent experiments, Lacasse and Ollier (Reference Lacasse and Ollier2015) and Tong et al. (Reference Tong, Thompson, Zhao and Lacasse2018) injected mid-lactation cows with a dopamine antagonist, domperidone. In both experiments, domperidone induced a gradual increase in PRL concentrations and milk production. Taken together, the results of all the experiments cited so far in this review support the view that PRL is galactopoietic in dairy ruminants. Accordingly, PRL inhibition could be used as a management tool to temporarily reduce milk production in situations where such a reduction could be beneficial. In the following sections, we will review experiments in which PRL inhibition was used to improve energy balance during the postpartum period or during acute nutritional stress or to facilitate drying-off.
Prolactin inhibition as a management tool for the postpartum period
The transition from late gestation to lactation in dairy ruminants is marked by nutritional, metabolic, hormonal and immunological changes that affect the incidence of metabolic diseases (Goff and Horst, Reference Goff and Horst1997). As the demand for nutrients needed for milk synthesis increases rapidly and exceeds the intake of nutrients from food, cows are in a state of negative energy balance (NEB) (Bauman and Currie, Reference Bauman and Currie1980). This results in lower blood glucose, calcium and phosphorus concentrations and the mobilisation of body reserves to provide additional energy, leading to elevated blood concentrations of non-esterified fatty acids (NEFA) and β-hydroxybutyric acid (BHBA) (Drackley, Reference Drackley1999; Busato et al., Reference Busato, Faissler, Küpfer and Blum2002; Drackley et al., Reference Drackley, Dann, Douglas, Janovick Guretzky, Litherland, Underwood and Loor2005). When excessive, these metabolic perturbations can lead to hypocalcaemia, ketosis, displaced abomasum and hepatic lipidosis.
Periparturient dairy cows also experience a state of immunosuppression, which can increase their susceptibility to uterine and mammary infections (Kehrli et al., Reference Kehrli, Nonnecke and Roth1989; Sheldon, Reference Sheldon2004). Metabolic perturbations (Goff et al., Reference Goff, Kimura and Horst2002) and immunosuppression (Kimura et al., Reference Kimura, Goff and Kehrli1999 and Reference Kimura, Goff, Kehrli, Harp and Nonnecke2002; Nonnecke et al., Reference Nonnecke, Kimura, Goff and Kehrli2003) were found to be greatly alleviated by mastectomy, indicating that the physiological demands placed on cows by the secretion of milk play a key role in periparturient immunosuppression. Ster et al. (Reference Ster, Loiselle and Lacasse2012) showed that peripheral blood mononuclear cell (PBMC) proliferation and interferon-γ secretion were lower when the cells were incubated with sera harvested in the postpartum period and that those parameters were inversely correlated with serum NEFA concentration. Similarly, the myeloperoxidase activity of polymorphonuclear neutrophils (PMN) was negatively correlated with the concentration of NEFA in blood (Hammon et al., Reference Hammon, Evjen, Dhiman, Goff and Walters2006). In vitro, a dose–effect relationship was found between NEFA concentrations and the inhibition of lymphocyte proliferation as well as the inhibition of PMN oxidative burst (Ster et al., Reference Ster, Loiselle and Lacasse2012). These results suggest that the metabolic disturbances resulting from the NEB are responsible for peripartum immunosuppression.
Improving the energy balance in the immediate postpartum period is very important in order to reduce disease incidence in dairy cows. Hence, improving the nutrient supply through the transition period has been the object of extensive research. Nevertheless, the reduction of the imbalance between nutrient supply and nutrient demand can also be reduced by temporarily decreasing the latter. Some studies have used once-a-day milking (Andersen et al., Reference Andersen, Friggens, Larsen, Vestergaard and Ingvartsen2004; Patton et al., Reference Patton, Kenny, Mee, O’Mara, Wathes, Cook and Murphy2006; Loiselle et al., Reference Loiselle, Ster, Talbot, Zhao, Wagner, Boisclair and Lacasse2009; O’Driscoll et al., Reference O’Driscoll, Olmos, Llamas Moya, Mee, Earley, Gleeson, O’Brien and Boyle2012) or incomplete milking (Carbonneau et al., Reference Carbonneau, de Passillé, Rushen, Talbot and Lacasse2012; Krug et al., Reference Krug, Morin, Lacasse, Santschi, Roy, Dubuc and Dufour2018; Morin et al., Reference Morin, Krug, Chorfi, Dubuc, Lacasse, Roy, Santschi and Dufour2018) to reduce milk production and, consequently, the NEB. Those strategies were effective in reducing the amount of milk produced by the cows and had a positive effect on their metabolism and health.
Implementing incomplete milking might be too complex for many dairy farms. Therefore, Vanacker et al. (Reference Vanacker, Ollier, Beaudoin, Blouin and Lacasse2017) tested the hypothesis that using quinagolide to slow down the rapid increase in milk production that follows calving will decrease metabolic and immunological perturbations. The first intramuscular injection of quinagolide (2 mg) was given as soon as possible after calving, and the following seven injections were given at 12-h intervals. The milk production of the quinagolide-treated cows was lower from days 2 to 6 (24.3±6.4 and 34.8±4.1 kg/day). There was no residual effect of quinagolide on milk production after day 6. The quinagolide injections increased blood glucose and calcium concentrations and decreased blood BHBA concentration during the first week of lactation indicating an improvement of energy balance. Blood phosphorus, urea and NEFA concentrations were not affected by the treatment. Quinagolide administration did not affect the phagocytic ability of PMNs but enhanced the proportion of those cells that underwent oxidative burst when stimulated. The proliferation of PBMCs was not improved but was correlated negatively with NEFA concentrations in the serum.
When PRL secretion at calving is reduced by the administration of quinagolide, the postpartum increase in milk production is slowed down without compromising overall dairy cow productivity. Slowing down the increase in milk production allows a reduction in metabolic stress and, in turn, a potential reduction in the incidence of diseases during the transition period.
Prolactin inhibition as a management tool during acute nutritional stress
As a result of several conditions, such as surgery, inability to stand up, milk fever and ketosis, cows are sometimes unable to eat the amount of feed required to support milk production. Consequently, high-yielding cows fall into severe NEB and must mobilise body reserves extensively to provide the additional nutrients required for milk production. As highlighted in the previous section, cows in an energy deficit experience metabolic perturbations and have a weakened immune system, which increases their susceptibility to infectious diseases. Therefore, strategies that improve energy status may limit immunosuppression in cows experiencing acute nutritional stress.
Reducing the milk production of cows under acute nutritional stress decreases their energy deficit. Large doses of glucocorticoids such as dexamethasone inhibit milk synthesis (Braun et al., Reference Braun, Bergman and Albert1970; van der Kolk, Reference van der Kolk1990) and are sometimes used in cows under acute nutritional stress to temporarily reduce their milk production. However, glucocorticoids are potent immunosuppressors and increase the risk of infections (Roth and Kaeberle, Reference Roth and Kaeberle1982). Therefore, Ollier et al. (Reference Ollier, Beaudoin, Vanacker and Lacasse2016) conducted an experiment to determine whether the inhibition of PRL could be used to reduce metabolic perturbations and immunosuppression in cows under acute nutritional stress. The cows were subjected to an acute nutritional stress created by a severe feed restriction (they were fed ~56% of their previous week’s DM intake) for 5 days. After 1 day of restriction, the cows were injected with either quinagolide or water twice a day for the following 4 days. A third group of cows received a single injection of dexamethasone. Feed restriction decreased milk production, however that drop was hastened in the quinagolide-treated cows and the dexamethasone-treated cows. Accordingly, the NEB was less severe in those cows than in the control cows. Feed restriction increased plasma NEFA and BHBA concentrations and decreased plasma glucose concentration, but those effects were attenuated by the PRL inhibitor. Dexamethasone injection also decreased BHB and NEFA concentrations initially but increased them subsequently. Although a negative correlation between serum NEFA concentration and PBMC proliferation was observed, the negative effect of serum harvested during the restriction period on mitogen-induced PBMC proliferation was not alleviated by quinagolide. Dexamethasone injection decreased the proportion of PMN capable of inducing oxidative burst confirming the negative effect of glucocorticoids on immunity. The quinagolide-induced reduction of milk production allows the reduction of NEB and associated metabolic stress without disturbing immune functions and compromising milk production during the rest of lactation in cows subjected to acute nutritional stress.
Prolactin inhibition as a management tool for drying-off
As their milk yield increases, drying-off has become a challenging period for dairy cows. Cows are often dried off while still producing large quantities of milk (Dingwell et al., Reference Dingwell, Kelton, Leslie and Edge2001), which greatly increases the vulnerability of the mammary gland to new intramammary infections (Rajala-Schultz et al., Reference Rajala-Schultz, Hogan and Smith2005). The mammary gland is much more resistant to infection once active involution is completed. Therefore, strategies that reduce milk production before dry-off, accelerate mammary gland involution or both could be important management tools.
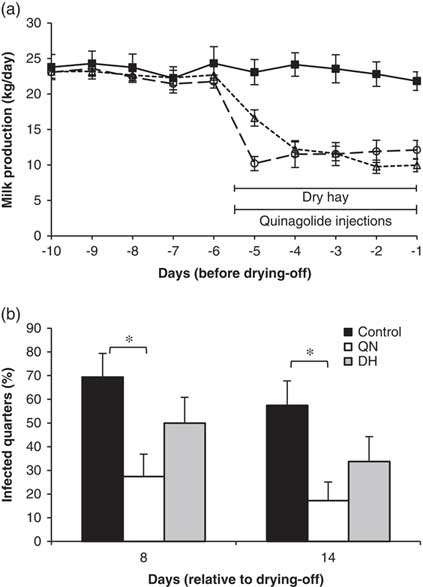
In this context, PRL inhibition could be used to reduce milk production at drying-off and accelerate the rate of mammary involution after cessation of milking. In one experiment, Ollier et al. (Reference Ollier, Zhao and Lacasse2013) injected late-lactation cows twice daily with quinagolide or water from 4 days before dry-off until 3 days after. The PRL inhibitor reduced milk production before dry-off by 20%. The increases in somatic cells and bovine serum albumin in milk during early involution were greater in the quinagolide-treated cows, suggesting that involution was accelerated. In another experiment, Ollier et al. (Reference Ollier, Zhao and Lacasse2014) compared the effects of injecting quinagolide from 5 days before dry-off until 13 days after with the effects of a drastic feed restriction in the days that preceded drying-off, a method commonly used to reduce milk production. The feed-restricted cows were fed dry hay only during the last 5 days before dry-off. A control group was neither feed-restricted nor injected with quinagolide. Both treatments decreased milk production at the time of drying-off and hastened mammary gland involution. Whereas quinagolide had little effect on blood metabolites, feed restriction increased blood concentrations of BHBA and NEFA and decreased blood concentrations of glucose and most amino acids. Serum harvested on the drying-off day from the hay-fed cows reduced the proliferation and interleukin-4 production of PBMCs, indicating immunosuppression. The same two treatments were repeated on another group of cows, but they were challenged by daily teat dipping in a solution containing Streptococcus agalactiae during the first week of the dry period (Ollier et al., Reference Ollier, Zhao and Lacasse2015). Although both feed restriction and quinagolide injections induced a major decrease in milk production, only the PRL-inhibition strategy reduced the incidence of new intramammary infections at drying-off (Figure 2).

Figure 2 (a) Milk production on the days preceding drying-off and (b) infection rate in mammary secretions of cows injected twice daily with quinagolide (4 mg) from 5 days before drying-off until 13 days after (○, long-dashed line; QN), cows fed dry hay only for the 5 days before drying-off (∆, short-dashed line; DH), and control cows (■, solid line). From day 1 to 7 after the last milking, all teats of each cow were dipped daily in a solution containing Streptococcus agalactiae at 5×107 cfu/ml. *P<0.05. This figure is adapted from Ollier et al. (Reference Ollier, Zhao and Lacasse2015).
Another dopamine agonist, cabergoline, was tested as a management tool to facilitate drying-off. The strategy used with this inhibitor was different from the one used with quinagolide: a single injection of cabergoline was administered just after the last milking before dry-off. Although the effect of cabergoline on milk yield could not be measured directly, udder pressure and firmness as well as milk leakage were reduced in the treated cows, indicating a reduction in milk synthesis (Bach et al., Reference Bach, De-Prado and Aris2015; Bertulat et al., Reference Bertulat, Isaka, de Prado, Lopez, Hetreau and Heuwieser2017). In addition, enhanced extracellular matrix remodelling in the mammary gland, the exfoliation of mammary epithelial cells into milk, and the changes observed in the composition of mammary secretions indicated that cabergoline treatment hastened mammary gland involution (Boutinaud et al., Reference Boutinaud, Isaka, Lollivier, Dessauge, Gandemer, Lamberton, De Prado Taranilla, Deflandre and Sordillo2016 and Reference Boutinaud, Isaka, Gandemer, Lamberton, Wiart, De Prado Taranilla, Sordillo and Lollivier2017). Even though the effect of cabergoline on resistance to intramammary infection was not tested, the more rapid increase in lactoferrin concentration, a bacteriostatic protein, in mammary secretions (Boutinaud et al., Reference Boutinaud, Isaka, Lollivier, Dessauge, Gandemer, Lamberton, De Prado Taranilla, Deflandre and Sordillo2016) may help to prevent intramammary infection.
In light of the research results obtained in various studies, a cabergoline-based product, Velactis, was commercialised in Europe and some Latin American countries. However, it was withdrawn from the European market after some cows were reported to suffer symptoms similar to a periparturient hypocalcaemia. The role of PRL in calcium homoeostasis is well established in fish (Flik et al., Reference Flik, Rentier-Delrue and Wendelaar Bonga1994), and bromocriptine was reported to decrease calcium absorption in rats (James et al., Reference James, Makeen, Foley, Stevens and Robinson1977). It is therefore reasonable to think that the inhibition of PRL might cause hypocalcaemia. However, an improved calcaemia was observed in periparturient cows treated with quinagolide (Vanacker et al., Reference Vanacker, Ollier, Beaudoin, Blouin and Lacasse2017), although PRL secretion was inhibited only for ~4 days. To determine whether PRL manipulations can affect calcium homoeostasis, we determined calcium concentrations in serum samples collected in lactating cows injected with quinagolide or water for 2 weeks and then treated with domperidone for the following 3 weeks (Tong et al., Reference Tong, Thompson, Zhao and Lacasse2018). Even though PRL blood concentration was altered by 30-fold, no significant change in serum calcium concentrations was observed (Figure 3). This finding indicates that lowering the PRL concentration is unlikely to cause hypocalcaemia.
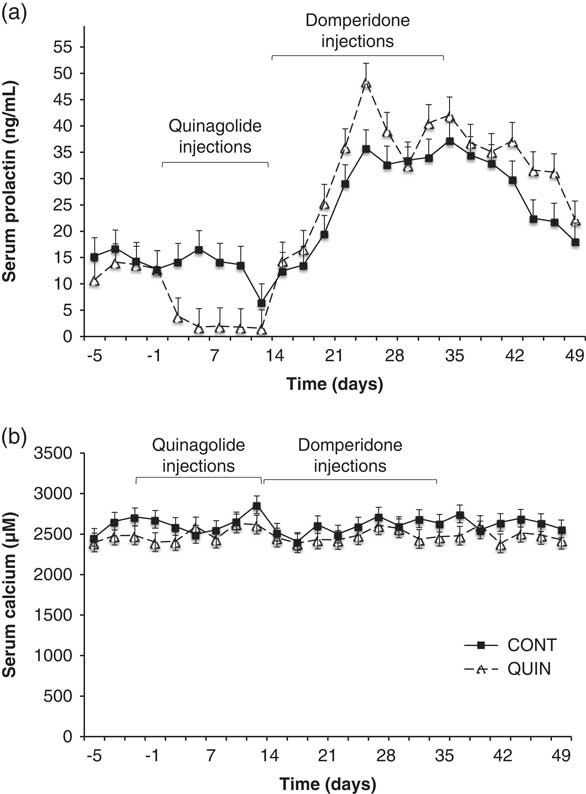
Figure 3 Serum concentrations of (a) prolactin and (b) calcium in dairy cows injected with either quinagolide (0.5 mg/ml; ∆, dashed line; n=9; QUIN) or water (2.0 ml; ■, solid line; n=9; CONT) from day 1 to 14. All cows were injected daily with domperidone (300 mg) from day 15 to 35. Serum prolactin was lower (P<0.05) in the quinagolide-treated cows from day 5 to 14. Serum calcium was not affected by the quinagolide or domperidone injections. Panel (a) is adapted from Tong et al. (Reference Tong, Thompson, Zhao and Lacasse2018).
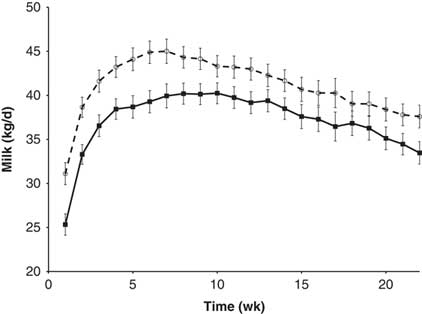
In some situations, it appears that the responsiveness of the mammary gland to the PRL galactopoietic signal is modulated by local or systemic factors (Lacasse et al., Reference Lacasse, Ollier, Lollivier and Boutinaud2016). The circulating level of PRL might also have an impact on mammary gland responsiveness to PRL. A short-day photoperiod during the dry period was found to reduce circulating PRL during that period and increase the subsequent milk production (Auchtung et al., Reference Auchtung, Rius, Kendall, McFadden and Dahl2005; Lacasse et al., Reference Lacasse, Vinet and Petitclerc2014). In Lacasse et al. (Reference Lacasse, Vinet and Petitclerc2014), milk production in the first 20 weeks of lactation was enhanced by about 10% by previous exposure to a short-day photoperiod during the dry period. Accordingly, the infusion of recombinant PRL into dry cows exposed to a short-day photoperiod during the dry period reduced the subsequent milk response to the photoperiod treatment (Crawford et al., Reference Crawford, Morin, Wall, McFadden and Dahl2015). In the previously cited studies on drying-off management, PRL was inhibited during the early dry period (Ollier et al., Reference Ollier, Zhao and Lacasse2013, Reference Ollier, Zhao and Lacasse2014 and Reference Ollier, Zhao and Lacasse2015). Even though those experiments were not designed to determine the effect of these treatments on milk production in the subsequent lactation, an increase in production was observed in the animals that had received the inhibitor (Figure 4), a result that supports the concept that PRL can influence responsiveness to its own signal. That would be a significant benefit of this strategy, and an experiment is currently ongoing to confirm this finding.

Figure 4 Milk production during the subsequent lactation of cows injected twice daily with quinagolide (○, dashed line) or water (■, solid line) before drying-off and during the early dry period (see Ollier et al., Reference Ollier, Zhao and Lacasse2013, Reference Ollier, Zhao and Lacasse2014 and 2015 for more details). Milk production was greater (P=0.02) in the quinagolide-treated cows. This figure is adapted from Lacasse et al. (Reference Lacasse, Ollier, Lollivier and Boutinaud2016).
These experiments show that PRL inhibition induces a sharp decrease in the milk production of cows in late lactation, accelerates mammary gland involution, and reduces susceptibility to intramammary infections in a S. agalactiae challenge. Taken together, these results indicate that the PRL-inhibition strategy could be an alternative tool for facilitating drying-off, especially in high-yielding cows. However, a better understanding of the cause of the side effects that have been reported is needed.
Conclusion
The results of several experiments now support the hypothesis that PRL is galactopoietic in dairy cows and goats. Our results suggest that PRL inhibition could be used as a management tool to reduce metabolic stress in cows during the transition period or to assist cows under acute nutritional stress. In addition, PRL inhibitors could also be used to facilitate the drying-off of high-producing dairy cows. Nevertheless, additional research is needed to ensure the safety of such approaches for the animals.
Acknowledgements
The authors would like to thank Séverine Ollier for her outstanding contribution and the teams at the Sherbrooke Research and Development Centre (Sherbrooke, QC, Canada) and at the UMR1348 PEGASE (Rennes, France) for taking care of the cows, helping with the blood sampling and providing technical assistance. The authors are grateful to Mary Varcoe, from the Translation Bureau, Public Services and Procurement Canada, for her careful editing of this manuscript.
Declaration of interest
The authors declare no conflict of interest.
Ethics statement
Not relevant.
Software and data repository resources
Data were not deposited in an official repository.