Vitamin D is a secosteroid hormone, recognised as a neuroprotective factor with a role to play in brain development (Harms Reference Harms, Eyles and McGrath2008; Eyles Reference Eyles, Burne and McGrath2013). Vitamin D promotes neurodevelopment and has a range of actions, such as promoting cell growth and differentiation, regulation of neurotransmission, immunomodulation, and antioxidant and anti-inflammatory effects. Vitamin D deficiency has been associated with various mental disorders, including mood disorders, psychotic disorders, autism and cognitive decline.
Vitamin D physiology
Vitamin D belongs to a group of fat-soluble vitamins. Its primary functions are to aid the intestinal absorption of calcium and phosphate, and to regulate bone mineralisation (Holick Reference Holick, Caballero, Allen and Prentice2013). The two main forms of vitamin D are: vitamin D3 or cholecalciferol, which is formed in the skin after exposure to sunlight, and vitamin D2 or ergocalciferol, which is synthetically produced by UV irradiation of ergosterol, a steroid found in fungi.
Vitamin D levels are influenced by environment and lifestyle. Endogenous synthesis following cutaneous exposure to ultraviolet B radiation is the primary source (Fig. 1). A smaller proportion is acquired from dietary sources. It is not a widely appreciated fact, but nutritional sources of vitamin D are relatively limited (Holick Reference Holick, Caballero, Allen and Prentice2013). Recently a genome-wide association study (GWAS) meta-analysis of 31 studies with a total of 79 366 individuals identified genetic variants at three loci (group component (GC), 7-dehydrocholesterol reductase (NADSYN1/DHCR7) and 25-hydroxylase (CYP2R1)) influencing vitamin D levels (Jiang Reference Jiang, O'Reilly and Aschard2018). However, the findings were suggestive of a relatively small heritability rate for vitamin D levels, indicating that modifiable environmental factors are the main determinant of vitamin D levels.
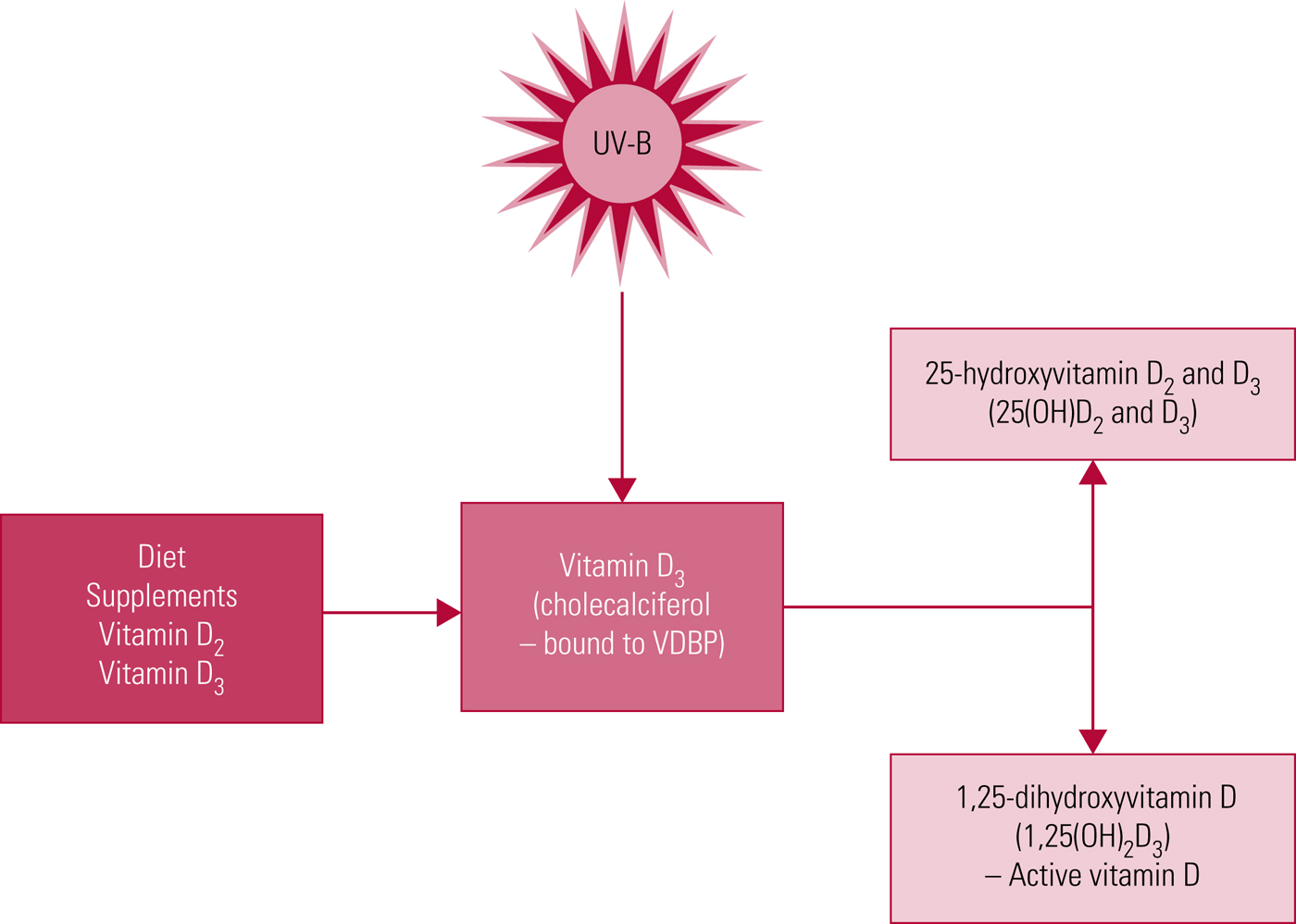
FIG 1 Vitamin D synthesis. UV-B, ultraviolet B radiation; VDBP, vitamin D binding protein.
Ultraviolet sunlight stimulation will depend on the season of the year, latitude and skin exposure. Vitamin D levels thus vary seasonally, with deficiency more common in winter and at higher latitudes, reflecting ambient levels of sunlight (Hypponen Reference Hypponen and Power2007), as well as in urban settings, owing to lifestyle choices and lower sunlight exposure (Holick Reference Holick1995). People with more pigmented skin need more sunlight to produce vitamin D, so are particularly affected by limited sun exposure; lower levels of vitamin D are consistently observed in Black and Asian populations (Ford Reference Ford, Graham and Wall2006). Older age is associated with lower vitamin D levels, when reduced sunlight exposure and decreased ability to synthesise vitamin D cutaneously with sunlight exposure are contributing factors.
Epidemiology of vitamin D deficiency
Vitamin D deficiency is a global problem; more than a billion people worldwide are believed to have suboptimal levels (Holick Reference Holick and Chen2008). A recent systematic review of 195 studies including 168 000 people from 44 countries (Hilger Reference Hilger, Friedel and Herr2014) identified that only 11% had 25(OH)D levels >75 nmol/L, the level classed as sufficient. A summary of results is given in Table 1.
TABLE 1 Vitamin D status in populations worldwide

25(OH)D, 25-hydroxyvitamin D. Source: Hilger et al (2014).
Classification of vitamin D status
Serum levels of 25(OH)D, the main circulating form of vitamin D, are usually taken as a proxy of vitamin D status (Holick Reference Holick and Chen2008; Ross Reference Ross, Manson and Abrams2011), as it is a more stable compound than the physiologically active form 1,25(OH)2D3, with higher serum concentrations and a longer half-life (approximately 20 days compared with 7 h) (Lips Reference Lips2007).
Controversy remains about what vitamin D levels are optimal (sufficient), insufficient and deficient, and we show typical thresholds in Box 1. The definitions of sufficiency as a vitamin D level >75nmol/L are based on observations relating to the role of 25(OH)D in calcium homeostasis and optimal calcium absorption. Parathyroid hormone levels decline with reducing concentrations of 25(OH)D, although this decline plateaus and reaches a nadir at 25(OH)D concentrations of 75–100 nmol/L (equivalent to 30–40 ng/mL) (Holick Reference Holick, Chen and Lu2007). Intestinal calcium absorption is optimal at concentrations >80 nmol/L (32 ng/mL) (Holick Reference Holick and Chen2008). The definition of vitamin D sufficiency of >50 nmol/L 25(OH)D is based on the observation that parathyroid hormone levels normalise with 25(OH)D concentrations of >50 nmol/L (Ross Reference Ross, Manson and Abrams2011); similar levels are required to prevent osteomalacia and to ensure optimal bone function (Ebeling Reference Ebeling2014).
The US Institute of Medicine recommends vitamin D levels >20 ng/mL (>50 nmol/L) to optimise skeletal benefits, based on trials in the general population (Ross Reference Ross, Manson and Abrams2011). Most recently, the Endocrine Society's clinical guidelines, based on studies of people at high risk for vitamin D deficiency, recommend that a 25(OH)D concentration >30 ng/mL (>75 nmol/L) be attained to improve outcomes (Holick Reference Holick, Binkley and Bischoff-Ferrari2011).
BOX 1 Definitions of vitamin D status
For the ranges below, vitamin D status is measured in terms of 25(OH)D serum concentration.
Most common thresholdsFootnote a
Deficiency: <50 nmol/L (20 ng/mL)
Insufficiency: 51–74 nmol/L (21–29 ng/mL)
Sufficiency: >75 nmol/L (>30 ng/mL)
More conservative thresholdsFootnote b
Deficiency: <25 nmol/L (<10 ng/mL)
Insufficiency: 25–50 nmol/L (10–20 ng/mL)
Sufficiency: >50 nmol/L (>20 ng/mL)
a. Holick & Chen (Reference Holick and Chen2008); US Institute of Medicine: Ross et al (Reference Ross, Manson and Abrams2011).
b. International Osteoporosis Foundation: Dawson-Hughes et al (Reference Dawson-Hughes, Mithal and Bonjour2010).
Vitamin D and depression
Is there a relationship?
Research exploring the relationship between suboptimal vitamin D levels and depression risk has provided inconsistent findings. Several narrative reviews assessing the association between low vitamin D levels and depression suggest an inconclusive relationship.
A systematic review and meta-analysis of observational studies (Anglin Reference Anglin, Samaan and Walter2013) concluded that vitamin D levels were inversely associated with the prevalence of depression. However, the observational nature of the included studies precluded drawing conclusions on a causal relationship.
There remains a paucity of longitudinal data investigating the relationship between vitamin D and depression. The few existing studies have provided inconclusive results. A recent large-scale population-based study of 3251 adults over 55 years of age that investigated long-term associations between vitamin D serum levels and depression (Jovanova Reference Jovanova, Aarts and Noordam2017) identified a cross-sectional association between low vitamin D and depression, but found no evidence for a longitudinal relationship. The cross-sectional relationship might be expected, as people who are depressed may be less able to engage in outdoor activity and may limit their sun exposure. However, if vitamin D is a risk factor for depression, then we would expect to find that vitamin D concentrations had a longitudinal association with depression and depressive symptoms, which was not found in that study (Jovanova Reference Jovanova, Aarts and Noordam2017). This replicated previous longitudinal data which failed to identify a longitudinal association between low vitamin D and depression (Chan Reference Chan, Chan and Woo2011; Toffanello Reference Toffanello, Sergi and Veronese2014), although it was contradictory to findings from two other longitudinal studies (May Reference May, Bair and Lappe2010; Milaneschi Reference Milaneschi, Shardell and Corsi2010), which identified a relationship between suboptimal vitamin D levels and the prospective onset of depression.
A meta-analysis of randomised controlled trials (RCTs) of vitamin D supplementation as a treatment for depression identified six RCTs with 1203 participants (72% females), including 71 with current depression (5 trials included participants at risk of depression and 1 trial included patients with depression). There was no significant effect of vitamin D supplementation on depression scores (SMD = −0.14, 95% CI −0.41 to 0.13, P = 0.32; OR = 0.93, 95% CI 0.54–1.59, P = 0.79) (Li Reference Li, Mbuagbaw and Samaan2014). Both this and the above-mentioned systematic review (Anglin Reference Anglin, Samaan and Walter2013) supported the conclusion of previous narrative reviews indicating that no clear causal relationship between suboptimal vitamin D levels and depression has been identified.
Vitamin D as a therapeutic agent: augmentation trials
In depression
Only one small randomised double-blind trial of vitamin D3 augmentation of a specific antidepressant medication in depressive disorder has taken place. Over an 8-week period there was a significant improvement in depressive symptoms in those whose fluoxetine 20 mg daily was augmented with 1500 IU of vitamin D3 (n = 20) compared with those who received fluoxetine alone (n = 20) (Khoraminya Reference Khoraminya, Tehrani-Doost and Jazayeri2013). A controlled open label trial of antidepressant (any) augmentation with a single oral dose of 300 000 IU of vitamin D3 (n = 24) showed a significant improvement in depressive symptoms over a 4-week period in comparison with use of antidepressant alone (n = 15) (Zanetidou Reference Zanetidou, Belvederi Murri and Buffa2011). Over 90% of the included cases treated with vitamin D3 augmentation had a 25(OH)D level <75 nmol/L (<30 ng/mL), and 80% of the comparison group treated with antidepressants only, had a 25(OH)D level <75 nmol/L. A later RCT showed efficacy for a single dose of 300 000 IU intramuscular vitamin D (n = 40) in improving depressive symptoms at 8 weeks following injection compared with placebo (n = 40), an effect not seen with the lower 150 000 IU dose (n = 40) (Mozaffari-Khosravi Reference Mozaffari-Khosravi, Nabizade and Yassini-Ardakani2013).
An RCT of 78 people aged 60 and older in receipt of treatment for depression identified a non-significant change in the mean depression score between those who also received 50 000 IU of vitamin D3 weekly for 8 weeks compared with those who received a weekly placebo (although the mean vitamin D level (22.57 ng/mL (s.d. = 6.2)) in the vitamin D group may be considered to have been optimal) (Alavi Reference Alavi, Khademalhoseini and Vakili2018). An earlier double-blind RCT of 50 000 IU vitamin D weekly compared with placebo found a non-significant decrease in depressive symptoms over an 8-week period (Sepehrmanesh Reference Sepehrmanesh, Kolahdooz and Abedi2016). A double-blind RCT of dialysis patients with depression did not identify a significant reduction in depressive symptoms following treatment with 50 000 IU vitamin D weekly for 52 weeks (n = 362) (Wang Reference Wang, Liu and Lian2016).
In bipolar depression
There has been a single RCT of vitamin D3 augmentation in bipolar depression, with no significant difference in depression symptom scores between those treated with 5000 IU of vitamin D3 daily (n = 16) and placebo (n = 17) after 12 weeks (Marsh Reference Marsh, Penny and Rothschild2017). This was despite a significantly higher mean increase in vitamin D levels in the augmentation group (9.9 ng/mL (s.d. = 8.2)) compared with the placebo group (1.3 ng/mL (s.d. = 4.3)).
Summary
The RCTs to date in mood disorders have been limited by small sample sizes and heterogeneity of study populations and vitamin D dosing techniques. These trials have produced inconsistent findings, which provide at best a mild signal for a beneficial effect of vitamin D on mood, but which is far from conclusive.
Vitamin D as a preventive agent
Effect of supplementation on depression symptom scores and mental health
In addition to trials of vitamin D as a therapeutic agent in depression, RCTs have investigated vitamin D as a preventive agent. A few RCTs have investigated vitamin D supplementation in improving depressive symptoms or depression scale scores, and those that have done so reported inconsistent findings: some showed a positive effect (Jorde Reference Jorde, Sneve and Figenschau2008; Khoraminya Reference Khoraminya, Tehrani-Doost and Jazayeri2013), whereas others found no significant association (Vieth Reference Vieth, Kimball and Hu2004; Kjærgaard Reference Kjærgaard, Waterloo and Wang2012; Yalamanchili Reference Yalamanchili and Gallagher2012). There was no association with improved depression and anxiety symptom scores in a cohort of young healthy adults supplemented with 5000 IU of vitamin D3 for 6 weeks compared with placebo (Dean Reference Dean, Bellgrove and Hall2011), although there was a low prevalence of vitamin D deficiency in the test population. A randomised trial of vitamin D3 supplementation with 800 IU/day in women aged 70 or over did not identify any significant improvement in mental health outcomes with supplementation, although the study was limited by a low level of depression in the study sample and the moderately low dose of vitamin D3 (Dumville Reference Dumville, Miles and Porthouse2006). A randomised trial investigating the effect of low-dose (600 IU daily) and high-dose (4000 IU daily) vitamin D3 (total n = 82) found significant improvements in well-being for those receiving the high-dose therapy at 6-month follow-up (Vieth Reference Vieth, Kimball and Hu2004). Other randomised trials have found benefits with vitamin D supplementation in seasonal affective disorder (n = 8 treated with 100 000 IU of vitamin D and n = 7 treated with phototherapy) (Gloth Reference Gloth, Alam and Hollis1999) and in improving depressive symptom scores in overweight or obese (body mass index BMI > 28 kg/m2) general hospital out-patients given 20 000 IU of vitamin D3 twice weekly compared with placebo (the participants were not vitamin D deficient nor were they clinically depressed) (Jorde Reference Jorde, Sneve and Figenschau2008).
As noted, these studies have not always focused on patients with clinical depression or vitamin D deficiency. Rather, most have involved vitamin D3 supplementation in general population samples, thus limiting interpretation of their findings and contributing to inconclusiveness of findings. These studies are further limited by being underpowered, with small sample sizes and heterogeneous study populations.
Vitamin D and psychotic disorders
Vitamin D insufficiency is highly prevalent in people with schizophrenia and other psychotic disorders (Suetani Reference Suetani, Saha and Eyles2017). In a cross-sectional study of 324 community-based people with established psychosis, 86% had suboptimal vitamin D levels (<20 ng/mL). In a systematic review (Adamson Reference Adamson, Lally and Gaughran2017), 63% met criteria for vitamin D deficiency (with the threshold level to define deficiency ranging from 10 to 40 ng/mL).
We found that vitamin D levels were lower on presentation with a first episode of psychosis than in matched healthy controls (total n = 138) (Crews Reference Crews, Lally and Gardner-Sood2013) and that 80% (n = 134) of individuals with a first episode had suboptimal vitamin D levels at time of first contact with services (Lally Reference Lally, Ajnakina and Singh2018). Lifestyle and physical health factors associated with an increased risk of vitamin D insufficiency or deficiency, such as smoking, increased BMI, social withdrawal and inactivity resulting in decreased sunlight exposure, are all more common in people with psychotic disorders.
Epidemiological studies have indicated that those born in late winter/early spring (Davies Reference Davies, Welham and Chant2003), at higher latitudes (Saha Reference Saha, Chant and Welham2006) and in urban settings have an increased risk of schizophrenia, leading to suggestions that this risk may be mediated by vitamin D deficiency. This association is further suggested by studies in Black African and Black Caribbean migrant populations, among whom vitamin D levels tend to be low. Black African and Black Caribbean migrant populations have increased rates of psychosis. Cross-sectional data in first-episode psychosis (FEP) and established psychosis have identified lower mean vitamin D levels in patients of Black African or Caribbean ethnicity compared with White patients (Lally Reference Lally, Gardner-Sood and Firdosi2016, Reference Lally, Ajnakina and Singh2018), although whether this might differentially affect clinical symptoms or symptomatic response to treatment has not been investigated. Prenatal vitamin D deficiency has been hypothesised to adversely affect fetal neural development, thus increasing the risk of schizophrenia (McGrath Reference McGrath1999). This possibility is supported by a Danish longitudinal case–control study which showed that vitamin D status in neonates was associated with the risk of schizophrenia (McGrath Reference McGrath, Eyles and Pedersen2010) and by a birth cohort study demonstrating an increased risk of schizophrenia in Finnish males not given vitamin D supplements during the first year of life (McGrath Reference McGrath, Saari and Hakko2004).
Vitamin D3 augmentation in schizophrenia
The only randomised trial to date investigating vitamin D3 augmentation in schizophrenia was conducted in a population of individuals receiving clozapine for treatment-resistant schizophrenia (all with baseline vitamin D levels <30 ng/mL). At 8 weeks, there was no significant difference in psychotic symptoms between those receiving 14 000 IU per week of vitamin D3 (n = 24) compared with placebo (n = 23), although a trend towards improved cognitive performance relating to attention and recall was detected (Krivoy Reference Krivoy, Onn and Vilner2017).
Vitamin D and clinical symptoms in FEP
A major limitation of work so far in FEP is the cross-sectional design of most studies, limiting any inference of a causal relationship between vitamin D and clinical status: it may as easily be that the relationship identified between symptoms and low vitamin D, be it in acute psychotic episodes (Yuksel Reference Yuksel, Altunsoy and Tikir2014) or in FEP (Graham Reference Graham, Keefe and Lieberman2015), may be the result, rather than a cause, of psychosis.
As mentioned above, we recently investigated the longitudinal relationship between vitamin D levels at time of first contact with services in FEP and clinical symptoms at 12 months, identifying a significant association between higher vitamin D levels at first contact for psychosis and lower negative symptoms and total psychotic symptoms at 12-month follow-up (Lally Reference Lally, Ajnakina and Singh2018). This is the first longitudinal assessment of vitamin D levels and associations with psychotic symptoms.
Vitamin D is considered to be neuroprotective and is postulated to have brain antioxidant properties, reducing oxidative stress (Wrzosek Reference Wrzosek, Łukaszkiewicz and Wrzosek2013; Nerhus Reference Nerhus, Berg and Kvitland2016; Mitra Reference Mitra, Natarajan and Ziedonis2017) by decreasing the production of the oxidant nitric oxide (Garcion Reference Garcion, Wion-Barbot and Montero-Menei2002) and increasing the production of antioxidants such as glutathione (Garcion Reference Garcion, Thanh and Bled1996; Wrzosek Reference Wrzosek, Łukaszkiewicz and Wrzosek2013). Previous studies have hypothesised that unmitigated oxidative stress can contribute to the development of negative symptoms of schizophrenia through a dysregulation of glutamate–gamma-aminobutyric acid excitatory/inhibitory responses (Sullivan Reference Sullivan and O'Donnell2012; Albayrak Reference Albayrak, Ünsal and Beyazyüz2013), while higher glutamate levels in the anterior cingulate cortex have been associated with increased negative symptoms in first-episode schizophrenia (Egerton Reference Egerton, Brugger and Raffin2012). Vitamin D's anti-inflammatory properties are supported by the finding that vitamin D supplementation can reduce levels of C-reactive protein (CRP), a marker of inflammation (Chen Reference Chen, Wan and Han2014). This is mirrored in established psychosis, in which an inverse relationship between vitamin D and CRP levels has been identified (Lally Reference Lally, Gardner-Sood and Firdosi2016).
Vitamin D and physical health in psychotic disorders
Vitamin D and cardiometabolic risk
Higher vitamin D levels have been associated with improved longer-term clinical outcomes in medical conditions, with observational studies showing inverse associations between circulating 25-hydroxyvitamin D and risks of death due to cardiovascular disease and cancer (Chowdhury Reference Chowdhury, Kunutsor and Vitezova2014). However, there is as yet no consistent evidence for routine supplementation.
To date, epidemiological evidence concerning the association between vitamin D and cardiometabolic risk factors in community-dwelling individuals with established psychotic illnesses is limited. For the first time in a population with established psychosis, we identified that those with the highest levels of vitamin D have a lower prevalence of metabolic syndrome (20.5%) compared with those in the lowest (39.1%), second (48.3%) and third quartile (43.1%) of vitamin D levels (all P < 0.01) (Lally Reference Lally, Gardner-Sood and Firdosi2016). This was the first large-scale study to have identified an association between decreased 25(OH)D levels and cardiovascular risk factors in psychotic illnesses. Of interest, we identified associations between hypertension and low 25(OH)D levels, which may be a causally related finding. This is suggested by findings in the general population, where low 25(OH)D levels are associated with a higher risk of incident cardiovascular disease and specifically hypertension (Wang Reference Wang, Pencina and Booth2008). The strongest correlations with low 25(OH)D levels were with factors related to high body fat (Lally Reference Lally, Gardner-Sood and Firdosi2016), which is supported by findings that those with increased adipose tissue stores (vitamin D, being fat soluble, is stored in adipose tissue) due to obesity have lower circulating levels of vitamin D because of this increased storage capacity (Wortsman Reference Wortsman, Matsuoka and Chen2000).
Vitamin D and bone mineral density
Osteoporosis is 2.5 times more common in schizophrenia than in controls, with 52% having low bone mass (Stubbs Reference Stubbs, de Hert and Sepehry2014) and significantly reduced bone mineral density (BMD) at the lumbar spine (Gomez Reference Gomez, Stubbs and Shirazi2016).
Only three studies to date have assessed associations between vitamin D and BMD in schizophrenia (Bergemann Reference Bergemann, Parzer and Mundt2008; Hallahan Reference Hallahan, Lyons and Doyle2008; Rey-Sanchez Reference Rey-Sanchez, Lavado-Garcia and Canal-Macias2009). In a cross-sectional study (Hallahan Reference Hallahan, Lyons and Doyle2008), 15 individuals with chronic schizophrenia, living in a long-stay residential unit, had BMD measures recorded by dual-energy X-ray absorption (DEXA) scan. There were no significant correlations between vitamin D levels (mean 23.8 nmol/L (s.d. = 8.9)) and BMD. A case–control study (Rey-Sanchez Reference Rey-Sanchez, Lavado-Garcia and Canal-Macias2009) measured BMD using quantitative ultrasound (QUS) in 73 people with schizophrenia (48 males), who were all being treated with antipsychotics. There was no significant correlation between 25(OH)D levels (females: mean 20.4 ng/mL (s.d. = 26.1) (equivalent to 51.0 nmol/L); males: mean 15.1 ng/mL (s.d. = 12.0) (equivalent to 37.8 nmol/L)) and phalangeal BMD values. However, a significant negative correlation between the parathyroid hormone and lower bone mass was identified in males and females (r = 0.347, P < 0.05). In another case–control study (Bergemann Reference Bergemann, Parzer and Mundt2008), 72 premenopausal women with schizophrenia (mean age 33.8 years (s.d. = 6.5), range 20.5–45.3 years) were compared with 71 age- and sex-matched healthy controls. Those with schizophrenia had no significant difference in BMD (T-score) compared with the controls. Those with schizophrenia had a significantly reduced mean 25(OH)D concentration of 16.3 ng/mL (s.d. = 7.9) compared with the controls (24.6 ng/mL (s.d. = 11.5); P < 0.001), although no significant correlation between serum 25(OH)D levels and BMD were reported in the schizophrenia group.
If an individual with schizophrenia has a history of fragility fractures, or evidence of reduced BMD or osteoporosis, then supplementary calcium and vitamin D should be prescribed as in the general population (Aspray Reference Aspray, Bowring and Fraser2014), along with any direct treatments for osteoporosis where indicated.
Managing vitamin D deficiency in psychosis and depression: do we know when to screen and treat?
As already noted, definitions of vitamin D deficiency vary. In the UK, the National Osteoporosis Society (now the Royal Osteoporosis Society) set the following serum 25(OH)D thresholds: ‘<30 nmol/L (12 ng/mL) is deficient; 30–50 nmol/L (12–20 ng/mL) may be inadequate in some people; >50 nmol/L (>20 ng/mL) is sufficient for almost the whole population’ (Aspray Reference Aspray, Bowring and Fraser2014). Public Health England recommends that all adults consider taking vitamin D supplements (400 IU/day) in the autumn and winter months, with year-round supplementation advised for those with darker skin pigmentation (Scientific Advisory Committee on Nutrition 2016). What does this mean for the clinical care of people with schizophrenia and depression? Should we test all patients for vitamin D deficiency? How should we interpret test results and what treatment might be considered? The answer is that we do not yet know whether and how to adapt the general population advice for use in people with psychosis and depression.
It is more likely than not that a person with established psychosis will have suboptimal vitamin D levels (Lally Reference Lally, Gardner-Sood and Firdosi2016), and a pragmatic approach is reasonable when considering vitamin D testing: as in high-risk general population groups, a presumptive diagnosis of insufficiency could be made, based on risk factors, without the need for (expensive) testing of vitamin D levels unless the individual is symptomatic (Aspray Reference Aspray, Bowring and Fraser2014).
It is perhaps most appropriate to measure vitamin D levels in summer or autumn, when a secular trend towards more optimal levels will be seen. It is reasonable to consider people with schizophrenia as a high-risk group for suboptimal vitamin D levels. The National Osteoporosis Society recommends that such patients, as a minimum, should be treated with lifestyle advice and over-the-counter vitamin D supplements at a dose of 400 IU/day (Aspray Reference Aspray, Bowring and Fraser2014).
In schizophrenia and depression, if the vitamin D level is measured and is <30 nmol/L (<12 ng/mL), then correction should be considered, with a loading dose of 40 000 IU of colecalciferol given orally weekly for 7 weeks and vitamin D level rechecked at 12 weeks to allow the level to plateau. If the level is now sufficient (i.e. >50 nmol/L (>20 ng/mL), then a maintenance dose of oral colecalciferol 800–2000 IU/day should be initiated, alongside dietary advice and engagement in outdoor activity. A similar maintenance regimen is advised for people with vitamin D insufficiency (30–50 nmol/L (12–20 ng/mL)) (Aspray Reference Aspray, Bowring and Fraser2014).
Lifestyle advice should be offered to all patients, and education that the best source of vitamin D is sensible levels of sunlight exposure. Spending 10–15 min in sunlight on most days of a week, with face and arms exposed, will suffice to ensure adequate vitamin D levels (Nowson Reference Nowson, McGrath and Ebeling2012).
Discussion
Vitamin D deficiency is associated with psychotic disorders, and with depression, as well as with many other chronic physical conditions. The question remains whether vitamin D is a causal factor or a consequence of these illnesses. Over 90% of people with established psychosis have suboptimal vitamin D levels, but depression rates in psychotic disorders are not that high, nor are persisting psychotic symptoms universally prevalent (Lally Reference Lally, Ajnakina and Stubbs2017). The observed associations could be due to reverse causation, the illness affecting the vitamin D levels, although our recent prospective study, while requiring replication, opens the possibility of a direct effect of vitamin D levels on outcomes in early psychosis (Lally Reference Lally, Ajnakina and Singh2018).
The evidence that vitamin D deficiency in early life may be a risk factor for later psychosis is somewhat stronger (Eyles Reference Eyles, Trzaskowski and Vinkhuyzen2018). It may be the case that vitamin D at suboptimal levels is no longer neuroprotective, perhaps owing to the loss of its antioxidant or anti-inflammatory effects, leaving the person more vulnerable to emerging illnesses such as psychosis or depression.
In terms of supplementation, randomised trials fail to indicate symptom improvements with vitamin D augmentation in schizophrenia and depression. Nevertheless, vitamin D testing and supplementation has crept into routine medical practice, with the assumption that optimisation of vitamin D levels will have longer-term benefits for physical health. However, evidence for this is lacking, even in the general population (Manson Reference Manson, Cook and Lee2019).
Conclusions
Vitamin D deficiency has been associated with poorer mental health, depression and psychotic disorders, as well as with chronic physical conditions. However, the evidence base establishing vitamin D as a potential cause rather than consequence of depression is lacking, although there is some evidence that developmental vitamin D deficiency may be pertinent to psychosis risk.
Well-designed clinical trials are needed to further study the relationship between repletion of vitamin D stores and clinical outcomes in patients with depression and schizophrenia before routine testing and supplementation can be recommended. In the meantime, the guidelines for the general population should be followed, bearing in mind that the risks of vitamin D deficiency in those with psychosis and depression are higher than in the general population.
MCQs
Select the single best option for each question stem
1 Evidence to suggest that vitamin D may be an aetiological factor in schizophrenia include all of the following except:
a increased incidence of schizophrenia at higher latitudes
b increased prevalence of schizophrenia in people of African ethnicity
c raised incidence of schizophrenia in urban settings
d association between lower dairy intake and schizophrenia
e increased incidence in those born during the winter.
2 As regards vitamin D augmentation in depression:
a vitamin D augmentation should be given to all people with depression
b the effective dose of vitamin D for augmentation in depression is well established
c vitamin D augmentation in depression is more effective in those without vitamin D deficiency
d vitamin D augmentation should be restricted to those who are housebound
e augmentation trials may be best focused on depressed patients with vitamin D deficiency.
3 As regards vitamin D deficiency in schizophrenia:
a low milk intake is a common cause of vitamin D deficiency schizophrenia
b most vitamin D does not come from food so dietary intake is not an important factor
c it explains why people living in rural areas have higher risk of schizophrenia
d it explains why schizophrenia is only found in people living at high latitudes
e it explains why those born in summer months have higher risk of schizophrenia.
4 The physiologically active form of vitamin D is:
a cholecalciferol
b 25(OH)2D3
c 1,25(OH)2D3
d 25(OH)3D3
e vitamin D2.
5 Vitamin D deficiency is defined by levels:
a <50 nmol/L
b <100 nmol/L
c <25 nmol/L
d <75 nmol/L
e <400 nmol/L.
MCQ answers
1 d 2 e 3 b 4 c 5 c



eLetters
No eLetters have been published for this article.