DHA is the most abundant very long-chain n-3 PUFA in the central nervous system(Reference Sastry1) and is therefore often considered an essential nutrient for the development of the brain(Reference Innis2). Evidence for an effect of dietary n-3 PUFA on cognitive development of human infants is still limited and inconclusive(Reference Heird and Lapillonne3, Reference Eilander, Hundscheid, Osendarp, Transler and Zock4). However, evidence from animal studies suggests that higher brain concentrations of DHA are associated with better cognitive performance(Reference McCann and Ames5).
Investigating whether dietary n-3 PUFA increase the proportion of n-3 PUFA in the human brain is complicated, as brain tissue sampling is not feasible in living human subjects. However, in animals we can examine the effect of n-3 PUFA consumption on the n-3 PUFA proportions in the developing brain. Although no animal species perfectly parallels the human situation, pigs resemble human subjects very closely in brain morphology, brain surface anatomy and postnatal brain development and maturation(Reference Dobbing and Sands6, Reference Duhaime7). Therefore, pigs confer significant advantages over other animal species for modelling the effects of dietary n-3 PUFA on the fatty acid composition of human brain tissue.
Several studies have investigated the effects of dietary n-3 PUFA on the fatty acid composition of the brain in newborn piglets provided with formula milk with or without n-3 PUFA(Reference Arbuckle, Rioux, Mackinnon, Hrboticky and Innis8–Reference Goustard-Langelier, Guesnet, Durand, Antoine and Alessandri17). The choice of newborn piglets arises from the fact that the brain growth spurt in pigs extends from about 6 weeks before birth to about 5 weeks afterwards, and embraces a phase of rapid deposition of lipids(Reference Dickerson and Dobbing18). However, it is largely unknown whether dietary n-3 PUFA still affect the fatty acid composition of the brain after this period of rapid brain growth in juvenile pigs.
Most studies in piglets that investigated the effects of dietary n-3 PUFA determined the fatty acid composition of the whole brain(Reference Arbuckle, Rioux, Mackinnon, Hrboticky and Innis8–Reference Lapillonne, DeMar, Nannegari and Heird16) and many reported higher proportions of n-3 PUFA in the brain tissue after n-3 PUFA feeding compared with control feeding(Reference Arbuckle, Rioux, Mackinnon, Hrboticky and Innis8–Reference Foote, Hrboticky, MacKinnon and Innis14). However, a limitation of such whole-brain analyses is that they do not take into account that brain development is not a homogeneous process in time or space. Various parts of the brain develop at different time points and grow with different speeds(Reference Rice and Barone19), which may have consequences for the responsiveness of different brain regions to n-3 PUFA. Effects of dietary n-3 PUFA on the whole brain may therefore mask regional heterogeneity in the brain lobes regarding the n-3 PUFA proportions and the response to dietary n-3 PUFA. Each hemisphere of both the human and the pig brain can be divided into the frontal, parietal, temporal and occipital brain lobes and in human subjects these separate brain lobes are all involved in specific cognitive functions(Reference Smith and Jonides20–Reference Grill-Spector, Kourtzi and Kanwisher23). The separate brain lobes may be differentially affected by n-3 PUFA consumption, which could be of importance for future research concerning dietary n-3 PUFA and brain function.
We investigated the effect of feeding dietary fish oil in a semipurified pig diet for a period of 8 weeks on the fatty acid composition of the frontal, parietal, temporal and occipital brain lobes in a randomized, controlled feeding trial in juvenile pigs aged 7 weeks at commencement.
Methods
The experimental protocol complied with the Guide for the Care and Use of Laboratory Animals (24) and was approved by the animal experiments committee of Amsterdam Medical Centre, the Netherlands. Some of the brain samples used in this study were derived from twenty-two pigs that were also involved in another study, designed to investigate effects of dietary n-3 PUFA on electrophysiological changes in isolated pig hearts(Reference Coronel, Wilms-Schopman and Den Ruijter25). However, the brain samples of these pigs were dissected before the electrophysiological experiments on the isolated pig hearts started, thus the study conditions were similar for all pigs in our experiment.
Animals and diets
Five-week-old male non-littermate pigs (Topigs 40 sow × Tempo boar), were purchased from a research piggery (V.O.F. van Beek, Lelystad, the Netherlands; n 37) and were housed in groups of two animals per pigpen in the animal care facilities of University Medical Centre Utrecht, the Netherlands. After 2 weeks of habituation on a regular pig chow diet, the 7-week-old pigs were randomly assigned to a semipurified pig diet containing either 4 % (w/w) fish oil, Marinol C-35 (n 19) or 4 % (w/w) high-oleic acid sunflower oil (HOSF; n 18) for a period of 8 weeks. The source of the fish oil was anchovy. The fish oil and HOSF (Loders Croklaan, Wormerveer, the Netherlands) were blended into the pig diets (Research Diets Services, Wijk bij Duurstede, the Netherlands) every 2 weeks and stored in a cold room (4°C). Each pig received 1 kg feed per d and all pigs consumed the complete amount of feed every day. We used block randomization with a block size of four to ensure approximately equal group sizes. Pigs entered the study in a phased procedure; i.e. two pigs started the trial each week. All study investigators as well as the laboratory and technical staff were blinded for the treatment allocation. Both diets contained similar amounts of total fat, carbohydrates and proteins and contained various vitamins and other essential food components (Table 1). Both diets were enriched with all-rac-α-tocopheryl acetate (40 mg/kg diet) to diminish in vivo oxidation of unsaturated fatty acids. We analyzed the fatty acid composition of both diets (Table 2) at three different time points during the intervention period.
Table 1 Macro- and micronutrient composition of the semipurified pig diets containing either fish oil (fish oil diet) or high-oleic acid sunflower oil (HOSF diet)
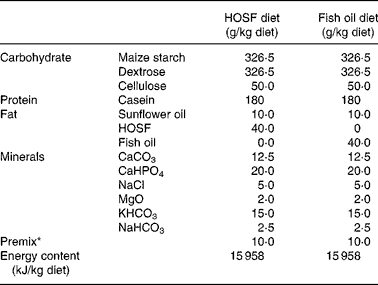
HOSF, high-oleic acid sunflower oil.
* The premix contained (mg/kg diet): MnO2 (70·0); FeSO4.7H2O (400·0); ZnSO4.H2O (300·0); NaSeO3.5H2O (0·2); Kl (0·5); CuSO4.5H2O (100·0); CoSO4.7H2O (2·5); thiamin hydrochloride (2·0); riboflavin (5·0); nicotinamide (30·0); calcium pantothenate (12·0); pyridoxine hydrochloride (3·0); cyanocobalamin (0·04); folic acid (1·0); biotin (0·1); ascorbic acid (50·0); choline chloride (1000·0); menadione (3·0); all-rac-α-tocopheryl acetate (40·0); retinyl acetate and retinyl palmitate (18·0); cholecalcipherol (0·045); maize starch carrier material (7962,62).
Table 2 Fatty acid composition of the semipurified pig diets containing either fish oil (fish oil diet) or high-oleic acid sunflower oil (HOSF diet)
(Means and standard deviations from three diet samples for each diet, taken at three different time points during the intervention period)
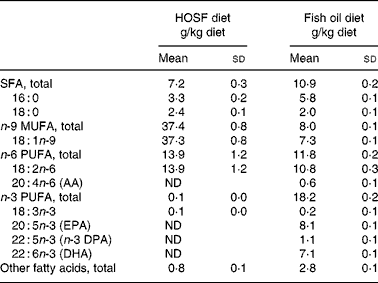
AA, arachidonic acid; ND, not detectable; DPA, docosa pentaenoic acid.
Tissue sampling and lipid analyses
After 8 weeks of feeding, the fasted pigs received 350 mg ketamine (Nimatek, Animal Health BV, the Netherlands) and 80 mg azaperone (Stresnil, Janssen, the Netherlands) intramuscularly and were anaesthetized with 20 mg pentobarbital/kg (Nembutal, CevaSate Animale) intravenously. Blood was collected, plasma and erythrocytes were extracted and stored at − 80°C until lipid extraction and fatty acid analysis. The cerebrum was excised and separated into the left and right cerebral hemispheres by a mid-saggital section. Samples from the frontal, temporal, parietal and occipital lobes of the left hemisphere of the cerebrum were dissected, frozen in liquid N2 and stored at − 80°C until lipid extraction and fatty acid analysis. Total lipids were extracted from all tissues according to the method of Folch et al. (Reference Folch, Lees and Sloane Stanley26). We analyzed the fatty acid composition in brain total lipids, plasma cholesteryl esters and erythrocyte phospholipids. The identity of the fatty acid peaks was shown by GC by comparing each peak's retention time to the retention times of fatty acids in synthetic standards of known composition. Fatty acid composition data are expressed as g/100g fatty acid methyl esters. The sum of all peak areas of the fatty acids identified was taken as 100 %. The percentages of fatty acid methyl ester from the diet samples were converted to g fatty acids per 100 g total lipid using lipid conversion factors (0·93 for fish oil and 0·96 for HOSF(Reference Weihrauch, Posati, Anderson and Exler27)) and converted into g fatty acids per 100 g diet using the total lipid content.
Statistical analyses
Normal distribution of the data was tested using Shapiro–Wilk tests, and final decisions regarding normal distribution of the data were based on visual inspection of normal probability plots. Equality of variances was tested using Levene's tests. Two-way ANOVA was used to evaluate the effects of diet and brain lobe on the fatty acid composition of the brain lobes. When significant F tests were obtained, Tukey's honest significant differences tests were applied for the post hoc comparison. Statistical significance was defined as P < 0·05. All statistical analyses were conducted using SAS version 9.1.3 (Statistical Analysis Software; SAS Institute, Cary, NC, USA).
Results
Thirty-seven pigs received the diets containing either fish oil or HOSF for a mean period of 57·2 (sd 2·8) d. The baseline fatty acid patterns in plasma and erythrocytes in the pigs assigned to the fish oil diet and those allocated to the HOSF diet were well balanced (Table 3). Brain samples from all four cerebral brain lobes were collected from fourteen pigs in the fish oil group and sixteen pigs in the HOSF group. Table 4 shows the fatty acid composition in total lipids of the frontal, temporal, parietal and occipital brain lobes of pigs fed the fish oil diet and the HOSF diet. DHA was the predominant n-3 PUFA and arachidonic acid was the predominant n-6 PUFA found in all brain lobes of the pigs.
Table 3 Fatty acid patterns in erythrocyte membranes and plasma cholesteryl esters of pigs at baseline and after 8 weeks on a semipurified pig diet containing either fish oil (fish oil diet, n 14) or high-oleic acid sunflower oil (HOSF diet, n 16)*
(Means and standard deviations or medians with interquartile ranges)
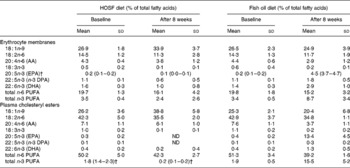
AA, arachidonic acid; DPA, docosapentaenoic acid; ND, not detectable.
* For details of diets and procedures, see Methods.
† Median values with interquartile ranges.
Table 4 Fatty acid composition in total lipids of frontal, parietal, temporal and occipital brain lobes of pigs fed a semipurified pig diet containing either fish oil (fish oil diet, n 14) or high-oleic acid sunflower oil (HOSF diet, n 16) for 8 weeks*
(Mean values and standard deviations)

ND, not detectable.
a,b,c Mean values with different superscript letters indicate significant differences between the brain lobes within each dietary intervention group, according to post hoc Tukey's honest significant differences tests.
* For details of diets and procedures, see Methods.
† Two-way ANOVA.
Pigs on the fish oil diet had higher proportions of DHA in the frontal, parietal and occipital brain lobes but not in the temporal lobe, compared with pigs fed the diet containing HOSF. The interaction between diet and brain lobe was significant for DHA (P = 0·03), indicating that the differences in DHA proportions between the brain lobes were not completely similar for the pigs on the fish oil diet compared with the pigs on the HOSF diet. In the fish-oil-fed pigs, the proportion of DHA was significantly lower in the temporal lobe compared with the other three brain lobes. However, in the pigs fed the HOSF diet, the proportion of DHA was significantly lower in the temporal lobe compared with the parietal lobe only, but not compared with the other two brain lobes. EPA and n-3 DPA together were hardly detectable in the brain lobes of the pigs on the HOSF diet and comprised about 1·7 % of total fatty acids in the fish-oil-fed pigs.
Pigs on the fish oil diet had significantly lower proportions of the n-6 PUFA arachidonic acid (C20 : 4n-6), adrenic acid (C22 : 4n-6) and osbond acid (C22 : 5n-6) in all four brain lobes compared with the pigs fed HOSF. A post hoc Tukey honest significant differences test on the differences between the brain lobes showed that the proportions of n-6 PUFA were different between the brain lobes in both intervention groups. The frontal lobe had significantly higher proportions of n-6 PUFA compared with the other three brain lobes.
Although the HOSF diet contained higher proportions of oleic acid (37·3 (sd 0·8) g/kg diet) compared with the fish oil diet (7·3 (sd 0·1) g/kg diet), there were no significant differences in the proportions of total n-9 MUFA between the HOSF diet group and the fish oil group. However, a post hoc Tukey honest significant differences test on the differences between the brain lobes showed that the temporal lobe had higher proportions of n-9 MUFA compared with the frontal lobe in both intervention groups.
With regard to the saturated fatty acids it appeared that the temporal lobe had lower proportions of total saturated fatty acids compared with the frontal lobe in both intervention groups.
Discussion
This study shows that a diet enriched with fish oil affects the fatty acid composition of the brain, even when supplied after the period of rapid brain growth in juvenile pigs. Moreover, we showed regional differences between specific cerebral brain lobes. Pigs on the fish oil diet had significantly higher proportions of DHA in the frontal, parietal and occipital brain lobes, but not in the temporal lobe. This suggests that the temporal lobe is less responsive to dietary fish oil compared with the other three brain lobes.
An important issue with regard to the interpretation of these data is that both intervention diets contained low amounts of α-linolenic acid. Dietary α-linolenic acid has been shown to support the synthesis and deposition of DHA in neural tissues, in the absence of a dietary source of DHA in pigs(Reference Arbuckle and Innis9). It is therefore possible that the insufficient amount of α-linolenic acid in combination with the absence of DHA in the HOSF diet may have caused a decrease in brain DHA.
We specifically addressed the effect of dietary fish oil, without this potentially disturbing effect of α-linolenic acid conversion into DHA. Our results suggest that the temporal lobe is less responsive to dietary fish oil with regard to DHA proportions compared with the other brain lobes, but that there are no differences in DHA proportions between the temporal, parietal and occipital brain lobes in the absence of dietary DHA. However, we have to keep in mind that our study design does not enable us to compare the effect of dietary fish oil on the fatty acid composition of the separate brain lobes with the effect of a normal pig chow diet containing sufficient amounts of α-linolenic acid.
The use of pigs as an animal model to study the effects of dietary fish oil on the fatty acid composition of the brain has distinct advantages. The pig brain resembles the human brain very closely in overall shape and structure. Both pigs and human subjects have brains with a highly convoluted surface (gyrencephalic brain), as opposed to rodents which have a smooth, lissencephalic brain. Moreover, pigs have a human-like distribution of grey and white matter in the brain, whereas rodents have little white matter(Reference Dobbing and Sands6, Reference Duhaime7). The timing of the brain growth spurt in relation to birth varies between animal species. However, pigs closely resemble the human brain development sequence as the brain growth spurt peaks at birth in both human subjects and pigs(Reference Dobbing and Sands6, Reference Duhaime7). Furthermore, the fatty acid composition of the pig brain corresponds well with the fatty acid composition of the human brain. The total proportion of saturated fatty acids in the pig's brains was approximately 36 %, with the major saturated fatty acids being palmitic acid (16 : 0) and stearic acid (18 : 0). This is comparable to the human infant brain, where palmitic acid and stearic acid are the major saturated fatty acids comprising about 35–50 % of total fatty acids in phosphoglycerides(Reference Svennerholm28). Oleic acid (18 : 1n-9) comprised about 18 % of total fatty acids in both the pig brain as well as the human infant brain(Reference Svennerholm28). DHA and arachidonic acid are the two major PUFA in both the pig and the human brain(Reference Martinez29, Reference O'Brien and Sampson30). Moreover, the DHA:arachidonic acid ratio in the frontal lobe in the HOSF pigs was approximately 0·9, which is consistent with the DHA:arachidonic acid ratio of approximately 0·8 in the forebrain of human infants(Reference Martinez29).
Random allocation of the pigs to the two treatment groups was applied to prevent selection bias and to generate groups that were roughly comparable in terms of responsiveness to the diet and baseline proportions of n-3 PUFA in the brain tissue. As the pigs were non-littermates, were all fed the same pig chow diet before entering the study, and were randomly allocated to the intervention groups, it is highly unlikely that differences in fatty acid handling due to lineage or differences in the pretreatment diet between the two groups would have occurred in our study that could have biased our results. Moreover, the fatty acid levels in plasma cholesteryl esters and erythrocyte membranes support there being no differences in n-3 PUFA proportions in these tissues at baseline between the two intervention groups.
A limitation for translating our findings to human subjects is that the dose of fish oil we applied was fairly high. The diets contained either 4 % (w/w) fish oil or 4 % (w/w) HOSF, which is 2·51 g fish oil or HOSF per 1000 kJ metabolizable energy. In human studies, normal doses used are 2–10 g fish oil/d, which corresponds to approximately 0·2–1·0 g fish oil per 1000 kJ energy intake. Thus, based on energy intake, the dose of fish oil in this pig study was about 2·5 to 12·5 times higher than in human trials. However, in view of the magnitude of the effects seen in pigs, it is likely that lower doses of fish oil will also have a notable effect on brain fatty acid composition.
The proportions of saturated fatty acids, n-9 MUFA, n-6 PUFA and n-3 PUFA in the pig brains observed in this study are comparable to the proportions reported in the whole brain in other pig studies(Reference Arbuckle, Rioux, Mackinnon, Hrboticky and Innis8, Reference Jimenez, Boza, Suarez and Gil15, Reference Blank, Neumann, Makrides and Gibson31). However, relatively little is known about the effects of dietary fish oil on the fatty acid composition of specific brain lobes in pigs. To the best of our knowledge, there is only one other study in pigs comparing the effect of dietary fish oil on the fatty acid composition of the four cerebral brain lobes. This study in newborn piglets showed no significant differences in DHA between the temporal lobe and the other brain lobes after 2 weeks of fish oil feeding, although the authors describe an apparent preservation of the temporal lobe with regard to the fatty acid composition in their discussion section(Reference Goustard-Langelier, Guesnet, Durand, Antoine and Alessandri17). However, our results are in agreement with a study in adult rats that showed lower proportions of DHA in the temporal lobe, compared with the parietal, frontal and occipital lobes after a diet with preformed DHA(Reference Levant, Ozias, Jones and Carlson32). Although this suggests that the temporal lobe could be less responsive to dietary fish oil compared to the other brain lobes, the number of animal studies comparing the four cerebral brain lobes is too limited to make definite inferences.
A hypothesis regarding the regional differences in fatty acid composition after dietary fish oil may be that the four cerebral brain lobes have different growth rates. It may be that regions associated with more primary functions (e.g. primary motor cortex, within the frontal lobe) develop earlier compared with regions that are involved with more complex and integrative tasks (e.g. temporal lobe)(Reference Gogtay, Giedd and Lusk33). An issue of concern is the interpretation given to the data. If the insensitivity for dietary fish oil in the temporal lobe would be confirmed in future studies in pigs, the question arises what a relative enrichment of DHA in specific brain lobes means, in particular when considering human brain development and function. Are cognitive functions that are linked to the temporal lobe in humans less likely to be influenced by dietary fish oil than cognitive functions linked to other brain lobes? If indeed the proportion of DHA in the brain would influence cognitive performance, our data would suggest that increasing fish oil consumption will poorly affect cognitive functions in which the temporal lobe is involved, such as verbal and visual memory(Reference Kennepohl, Sziklas, Garver, Wagner and Jones-Gotman34). Moreover, we saw significantly lower levels of n-6 PUFA, arachidonic acid in particular, in all four brain lobes in the pigs on the fish oil diet compared with the HOSF pigs, which may also have consequences for cognitive development. However, the relation between structure and function in the brain is not very well defined. Moreover, as the evidence for effects of dietary fish oil on specific cognitive functions at present is still limited and inconclusive for infants and children(Reference Eilander, Hundscheid, Osendarp, Transler and Zock4, Reference McCann and Ames5), these contentions are highly speculative.
The responsiveness of the developing brain to nutrients depends on whether a nutrient actually reaches the brain as well as on the timing of exposure. It is not well defined how long-chain PUFA accumulate in the brain, although it has been suggested that direct transport from the plasma is more important for brain accretion of DHA than local de novo synthesis(Reference Qi, Hall and Deckelbaum35). The transport of long-chain PUFA across cell membranes is suggested to be mediated by passive diffusion through the phospholipid bilayer(Reference Hamilton and Kamp36) or facilitated by membrane- and cytoplasma-associated proteins(Reference Glatz, van Nieuwenhoven, Luiken, Schaap and van der Vusse37). However, there is little information regarding passive diffusion of fatty acids through the blood–brain barrier and the blood–cerebrospinal fluid barrier. On the other hand, a number of fatty acid transporter proteins have been identified in the brain, indicating that facilitated transport may be the major mechanism for long-chain PUFA transport into the brain(Reference Qi, Hall and Deckelbaum35). DHA is mainly incorporated into phosphatidylethanolamine and phosphatidylcholine, whereas arachidonic acid is mainly incorporated into phosphatidylinositol and phosphatidylcholine(Reference Qi, Hall and Deckelbaum35). In this study, we measured the fatty acid composition of total lipids, and not the individual lipid classes. The total lipid fraction includes any NEFA, which may have originated from hydrolysis of fatty acid which may occur during the post mortem period. Another factor regarding the responsiveness of the developing brain for nutrients depends on the timing of exposure. Our study shows that dietary DHA is efficiently incorporated in the brain of juvenile pigs. This suggests that the juvenile pig brain is apparently still responsive to dietary differences in fatty acid composition, although the period of rapid lipid deposition which is associated with the brain growth spurt(Reference Flynn38) has ended.
In summary, our study shows that increasing the dietary intake of EPA and DHA through fish oil feeding in juvenile pigs after their brain growth spurt resulted in higher proportions of DHA and lower proportions of n-6 PUFA in the frontal, parietal, and occipital brain lobes. The fatty acid composition of the temporal brain lobe appears to be less responsive to dietary differences in DHA. The effects of lower dietary dosages of fish oil on regional differences in brain n-3 PUFA as well as the consequences of these differences for cognitive development and performance should be the focus for future investigation.
Acknowledgements
The authors gratefully acknowledge Nico Attevelt, Johan van Amerongen, Paulien Scholten, Ron Timmermans, Elly van Zwol, Janine Grouw, Charly Belterman, Betty van der Struijs and Marieke Bos for their valuable contributions to this study. The study was supported by the Top Institute Food and Nutrition (the former Wageningen Centre for Food Sciences), Wageningen, the Netherlands. P. L. Z. currently works at the Unilever Food and Health Research Institute (Vlaardingen, the Netherlands). Unilever markets food products, among which are products high in unsaturated fatty acids. None of the authors has any financial or personal conflict of interest to disclose.





