Methionine is an essential amino acid with a recommended daily intake of 1–2 g per d for human individuals(Reference Young, Wagner, Burini and Storch1). Most methylation reactions in the body, e.g. methylation of RNA and DNA or synthesis of creatine or adrenaline, rely on methionine metabolism(Reference Fonseca, Guba and Fink2). In the respective pathway, methionine is coupled to ATP leading to formation of S-adenosylmethionine (SAM), which is the main methyl donor in mammals(Reference Finkelstein3). A product of the methyl transfer reactions is S-adenosylhomocysteine (SAH), which can be further catabolized to homocysteine by SAH-hydrolase(Reference Kloor, Stumvoll, Schmid, Kompf, Mack and Osswald4). Homocysteine is remethylated by a vitamin B12- and folate-dependent pathway to methionine or metabolized via formation of cystathionine with serine to α-ketobutyrate and cysteine or secreted into the plasma(Reference Refsum, Guttormsen, Fiskerstrand and Ueland5).
Because methionine metabolism is the only known source for homocysteine in mammals, excessive methionine uptake might lead to the various pathophysiological consequences associated with hyperhomocysteinaemia(Reference Finkelstein3). Elevated plasma homocysteine concentration (hyperhomocysteinaemia) is regarded as an independent risk indicator for cardiovascular events(Reference De Bree, Verschuren, Kromhout, Kluijtmans and Blom6) and is associated with renal dysfunction(Reference van Guldener, Stam and Stehouwer7), neuronal diseases(Reference Allen, Stabler and Lindenbaum8) and complications in pregnancy(Reference El Khairy, Vollset, Refsum and Ueland9, Reference Ingec, Borekci and Kadanali10). Elevated plasma homocysteine may be caused by enzyme deficiencies(Reference Reish, Townsend, Berry, Tsai and King11, Reference Blom12) or by malnutrition, e.g. vitamin depletion(Reference Allen, Stabler and Lindenbaum8, Reference Herrmann and Obeid13) or a high methionine intake(Reference De Bree, Verschuren, Kromhout, Kluijtmans and Blom6, Reference Bellamy, McDowell, Ramsey, Brownlee, Bones, Newcombe and Lewis14).
Several theories have been put forward to explain the pathomechanisms associated with hyperhomocysteinaemia. Under in vitro conditions, homocysteine can interact with various enzymes, leading to an inhibition of the respective enzymatic reaction. This has been described for tyrosinase(Reference Reish, Townsend, Berry, Tsai and King11), histidine ammonia-lyase(Reference Klee15) or dimethylargininase (DDAH)(Reference Stühlinger, Oka and Graf16). Inhibition of DDAH may consequently lead to accumulation of asymmetrical dimethylarginine (ADMA), which is an inhibitor of endothelial nitric oxide synthase (eNOS). Elevated ADMA diminishes production of nitric oxide and contributes to endothelial dysfunction, making elevated plasma ADMA levels a risk factor for CVD(Reference Böger, Lentz, Bode-Böger, Knapp and Haynes17). Adenosine is another known regulator of vessel diameter(Reference Deussen18). Extracellular adenosine concentrations might be lowered in hyperhomocysteinaemia due to a reversed SAH-hydrolase reaction(Reference Chen, Li and Zou19, Reference Riksen, Rongen, Blom, Russel, Boers and Smits20). Because this bidirectional pathway favours intracellular production of SAH, if homocysteine concentrations are increased, this may result in net consumption of adenosine and hence lowered adenosine concentrations(Reference Borst, Deussen and Schrader21). Under those conditions, increased homocysteine concentrations may be reflected by elevated SAH plasma levels(Reference Kerins, Koury, Capdevila, Rana and Wagner22, Reference Yi, Melnyk, Pogribna, Pogribny, Hine and James23). Changes of tissue SAH concentrations are an index for disorders in transmethylation metabolism, which, on one hand, may result in hypermethylation (more SAH is formed) and, on the other hand, in hypomethylation (SAH inhibits various methyltransferases)(Reference Perna, Capasso, Lombardi, Acanfora, Satta and Ingrosso24, Reference Ingrosso, Cimmino and Perna25).
A previous study in human subjects has addressed short-term effects (1–7 d) of methionine or methionine-enriched diet (MET) on homocysteine plasma levels(Reference Ward, McNulty, Pentieva, McPartlin, Strain, Weir and Scott26). It was found that plasma homocysteine was unaltered. The present study, conducted in rats, was designed to explore the impact of elevated methionine supplementation over a longer period of time and to assess metabolic consequences more comprehensively with regard to different organs, in addition to the commonly used plasma measurements. With regard to homocysteine-related diseases reported in literature we chose to analyse kidney(Reference van Guldener, Stam and Stehouwer7), heart(Reference De Bree, Verschuren, Kromhout, Kluijtmans and Blom6) and brain(Reference Allen, Stabler and Lindenbaum8). In addition, liver was analysed, because of its special importance for homocysteine metabolism, which includes a second remethylation pathway with betaine as methyl donor and a particularly high activity of the enzymes involved in homocysteine metabolism(Reference Finkelstein3). Measurements of spleen were included, because this organ is a major blood storage. Furthermore, the potential cardiovascular risk as mediated via homocysteine pathogenicity was addressed by measurement of plasma concentrations of adenosine and dimethylarginine (symmetric and asymmetric). Cholinergic mesenteric artery relaxation was quantified as a model of endothelium-dependent vessel function. An effect of homocysteine feeding on cholinergic aorta relaxation has been shown in literature(Reference Dimitrova, DeGroot, Pacquing, Suyderhoud, Pirovic, Munro, Wieneke, Myers and Kim27). We decided to use a methionine, not a homocysteine, feeding model, as the concentrations of homocysteine in food are far lower than those of methionine(Reference Pexa, Fischer, Deussen and Henle28). The methionine supplementation was chosen to reflect the upper range that may be consumed additionally during over-nutrition in human individuals.
Methods
Animals and tissue preparation
The experimental project was approved by the University Commission on Animal Experiments with regard to the animal welfare regulations of Germany, which conform to National Institutes of Health guidelines. A written permission for the study was issued by the German local authorities (24-9168·11-1-2004-16).
Male Wistar rats (Charles River Laboratories, Sulzfeld, Germany) were fed either a normal (control, n 9) or MET (n 9) with 4 g/l methionine (Sigma, Taufkirchen, Germany) in the drinking water ad libitum (approximately 40 ml/d). The rationale for choosing this condition was to reflect a 3–4-fold increased methionine intake, as it might occur in human individuals due to over-nutrition. The regular pellet diet (Ssniff, Soest, Germany) contained 0·35 g methionine per 100 g and was given to both groups of rats ad libitum (approximately 20 g/d). A duration of 4 weeks feeding was chosen to induce steady state hyperhomocysteinaemia(Reference Morita, Kurihara, Yoshida, Saito, Shindo, Oh-Hashi, Kurihara, Yazaki and Nagai29).
After 4 weeks, animals were killed by CO2 exposure, blood was drawn from the vena cava in Li-heparin tubes (Sarstedt, Nuernbrecht, Germany) and plasma was separated from corpuscles immediately after collection by centrifugation at 2000 g. Leucocytes were removed and erythrocytes were washed with 0·9 % NaCl solution and stored at − 80°C for analysis of homocysteine. Liver, heart, spleen, kidney and brain were immediately frozen in liquid N2 and stored at − 80°C for analysis of metabolites. Additionally, tissue samples of liver and lung were frozen at − 20°C for western blot analysis. For test of vascular function, the mesentery and attached tissue was removed and placed into ice-cold physiological saline solution containing 119 mmol/l NaCl, 4·7 mmol/l KCl, 1·17 mmol/l MgSO4, 1·18 mmol/l KH2PO4, 25 mmol/l NaHCO3, 5·5 mmol/l glucose, 0·027 mmol/l EDTA and 2·5 mmol/l CaCl2.
Isometric force measurement
Vessel function was determined on a Mulvany myograph (Power Lab/400; AD-Instruments, Spechbach, Germany) connected to a PC by interface Model 410A (Danish Myo Technologies, Aarhus, Denmark) using mesenteric arterial vessels(Reference Buus, VanBavel and Mulvany30). The software Chart v4.1.2 (AD Instruments, Spechbach, Germany) was used for data acquisition. Freshly isolated mesenteric arteries were cleared from surrounding adipose tissue and cut into segments of about 2 mm width. The rings were carefully mounted in a computer-linked force transducer located in a 10 ml organ chamber (Mitutoyo, Kawasaki, Japan) filled with physiological saline solution. The organ bath was warmed to 37°C and continuously bubbled with 5 % CO2 and 95 % O2 to maintain pH at 7·4. Rings were equilibrated with a resting tension equivalent to that obtained by exposure to an intraluminal pressure of 100 mm Hg. Maximum contraction was obtained by exposure of vessel rings to K-enriched physiological saline solution, containing 123·7 mmol/l KCl, 1·17 mmol/l MgSO4, 1·18 mmol/l KH2PO4, 25 mmol/l NaHCO3, 5·5 mmol/l glucose, 0·027 mmol/l EDTA and 2·5 mmol/l CaCl2. Vasoconstriction was induced with 7·5 μmol/l noradrenalin. Endothelium-dependent vasorelaxation of mesenteric arteries was determined using acetylcholine (1 to 3 μmol/l) and endothelium-independent relaxation using sodium-nitroprusside (0·3 to 3 μmol/l). EC50-values were calculated from these concentration-response curves.
Western blot analysis of endothelial nitric oxide synthase and inducible nitric oxide synthase
Proteins were isolated by chloroform/methanol precipitation and protein content was determined by amidoblack method as published(Reference Dieckmann-Schuppert and Schnittler31). Identical amounts of protein were used for each lane. For western blot analysis of eNOS from lung and liver, proteins were separated by 7·5 % SDS-PAGE. For analysis of inducible nitric oxide synthase (iNOS), proteins were separated on a 5 % SDS-polyacrylamide gel. Separated proteins were transferred to nitrocellulose membranes (Amersham, Munich, Germany) by electro blotting. Anti-eNOS and anti-iNOS were purchased from BD Pharmingen (Heidelberg, Germany) and anti-β-tubuline was purchased from Sigma (Taufkirchen, Germany). eNOS and iNOS were detected by ECL western blotting detection reagents (Amersham, Munich, Germany). Horseradish peroxidase-conjugated goat anti-rabbit IgG and sheep anti-mouse IgG were purchased from Dianova (Hamburg, Germany). Protein expression was calculated as intensity of blotting bands relative to β-tubuline.
Liquid chromatography–tandem MS analysis of arginine, asymmetrical and symmetrical dimethylarginine
ADMA and symmetrical dimethylarginine (SDMA) were determined together with arginine by liquid chromatography–tandem MS as described previously(Reference Schwedhelm, Tan-Andresen, Maas, Riederer, Schulze and Boger32). In brief, samples (50 μl) were precipitated with 100 μl acetone and supernatants were derivatized with 100 μl 1 m-HCl in butanol (20 min, 65°C) after evaporation. Deuterated ADMA (2 μmol/l; d6-ADMA) and 50 μmol/l deuterated arginine (d7-arginine) were added as internal standards prior to precipitation. Butyl ester derivatives of arginine, ADMA and SDMA were analysed in the electron spray ionisation (ESI) positive mode. The daughter ions m/z 70, 214 and 228 were scanned for arginine, ADMA and SDMA, respectively. The daughter ions with m/z ratios of 77 and 220 were scanned for the internal standards d7-arginine and d6-ADMA, respectively. Peak area ratio was determined and concentrations (μmol/l) were calculated according to calibration curves.
HPLC analysis of homocysteine
Plasma total homocysteine levels were measured by HPLC with a ready-to-use kit (Immundiagnostic, Bensheim, Germany) after reduction of disulfide bounds. HPLC analysis was performed on a Waters Alliance liquid chromatography module (Waters, Eschborn, Germany) with a Merck-Hitachi fluorescence detector (Merck, Darmstadt, Germany) working at excitation/emission wavelengths of λex 385 nm and λem 515 nm. Separation was carried out on a Novapac RP-C18 column (Waters). Tissue and erythrocyte sample preparations were as follows. Tissue samples were homogenized with a half-automated Potter homogenizer in ice-cold 0·1 mol/l perchloric acid (6 ml per g tissue). Samples were neutralized immediately after homogenization with 1 mol/l K3PO4. Erythrocytes (500 μl) were disrupted with 300 μl 0·1 mol/l perchloric acid and then immediately neutralized.
HPLC analysis of plasma adenosine and S-adenosylhomocysteine
HPLC analysis was based on 1, N Reference De Bree, Verschuren, Kromhout, Kluijtmans and Blom6-etheno-derivatization of the adenine body. Derivatization and HPLC conditions were adapted from Haink & Deussen(Reference Haink and Deussen33) with few modifications. In 200 μl plasma samples, proteins were precipitated with 100 μl perchloric acid (1 mol/l) and separated by centrifugation for 15 min at 20 000 g and 4°C. Samples were neutralized with K3PO4 (1 mol/l) and precipitated potassium perchlorate was removed by centrifugation for 15 min at 20 000 g and 4°C. From the supernatant, 143 μl were added to 51 μl citrate-phosphate-buffer (containing 0·1 mol/l citric acid and 0·2 mol/l Na2HPO4, pH 4). Derivatization was started by addition of 6 μl chloracetaldehyde (40 %; Merck). HPLC analysis was performed on the Waters Alliance liquid chromatography module connected to a Merck Hitachi fluorescence detector (λex 280 nm, λem 410 nm). Separation was carried out on an XTerraMS-C18 column (4·6 × 50 mm, particle size 5 μm; Waters) using a linear phosphate-buffer/acetonitrile gradient (from 6 to 24 % acetonitrile over 5·6 min) with ion-pair reagent tetrabutylammoniumsulfate (5·7 mmol/l) at pH 5·8. Retention time was 1·0 min for SAH and 1·3 min for adenosine.
HPLC analysis of S-adenosylmethionine and S-adenosylhomocysteine in tissues
HPLC analysis was based on 1, N Reference De Bree, Verschuren, Kromhout, Kluijtmans and Blom6-etheno-adenosine derivatives of SAM and SAH as described previously(Reference Loehrer, Tschopl, Angst, Litynski, Jager, Fowler and Haefeli34) with few modifications. In brief, tissues were homogenized, acidified and neutralized as described earlier for tissue homocysteine analysis. Supernatant (200 μl) was adjusted to pH 5 with 50 μl ammonium acetate buffer (0·6 mol/l, pH 5). Derivatization procedure was started by addition of 50 μl chloracetaldehyde (40 %; Merck). After 8 h at 39°C, samples were frozen to − 20°C until day of analysis. HPLC analysis was performed on a Waters Alliance liquid chromatography module connected to a Merck Hitachi fluorescence detector (λex 280 nm, λem 410 nm). Separation was carried out on a RP-C18 column (3·9 × 150 mm Nova-Pak RP 18, 4 μm; Waters) applying a linear ammonium acetate/acetonitrile gradient (from 2 to 70 % acetonitrile over 15 min) with ion-pair reagent pentanesulfonic acid (10 μmol/l) at pH 5. Retention times were 4·8 min for SAM and 8·9 min for SAH.
GC-flame ionization detection analysis of methionine and other amino acids
GC with flame ionization detection was performed to measure the plasma and tissue concentrations of amino acids. Tissues were homogenized in ice-cold 0·1 mol/l perchloric acid. Precipitated proteins were removed by centrifugation for 15 min at 20 000 g and 4°C. Plasma was used for the test without protein precipitation. Sample preparation and analysis were performed with a ready-to-use kit (EZ:Faast; Phenomenex, Aschaffenburg, Germany). GC-flame ionization detection analysis was performed on a CP 3380 GC system (Varian, Darmstadt, Germany).
Statistical analysis
The experiment number necessary to detect intergroup differences was estimated by power analysis (α = 0·05; β = 0·8). Data are presented as mean values with their standard errors of the mean. Distribution of data was evaluated by Kolmogorov–Smirnov test. To assess differences of mean values between groups Student's t test for unpaired data was used (two-sided). Correlation coefficient r was calculated according to Pearson in the methionine feeding group. A value of P < 0·05 was considered to indicate a statistically significant difference. Statistics were performed using SPSS for Windows (version 12.0; SPSS Inc.).
Results
Plasma concentrations of cardiovascular risk indicators
Rats consumed on average 20 g pellets per d and drank on average 40 ml water. Calculated average methionine intake was 70 mg per d in the control group and 230 mg per d in the high methionine group, respectively (3·3-fold elevated intake). Plasma concentrations of risk indicators for disturbed cardiovascular functions are summarized in Table 1. Plasma homocysteine concentration was doubled (P < 0·05) in animals fed MET. Plasma concentrations of ADMA were not altered significantly by the MET. However, plasma concentrations of SDMA were significantly elevated in rats fed the MET. There was no significant change of arginine plasma level. Plasma concentrations of SAH and adenosine were not significantly altered by the MET.
Table 1 Cardiovascular risk indicators in blood plasma†
(Values are means with their standard errors)
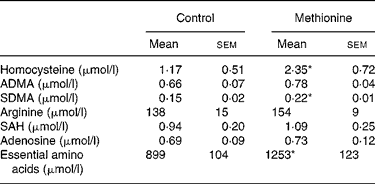
ADMA, asymmetrical dimethylarginine; SDMA, symmetrical dimethylarginine; SAH, S-adenosylhomocysteine.
* Mean values were significantly different from control (P < 0·05).
† For details of diets and procedures, see Methods.
Effects on vessel function
Examined vessel segments did not differ significantly between rats fed MET or a normal methionine diet with regard to length (control 1·7 (sem 0·2) mm, MET 1·7 (sem 0·2) mm) and internal diameter (control 316 (sem 64) μm, MET 308 (sem 54) μm). Acetylcholine and sodium nitroprusside, respectively, evoked the typical concentration-related vasorelaxations in the mesenteric vessel preparations. Half maximal relaxations were obtained at 7·19 (sem 0·78 × 10− 8) mol/l for acetylcholine and 7·24 × 10− 5 (sem 1·38 × 10− 5) mol/l for sodium nitroprusside in vessels from control rats. In animals receiving MET, the endothelium dependent (7·49 × 10− 8 (sem 1·66 × 10− 8) mol/l) and independent (4·43 × 10− 5 (sem 0·90 × 10− 5) mol/l) vasorelaxations were similar to those assessed in the control group. eNOS expression was similar in lung and liver extracts of rats fed a control diet (Fig. 1). iNOS expression was lower than eNOS expression as assessed in lung. There was no statistically significant difference in iNOS or eNOS expression in rats fed MET as compared with animals fed the control diet.

Fig. 1 Expression of inducible (iNOS) and endothelial nitric oxide synthase (eNOS). (A) Representative examples of western blotting bands of eNOS and tubuline from liver; (B) density of blotting bands relative to tubuline from liver and lung. ■, Methionine-enriched diet (MET); ![]() , control diet (Control). Data are presented as means with their standard errors of the mean for nine animals per group. For details of diets and procedures, see Methods.
, control diet (Control). Data are presented as means with their standard errors of the mean for nine animals per group. For details of diets and procedures, see Methods.
Tissue concentration of homocysteine and its precursors
Tissue levels of homocysteine largely differed among organs of control rats. Highest homocysteine tissue levels were found in liver (34·2 (sem 5·0) nmol/g) and lowest levels were found in kidney (8·6 (sem 1·3) nmol/g) (Fig. 2). MET had no significant effect on homocysteine levels of liver, heart, kidney and brain. However, the homocysteine level of spleen was significantly (P < 0·05) elevated in rats that had received MET. Homocysteine level of erythrocytes was unaltered in rats fed MET (1·4 (sem 0·5) μmol/l) as compared with control animals (1·3 (sem 0·5) μmol/l). To address relationships between plasma and spleen homocysteine, the respective correlations were calculated using the data from individual experiments. Within the methionine group, homocysteine was elevated in plasma and spleen, but no significant correlation existed (r − 0·177; P = 0·512). There was also no significant correlation between erythrocyte and spleen homocysteine levels (r − 0·144; P = 0·596).
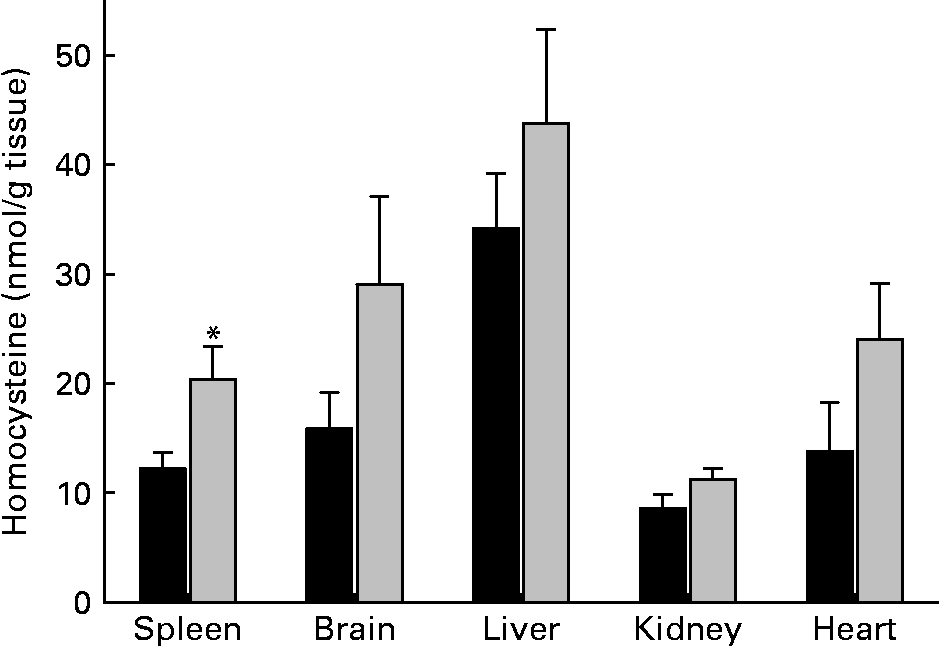
Fig. 2 Tissue concentrations of homocysteine in rats. ■, Control diet; ![]() , methionine-enriched diet. Data are presented as means with their standard errors of the mean for nine animals per group. *Mean value was significantly different from control (P < 0·05). For details of diets and procedures, see Methods.
, methionine-enriched diet. Data are presented as means with their standard errors of the mean for nine animals per group. *Mean value was significantly different from control (P < 0·05). For details of diets and procedures, see Methods.
While 4 weeks of MET was associated with enhanced homocysteine plasma levels (Table 1), no significant elevation of plasma methionine was found in animals fed MET (71 (sem 14) μmol/l) compared with the control group (50 (sem 6) μmol/l). Tissue concentrations of methionine, SAM, SAH and the ratio of both substances, often referred to as ‘transmethylation potential’, are summarized in Table 2. From the various organs studied, only the spleen showed a significantly elevated methionine concentration after MET. Spleen also showed the highest methionine level among the studied organs, followed by kidney. Under control conditions, SAM level was highest in liver and approximately 50 % lower in heart, spleen and kidney. Brain SAM level was only one-third of that in liver. MET did not significantly change SAM levels in any organ studied. Control SAH level was highest in liver followed by kidney (40 % lower). Brain, heart and spleen showed SAH levels in the order of 10 to 20 % of those found in liver. MET further enhanced liver SAH level, but did not change SAH levels of other organs studied. Despite the increase of liver SAH, the transmethylation potential was not significantly decreased in this organ. The sum of essential amino acids (valine, leucine, isoleucine, threonine, methionine, phenylalanine, lysine, tryptophan) was significantly elevated in plasma of rats fed MET (Table 1), but unchanged in organ tissues (data not shown).
Table 2 Methionine, S-adenosylmethionine (SAM), S-adenosylhomocysteine (SAH) and transmethylation potential (SAM/SAH) in various tissues under control conditions and 4 weeks of methionine-enriched diet (nmol/g)†
(Values are means with their standard errors)
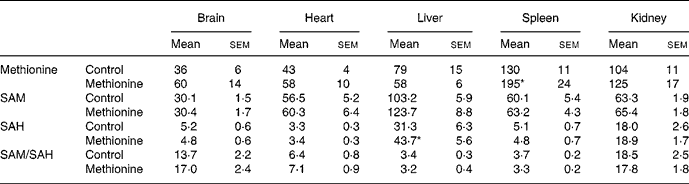
* Mean value was significantly different from control (P < 0·05).
† For details of diets and procedures, see Methods.
Discussion
High methionine uptake has been discussed controversially as a potential risk for elevation of plasma homocysteine(Reference Fonseca, Guba and Fink2, Reference De Bree, Verschuren, Kromhout, Kluijtmans and Blom6, Reference Bellamy, McDowell, Ramsey, Brownlee, Bones, Newcombe and Lewis14, Reference Lentz35). In human subjects, a single application of a high methionine dose (0·1 g/kg) resulted acutely in increased plasma homocysteine(Reference Bellamy, McDowell, Ramsey, Brownlee, Bones, Newcombe and Lewis14). However, a diet containing twice the daily intake (2·1 g/d instead of 1·1 g/d) in volunteers showed no significantly elevated plasma homocysteine after 1 week(Reference Ward, McNulty, Pentieva, McPartlin, Strain, Weir and Scott26). Because the rat model permits the assessment of organ metabolite levels in addition to plasma levels, this approach may provide interrelations of methionine and homocysteine metabolism and thus add important information to human studies. In the present study, 4 weeks of MET resulted in a 2-fold elevated homocysteine plasma level in male Wistar rats. Our experiments reflect conditions like those found in moderate hyperhomocysteinaemia, e.g. observed during malnutrition(Reference De Bree, Verschuren, Kromhout, Kluijtmans and Blom6). One important question to be addressed in the present study is whether the doubling of plasma homocysteine over 4 weeks of MET resulted in changes of endothelium-dependent mesenteric artery function as a standard model of arterial vessel relaxation(Reference Buus, VanBavel and Mulvany30). We found no significant difference between the vessel function of mesenteric arteries of animals fed MET compared with controls. Other studies(Reference Ungvari, Pacher, Rischak, Szollar and Koller36) found significantly altered arteriolar function in rats in response to 4 weeks of MET. However, it should be noted, that these authors used far higher feeding doses of methionine (1 g/kg body weight per d), which resulted in a higher increase in plasma homocysteine (from 7·1 to 23·6 μmol/l), as compared with the present study. The methionine dose applied in the present study was chosen to reflect the upper limit of human malnutrition (3–4-fold elevation of methionine uptake). Thus, pathophysiological effects reported in the earlier study(Reference Ungvari, Pacher, Rischak, Szollar and Koller36) are unlikely to result from malnutrition.
We tested for an influence of the diet on eNOS expression in lung and liver and iNOS expression in lung, but did not find any altered expression. Homocysteine was described to stimulate the expression of iNOS in macrophages in vitro (Reference Woo, Cheung, Chan and Siow37). We were unable to prove this effect in vivo. We conclude from our study that 2-fold elevation of plasma homocysteine induced by MET (3·3-fold elevation of methionine uptake) has no impact on endothelium-dependent mesenteric artery relaxation and no pronounced effects on eNOS and iNOS expression.
As many of the proposed effector mechanisms of hyperhomocysteinaemia are based on direct interactions of homocysteine with enzymes inside cells, e.g. inhibition of DDAH by homocysteine leading to an elevated concentration of ADMA, we have tested whether the homocysteine content in erythrocytes, spleen, brain, liver, kidney and heart was altered after 4 weeks of MET. A significant elevation of tissue homocysteine was only found in spleen. Because spleen is a major blood storage, we considered whether homocysteine present in plasma, erythrocytes or leucocytes might have contributed to the augmented homocysteine concentration in this organ. However, correlations of homocysteine concentrations in plasma and spleen and in erythrocytes and spleen did not reveal a significant relationship. Thus, stored plasma or erythrocytes cannot explain elevated spleen homocysteine. Because homocysteine concentrations of leucocytes were not measured in the present study, leucocytes remain a possibility to explain the elevation of homocysteine levels in spleen. In this respect, it is noted here that cultured mononuclear blood cells have been described to exhibit an enhanced secretion of homocysteine under conditions of elevated methionine concentration(Reference Schroecksnadel, Frick, Wirleitner, Schennach and Fuchs38).
Disorders in transmethylation metabolism are another commonly described effector mechanism of hyperhomocysteinaemia. Although SAM and SAH may decrease shortly after administration of methionine(Reference Young and Shalchi39), in long-term experiments, as performed here, elevations of both metabolites are expected(Reference Loehrer, Tschopl, Angst, Litynski, Jager, Fowler and Haefeli34). The increase of the transmethylation product SAH in liver after methionine feeding (Table 2) may be explained by the following two alternatives: first, by substrate saturation of SAH-hydrolase(Reference Finkelstein3); second, by reversed action of this bidirectional enzyme, when homocysteine concentrations are raised(Reference Deussen, Borst and Schrader40). Liver homocysteine concentrations were not significantly altered. Thus, an enhanced synthesis of SAH from adenosine and homocysteine is unlikely and the enhanced SAH level most likely indicates an accelerated methionine metabolism via transmethylation reactions and a rate-limitation of this pathway by activity of SAH-hydrolase.
Elevated homocysteine has been associated with elevated levels of ADMA, because homocysteine may inhibit DDAH, an ADMA catabolizing enzyme(Reference Stühlinger, Oka and Graf16, Reference Böger, Lentz, Bode-Böger, Knapp and Haynes17). As shown in Table 1, the mean ADMA plasma concentration was higher in the methionine group as compared with control animals, but this difference did not reach statistical significance. Another link between homocysteine and arginine metabolism is the transmethylation of arginine to SDMA or ADMA(Reference Böger, Bode-Böger, Sydow, Heistad and Lentz41). As SDMA plasma concentrations were significantly elevated in the methionine enriched-fed animals compared with the control group, an enhanced methylation of arginine might explain the trend of ADMA elevation rather than inhibited ADMA degradation via DDAH. The fact that ADMA was not significantly changed in the current study is compatible with the finding that acetylcholine-induced relaxation of mesenteric arteries was also unchanged after 4 weeks of an enriched methionine diet, which caused plasma homocysteine levels to double.
Study limitations
Methionine supply in the present study was scaled to reflect conditions of maximal dietary intake of human individuals. However, results obtained in experimental models should be treated with caution. When methionine is supplied in drinking water, the food uptake could be lowered, which might lead to a shortage of essential amino acids(Reference Sugiyama, Kushima and Muramatsu42, Reference Rowling, McMullen, Chipman and Schalinske43). This effect might have influenced the results of the study, as a high methionine diet is typically not induced by enhanced uptake of the pure substance methionine but by over-nutrition, comprising a mix of various amino acids. Because we did not find an alteration of total amino acid content of plasma and of examined tissues, it is unlikely that MET disturbed food uptake in our study.
The present study was conducted in the rat, which has served as a model for studying homocysteine metabolism before(Reference Sugiyama, Kushima and Muramatsu42–Reference Fell and Steele45). As shown in Table 1, plasma homocysteine level in the rat is considerably lower than that in man (6 to 12 μmol/l). Therefore, absolute levels should not be compared.
Taken together, we have found a significant, 2-fold elevation of plasma homocysteine following 4 weeks of MET. However, no alterations in the mesenteric artery vessel dilator function were observed. Furthermore, no alterations of risk indicators such as elevated plasma ADMA concentration, elevated plasma SAH or lowered plasma adenosine concentration were evident. We conclude that a 2-fold elevation of plasma homocysteine induced by 4 weeks of MET had only a small impact on tissue metabolites related to methionine and no significant impact on mesenteric artery dilatory function.
Acknowledgements
We thank Mariola Kastner, Sandra Tuchscheerer, Bianca Mueller and Birgit Zatschler for their technical assistance.





