Introduction
Toxoplasma gondii is an Apicomplexa parasite distributed worldwide that is responsible for toxoplasmosis, a disease of great medical and veterinary relevance. The parasite is capable of infecting all homeothermic organisms, including humans (Delgado et al., Reference Delgado, Zúquete, Santos, Basto, Leitão and Nolasco2022). The prevalence of T. gondii in México is 43.9% in the human population, with the Neotropical region having the highest rate of antibodies against this parasite (Caballero-Ortega et al., Reference Caballero-Ortega, Uribe-Salas, Conde-Glez, Cedillo-Pelaez, Vargas-Villavicencio, Luna-Pastén, Cañedo-Solares, Ortiz-Alegría and Correa2012). The interest in characterizing this protozoan in the Neotropical region of México is due to the combination of climatic and biological factors that favour the wide genetic diversity of T. gondii, as has been reported in several South American countries.
To date, at least 318 genotypes have been reported worldwide, and tropical regions usually have greater genetic diversity (Shwab et al., Reference Shwab, Zhu, Majumdar, Pena, Gennari, Dubey and Su2014; Meireles et al., Reference Meireles, Bezerra, Andrade, Cassiano, Pena, Alves, Francisco and de Andrade2022). Previous reports about the genotyping of T. gondii isolates obtained from feral cats and stray dogs from Quintana Roo and Chiapas, respectively, revealed the great genetic diversity of this protozoan and strong infection pressure, with individuals infected with up to 5 different T. gondii strains in the same urban environment shared by different species, including humans (Valenzuela-Moreno et al., Reference Valenzuela-Moreno, Rico-Torres, Cedillo-Peláez, Luna-Pastén, Méndez-Cruz, Lara-Martínez, Correa and Caballero-Ortega2019, Reference Valenzuela-Moreno, Rico-Torres, Cedillo-Peláez, Luna-Pastén, Méndez-Cruz, Reyes-García, Correa, Alves, Pena and Caballero-Ortega2020, Reference Valenzuela-Moreno, Méndez-Cruz, Rico-Torres, Cedillo-Peláez, Correa and Caballero-Ortega2022b). Campeche is a southeastern Mexican state that has favourable climatic conditions for the replication and dissemination of this protozoan, since 80% of its territory is rainforest. More than 4379 species of vertebrate animals live in this area, of which the family Felidae is represented by the domestic cat as well as by 5 of the 6 species of wild felines reported for México (Guzmán-Soriano et al., Reference Guzmán-Soriano, Guiascón and Cú-Vizcarra2013; CONABIO, 2021). However, studies of this zoonotic agent in this state are scarce. To date, there is only one study that attempted to isolate T. gondii DNA in tissues of wild bat (n = 25) from the State of Campeche, where all of the analysed animals were negative for the B1 gene by PCR (Torres-Castro et al., Reference Torres-Castro, Muñoz-Dueñas, Hernández-Betancourt, Bolio-González, Noh-Pech, Peláez-Sánchez and Sosa-Escalante2019). Therefore, the aim of this work was to identify, isolate and genotype T. gondii in sentinel hosts such as dogs (Canis familiaris) and free-range chickens (Gallus gallus) from Campeche, México, to determine which genotypes are circulating in this particular region and to predict the degree of virulence using an extended panel of 15 biomarkers assayed by the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) approach.
Materials and methods
Tissue samples
From February to June 2021, stray dogs and free-range chickens from Escárcega (18°37′1″ N, 90°43′1″ W) and Calakmul (19°12′–17°48′ N, 89°09′–90°28′ W), respectively, both municipalities of Campeche, were captured and euthanized to collect blood and tissue samples. These counties were located 107 km apart from each other. The body condition of dogs was evaluated according to the Purina® Body Condition Tool (http://www.purina.co.uk), which ranges from 1 (severely underweight) to 9 (clinically obese), where 5 is considered the ideal score. Samples from both animal species were collected in collaboration with the Facultad de Ciencias Agropecuarias of the Universidad Autónoma de Campeche (UAC), México, and the Departamento de Observación y Estudio de la Tierra, la Atmósfera y el Océano, of Colegio de la Frontera Sur (ECOSUR). The stray dogs were anaesthetized with tiletamine-zolazepam (7.5 mg kg−1 body weight, Zoletil-Virbac®, México), and once a dog was placed on an anaesthetic plane, 12 mL of whole blood was obtained from the cephalic vein for DNA extraction. Subsequently, an intravenous overdose of sodium pentobarbital (25 mg kg−1, Dolethal®, Vetoquinol, France) was administered to cause cardiorespiratory arrest and complete the euthanasia procedure. Free-range chickens were euthanized by cervical dislocation. Euthanasia procedures were performed in accordance with the guidelines of the Mexican Official Standard NOM-033-SAG/ZOO-2014 and Law for the Protection and Welfare of Animals of the State of Campeche. Necropsies were carried out following the methodology described by Schuneman and Constantino (Reference Schuneman and Constantino2002), and samples of heart, lung, liver, spleen, striated muscle (diaphragm and right gracilis) and brain from both species were obtained to attempt molecular diagnosis, isolation of the parasite by bioassay in mice, and histopathological analysis.
ELISA
Detection of IgG anti-T. gondii in dogs was carried out as previously described with slight modifications (Cedillo-Peláez et al., Reference Cedillo-Peláez, Díaz-Figueroa, Jiménez-Seres, Sánchez-Hernández and Correa2012). Briefly, ELISA plates (NUNC Maxisorb) were sensitized with 100 μL of crude extract of tachyzoites from T. gondii strain RH (type I) (2 μg mL−1). Sera and goat anti-dog IgG H&L (HRP) (Abcam, NL, ab112852) were diluted 1:200 and 1:8000 in 0.05% PBS-T20, respectively. Detection of the antigen–antibody immunocomplexes was carried out with a chromogen–substrate solution for peroxidase (H2O2/O-phenylendiamine Sigma-Aldrich, MA, USA). The reaction was stopped with 50 μL of 1 N sulphuric acid. Negative and positive sera from previously tested dogs were included in each plate (Cedillo-Peláez et al., Reference Cedillo-Peláez, Díaz-Figueroa, Jiménez-Seres, Sánchez-Hernández and Correa2012). The cutoff point was calculated by obtaining the average absorbance of the negative controls plus 3 standard deviations, and the reactivity index (RI) was calculated by dividing the absorbance of each serum sample by the cutoff point. Sera with IR >1.0 were considered positive. Detection of anti-T gondii antibodies in chicken sera was not performed since no blood samples were obtained.
Western blot
Immunoblotting was carried out as previously described and adapted to test dog sera (Caballero-Ortega et al., Reference Caballero-Ortega, Quiroz-Romero, Olazarán-Jenkins and Correa2008). In brief, T. gondii proteins from 20 million tachyzoites of the RH strain (type I) were separated by electrophoresis. The proteins were electrotransferred to a nitrocellulose membrane, strips of 3 mm width were cut, and serum samples diluted 1:200 in PBS-T20 were added. Then, goat anti-Dog IgG H&L (HRP) diluted 1:2000 in PBS-T20 was added. Immune complexes were detected using a substrate/chromogen solution with 4-chloro-1-naphthol (Sigma-Aldrich, MA, USA). Sera were considered positive when at least 3 diagnostic bands were detected on the nitrocellulose membrane.
Bioassay in mice
Parasite isolation was carried out under the NOM-062-ZOO-1999 guidelines for the handling and care of mice and as previously described (Rico-Torres et al., Reference Rico-Torres, Del Viento-Camacho, Caballero-Ortega, Besné-Mérida, Luna-Pastén, Correa and Palma-García2015). Briefly, the brain and pooled heart and diaphragm tissue from each dog or free-range chicken were manually macerated in a mortar in sterile PBS (pH 7.2) with 10 000 IU mL−1 penicillin G. Then, 1.0 mL homogenate was used to intraperitoneally inoculate two BALB/c mice per dog or chicken. All inoculated mice were examined daily for 45 days for signs of T. gondii infection. Animals with severe signs of illness, such as bristly hair, lethargy, ascites, loss of body weight and neurological signs, were euthanized, and imprints of the lung and brain were observed under a compound microscope to search for tachyzoites or tissue cysts (Fernández-Escobar et al., Reference Fernández-Escobar, Calero-Bernal, Regidor-Cerrillo, Vallejo, Benavides, Collantes-Fernández and Ortega-Mora2021). One week after inoculation, peritoneal lavages with sterile PBS were performed to obtain tachyzoites and proliferate them in NIH 3T3 mouse fibroblast culture (ATCC® CRL-1658™), as previously described (Khan and Grigg, Reference Khan and Grigg2017). The surviving mice were euthanized 45 days after inoculation and were considered infected if tachyzoites or tissue cysts were detected in any of their tissues.
DNA extraction
Peritoneal exudates with apicomplexan parasites from infected mice, blood buffy coat and tissue samples from dogs and chickens were subjected to DNA extraction (Brenier-Pinchart et al., Reference Brenier-Pinchart, Capderou, Bertini, Bailly, Fricker-Hidalgo, Varlet-Marie, Murat, Sterkers, Touafek, Bastien and Pelloux2015) following the manufacturer's instructions (Qiagen Gentra® Puregene® Tissue Kit, Hilden, Germany). The DNA obtained was quantified with a spectrophotometer (Thermo Scientific Nanodrop™ 1000, MA, USA) and kept at −20°C until use.
PCR and genotyping
To detect T. gondii DNA in the tissue samples and peritoneal exudates, endpoint and real-time PCR were performed to amplify the 200 to 300-fold repetitive 529 bp non-coding sequence -SeqRep529- (Tox4-5) and 35-fold repetitive B1 gene. Parasite burden in tissues was also determined by standard curves generated by spiking 50 to 5 × 106 tachyzoites of the RH strain in tissues of uninfected mice. Data were analysed using the Step One software 2.0. The linear regression coefficients (R 2) and amplification efficiencies were calculated (Homan et al., Reference Homan, Vercammen, De Braekeleer and Verschueren2000; Kompalic-Cristo et al., Reference Kompalic-Cristo, Frotta, Suárez-Mutis, Fernandes and Britto2007; Cedillo-Peláez et al., Reference Cedillo-Peláez, Rico-Torres, Salas-Garrido and Correa2011). The isolates of T. gondii obtained were also tested by endpoint PCR for the SeqRep529 marker. Positive samples for at least one of the molecular assays were included in a multilocus nested PCR (Mn-PCR-RFLP) to amplify 10 polymorphic markers: SAG1, alt. SAG2, SAG3, BTUB, GRA6, c22-8, c29-2, L358, PK1 and Apico (Su et al., Reference Su, Shwab, Zhou, Zhu and Dubey2010). Additionally, clinical samples and DNA from T. gondii isolates were included in the Mn-PCR-RFLP to genotype CS3, ROP16, ROP17, ROP5, and ROP18 virulence markers as previously described (Pena et al., Reference Pena, Gennari, Dubey and Su2008; Shwab et al., Reference Shwab, Jiang, Pena, Gennari, Dubey and Su2016). All PCR assays (real time and endpoint) were carried out with AmpliTaq Gold™ polymerase (Thermo Fisher Scientific, cat. 4311806, MA, USA). Internal and external primers of the CS3 marker had the same melting temperature (t m) as the oligonucleotides of the ROP markers; therefore, they were included together in the Mn-PCR-RFLP. All amplicons were digested with specific restriction enzymes (New England, Biolabs®, USA and Thermo Scientific®, USA) as previously described (Pena et al., Reference Pena, Gennari, Dubey and Su2008; Su et al., Reference Su, Shwab, Zhou, Zhu and Dubey2010). DNA samples of strains representative of the three archetypal lineages, RH, Me49 and VEG (Toxoplasma gondii ATCC® 50611 ™ and 50861™) reference strains (types I, II and III, respectively), were included as positive controls, while sterile water was used as a negative control. Nested PCR products were resolved in 1.5% agarose-TBE gels stained with ethidium bromide (EtBr). Additional controls were added to ensure that there was no DNA contamination (positive and negative reamplification controls). All restriction products were resolved in 3% agarose-TBE gels stained with EtBr and digitalized.
Histopathology and immunohistochemistry (IHC)
Tissues fixed in 10% buffered formalin were processed for analysis by the conventional technique for histology and stained with hematoxylin and eosin. For immunohistochemistry, samples were processed using the streptavidin–biotin–peroxidase complex (Histostain-Plus, Invitrogen, USA) as previously described, with slight modifications (Valenzuela-Moreno et al., Reference Valenzuela-Moreno, Carmona-Muciño, Cedillo-Peláez, Rico-Torres, Luna-Pastén, Hernández-Rodríguez and Caballero-Ortega2022a). Briefly, paraffin-embedded sections were cut and mounted on electrocharged slides (Kling-On-Hier, Biocare). Subsequently, the tissues were incubated with serum from T. gondii-positive mice experimentally infected with the T. gondii Me49 strain (dilution 1:300). The slides were then incubated with a secondary multispecies biotinylated antibody (Invitrogen, USA), followed by incubation with streptavidin-peroxidase. Immune complexes were revealed with the commercial Betazoid DAB solution, chromogen 3,3′ diaminobenzidine (Biocare®). Liver and spleen sections of mice infected with T. gondii strain Me49 were used as positive controls, and the primary antibody was replaced with PBS as a negative control. Histological and IHC sections were examined by light microscopy (Zeiss Axiostar plus; Göttingen, Germany).
Results
Eleven stray dogs were captured, all of which were adults (>1 year old). Their body condition ranged from severe to slightly underweight; externally, most of the stray dogs had skin diseases (scabies) and other ectoparasite infestations (fleas and ticks not identified) of varying degrees. No lesions compatible with acute or chronic T. gondii infection were found during necropsy and histopathological analyses. In the brain of dog #8, only one T. gondii-immunopositive tissue cyst was observed. The frequency of IgG anti-T. gondii antibodies found in dogs by ELISA and Western blot was 72.7% and 100%, respectively.
Molecular identification and parasite load
Six out of eleven dogs (54.5%) were positive in at least one tissue sample according to endpoint PCR and qPCR. The parasite load was between 50 and 4025 tachyzoites/mg of tissue. Two T. gondii isolates from dog #8 were obtained, one from the heart/diaphragm pool (TgDogMxCam8a) and one from the brain (TgDogMxCam8b), both showing the ToxoDB #116 genotype. In addition, in the spleen of the same dog, mixed infection (I + II + III) was detected for the SAG3 gene (Table 1).
Table 1. PCR-RFLP genotypes from clinical samples and Toxoplasma gondii isolates obtained from stray dogs and free-range chickens in Campeche State, Mexico

*Isolates obtained from the same animal were designated as ‘a’ and ‘b’ (heart and brain, respectively). +The expected amplicon was obtained but could not be subjected to RFLP. ND: Not done.
Seven free-range chickens were sampled, all of them were adults. Two T. gondii isolates (TgCkMxCam1a and TgCkMxCam1b) from the heart and brain of chicken #1 were obtained, and both were the ToxoDB #38 genotype. The parasite load found in this chicken was more than 35 000 tachyzoites per mg of tissue (Table 1).
Genotyping of virulence markers
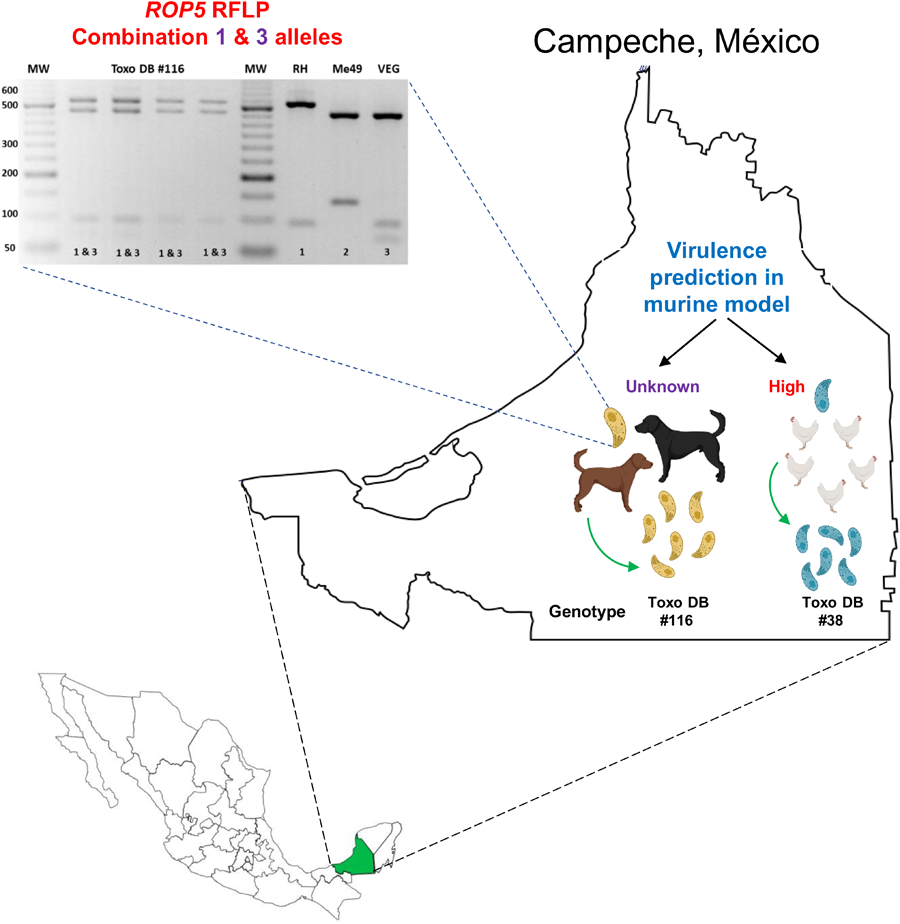
The two isolates from dog #8 have alleles III and 3 for CS3 and ROP18, respectively, while ROP16 and ROP17 have type 1 alleles (Table 2). For the ROP5 locus, a different restriction pattern was found (Fig. 1); it resembled that of the combination of types 1 and 3. Although the combination of alleles at the CS3 and ROP18 loci predicts low virulence in mice for the two TgDogMxCam8 isolates, the true virulence prediction ROP18/ROP5 is unknown due to the combination of type 1 and 3 alleles of ROP5; alleles 1 and 3 contain a virulent cluster, while allele 2 is avirulent. The partial genetic characterization of the virulence markers of a clinical sample from dog #1 showed that this could be a highly virulent strain of T. gondii (ROP18/ROP5 4/1) in the mouse model (Table 2).
Table 2. Virulence multilocus PCR-RFLP typing of clinical samples and Toxoplasma gondii isolates from stray dogs and free-range chickens in Campeche State, México

*Isolates obtained from the same animal were designated as ‘a’ and ‘b’ (heart and brain, respectively). &Strains of type I and III have allele 1. ^Strains of type II and III have allele 2. +Based on Shwab et al. (Reference Shwab, Jiang, Pena, Gennari, Dubey and Su2016). &Genotyping was performed from original tissues of the host. ♦Given the possible ROP18/ROP5 combinations (3/3 and 3/1), it could be predicted as a low-virulence strain.

Figure 1. Representative PCR-restriction fragment length polymorphism pattern of T. gondii ROP5 determined in TgDogMxCam8. A novel ROP5 locus showing a recombinant 1&3 ROP5 RFLP pattern in one dog from Escárcega, Campeche. D8a: TgDogMxCam8a; D8b: TgDogMxCam8b. PCR-RFLP was performed on peritoneal lavage (*) and cell culture (^) samples. Virtual digestion was performed with the Benchling digestion tool (www.benchling.com). RH, Me49 and VEG are type I, II and III reference strains, respectively. MW: molecular weight marker of 50 bp.
Considering the combination of results obtained for the virulence markers, particularly for ROP18/ROP5 1/3, high virulence was predicted in the murine model for the 2 TgCkMxCam1 isolates (Table 2). All blood samples from the included individuals were negative for T. gondii DNA detection.
Discussion
In México, there are multiple reports regarding T. gondii frequency in wild and domestic species, including dogs, animals that are usually in close contact with humans and can serve as sentinels of its infection pressure. Chickens have also been used to demonstrate the presence of the parasite in a given region due to their ground feeding habit (Costa et al., Reference Costa, Barbosa, Prado, Domann and Rezende2021). However, to date, there have been few studies reporting the infection of this parasite in Campeche. Therefore, the objective was to identify and genetically characterize the parasite in tissues of stray dogs and free-range chickens living in Campeche.
The high frequency of IgG anti-T. gondii antibodies found in dogs (72.7% and 100% by ELISA and Western blot, respectively) and molecular detection results for T. gondii DNA reflect the high infective pressure in which the individuals from Campeche, as was reported a few years ago in the human population of this state (Caballero-Ortega et al., Reference Caballero-Ortega, Uribe-Salas, Conde-Glez, Cedillo-Pelaez, Vargas-Villavicencio, Luna-Pastén, Cañedo-Solares, Ortiz-Alegría and Correa2012). These results show the high infection pressure of T. gondii in urban and rural environments shared by different species, including humans. In this study, none of the tested animals were positive for the presence of T. gondii DNA in peripheral blood, in contrast to the results obtained in other southeastern states (Valenzuela-Moreno et al., Reference Valenzuela-Moreno, Rico-Torres, Cedillo-Peláez, Luna-Pastén, Méndez-Cruz, Lara-Martínez, Correa and Caballero-Ortega2019, Reference Valenzuela-Moreno, Rico-Torres, Cedillo-Peláez, Luna-Pastén, Méndez-Cruz, Reyes-García, Correa, Alves, Pena and Caballero-Ortega2020). This was probably due to the season of the year (November and December) in which the samples were collected in Quintana Roo and Chiapas, months when the average rainfall is higher, and the oocysts can be transported into and stagnate in puddles where stray animals can drink them (CONAGUA, 2023).
DNA of T. gondii was determined in some tissues of the captured animals, and genotyping was achieved (partially in some cases), including those from which the 4 isolates were obtained. Although few alleles could be typed in most of the clinical samples, triple infection (SAG3 alleles I + II + III) was detected in the spleen of dog #8, a result that is quite common in the southeastern region of México and has also been reported in cases of congenital toxoplasmosis in the central zone of the country (Rico-Torres et al., Reference Rico-Torres, Valenzuela-Moreno, Luna-Pastén, Figueroa-Damián, Gómez-Toscano, Hernández-Delgado, Escobedo-Torres and Correa2018; Valenzuela-Moreno et al., Reference Valenzuela-Moreno, Rico-Torres, Cedillo-Peláez, Luna-Pastén, Méndez-Cruz, Lara-Martínez, Correa and Caballero-Ortega2019, Reference Valenzuela-Moreno, Rico-Torres, Cedillo-Peláez, Luna-Pastén, Méndez-Cruz, Reyes-García, Correa, Alves, Pena and Caballero-Ortega2020). In tissues where T. gondii isolates were obtained, almost all genetic markers were genotyped, and the genetic profiles of the clinical sample and the isolate were correlated, suggesting that these tissues had a higher parasite load.
Six isolates of T. gondii had previously been obtained from chickens in the central region of México (Dubey et al., Reference Dubey, Morales and Lehmann2004). Four out of 6 of these isolates were the archetypal type III clone (ToxoDB #2). Several isolates have also been obtained from dogs, demonstrating 4 different genotypes (ToxoDB #8, #28, #73 and #74) (Dubey et al., Reference Dubey, Velmurugan, Alvarado-Esquivel, Alvarado-Esquivel, Rodríguez-Peña, Martínez-García, González-Herrera, Ferreira, Kwok and Su2009; Valenzuela-Moreno et al., Reference Valenzuela-Moreno, Rico-Torres, Cedillo-Peláez, Luna-Pastén, Méndez-Cruz, Reyes-García, Correa, Alves, Pena and Caballero-Ortega2020; Rico-Torres et al., Reference Rico-Torres, Valenzuela-Moreno, Luna-Pastén, Cedillo-Peláez, Correa, Morales-Salinas, Martínez-Maya, Alves, Pena and Caballero-Ortega2023). Similarly, we described the ToxoDB #116 genotype in 2 Bennett's wallabies (Macropus rufogriseus) from the centre plateau of México that had died due to acute disseminated toxoplasmosis. This genotype has also been reported in Argentina, Brazil, Peru and Venezuela (Dubey et al., Reference Dubey, Velmurugan, Chockalingam, Pena, de Oliveira, Leifer, Gennari, Oliveira and Su2008; Rajendran et al., Reference Rajendran, Su and Dubey2012; Andrade et al., Reference Andrade, Pinheiro, Cunha, Carneiro, Andrade Neto and Vitor2013; Shwab et al., Reference Shwab, Zhu, Majumdar, Pena, Gennari, Dubey and Su2014; Rêgo et al., Reference Rêgo, Costa, Baraviera, Pinto, Bessa, Lopes and Vitor2017; Bernstein et al., Reference Bernstein, Pardini, Moré, Unzaga, Su and Venturini2018; Valenzuela-Moreno et al., Reference Valenzuela-Moreno, Carmona-Muciño, Cedillo-Peláez, Rico-Torres, Luna-Pastén, Hernández-Rodríguez and Caballero-Ortega2022a). On the other hand, we discovered the ToxoDB #38 genotype in Campeche, which has only been found in Colombia, where it has been isolated from dogs, cats and chickens (Dubey et al., Reference Dubey, Su, Cortés, Sundar, Gomez-Marin, Polo, Zambrano, Mora, Lora, Jimenez, Kwok, Shen, Zhang, Nieto and Thulliez2006, Reference Dubey, Cortés-Vecino, Vargas-Duarte, Sundar, Velmurugan, Bandini, Polo, Zambrano, Mora, Kwok, Smith and Su2007; Rajendran et al., Reference Rajendran, Su and Dubey2012).
Considering all typed markers, there is a predominance of type I and III alleles (with the exception of ToxoDB #116, which has a type II allele at c22-8). Our results are in agreement with most of the isolates that have been obtained in the Mexican Neotropical region and with what has been reported from Central and South American countries with a predominance of strains related to genotypes I and III but few or no type II variants (Rajendran et al., Reference Rajendran, Su and Dubey2012; Valenzuela-Moreno et al., Reference Valenzuela-Moreno, Rico-Torres, Cedillo-Peláez, Luna-Pastén, Méndez-Cruz, Reyes-García, Correa, Alves, Pena and Caballero-Ortega2020, Reference Valenzuela-Moreno, Carmona-Muciño, Cedillo-Peláez, Rico-Torres, Luna-Pastén, Hernández-Rodríguez and Caballero-Ortega2022a; Rico-Torres et al., Reference Rico-Torres, Valenzuela-Moreno, Luna-Pastén, Cedillo-Peláez, Correa, Morales-Salinas, Martínez-Maya, Alves, Pena and Caballero-Ortega2023)
For the virulence markers, we identified three ROP18/ROP5 virulence profiles in 7 samples (3 tissue samples and 4 T. gondii isolates), 2 of which had predicted high virulence in a murine model (4/1 and 1/3) and one with unknown virulence due to the new ROP5 combination (3/1&3). The virulence profile of the strains from the heart of chicken #1, TgCkMxCam1a and TgCkMxCam1b coincides with the profile described for the other ToxoDB #38 isolates from Colombia (TgCtCo4, 10, 11 and TgDgCo17), a genotype that remains unaltered for the 5 PCR-RFLP virulence markers, despite the locations being over 2400 km away from each other and more than 17 years having elapsed since the profile was first described (Dubey et al., Reference Dubey, Su, Cortés, Sundar, Gomez-Marin, Polo, Zambrano, Mora, Lora, Jimenez, Kwok, Shen, Zhang, Nieto and Thulliez2006, Reference Dubey, Cortés-Vecino, Vargas-Duarte, Sundar, Velmurugan, Bandini, Polo, Zambrano, Mora, Kwok, Smith and Su2007). It would be interesting to perform microsatellite analysis to define how related these strains are.
The profile of the ToxoDB #116 strains described here is different from that previously reported in México because the latter has a ROP5 type 3 allele and the strains described here have the recombinant 1&3 allele. This ROP5 result is exceptional, not previously reported, and it could be due to a mixed infection of two strains carrying alleles 1 and 3, but the same result was obtained in the 2 isolates and the clinical samples from the dog #8. In addition, the RFLP results of the other 14 genotyping markers did not show evidence of mixed infection. The ROP5 locus is an unusual segment of the T. gondii genome formed by tandem paralogous genes: ROP5a, ROP5b and ROP5c. These paralogous genes have different repeats and arrays between the 3 archetypical strains (Reese et al., Reference Reese, Zeiner, Saeij, Boothroyd and Boyle2011). Therefore, it is possible that during recombination events in the intestine of a cat, intragenic recombination occurred at the ROP5 locus, and the dog #8 ingested a strain with the recombinant ROP5 1&3 locus. The ToxoDB #116 genotype carries a CS3 and ROP18 type III allele (avirulent in mice); however, the role of the recombinant 1&3 ROP5 allele in the virulence of T. gondii is still unknown (Pena et al., Reference Pena, Gennari, Dubey and Su2008). On the other hand, ToxoDB #38 isolates have a type I allele at the CS3 marker that has been associated with high virulence in the murine model and correlates with the prediction of ROP18/ROP5. Finally, we found the combination 4/1 ROP18/ROP5 again in México. This fact is highly significant because this pattern can cause up to 100% mortality in the murine model, and it has only been reported a couple of times previously, suggesting that some Mexican strains could pose a high risk to the human and nonhuman animal populations (Shwab et al., Reference Shwab, Jiang, Pena, Gennari, Dubey and Su2016; Rico-Torres et al., Reference Rico-Torres, Valenzuela-Moreno, Luna-Pastén, Cedillo-Peláez, Correa, Morales-Salinas, Martínez-Maya, Alves, Pena and Caballero-Ortega2023).
In conclusion, ToxoDB #38 and #116 genotypes were found in free-range chickens and stray dogs, respectively. The latter variant presents an atypical virulence genotype not previously reported and could be endemic to this region of México. Of the samples that could be genotyped for virulence markers, two profiles would be of high virulence, and one of low virulence. It will be crucial to determine their phenotypes to corroborate the virulence profiles of the T. gondii isolates.
Data availability statement
This section is only required if data is available elsewhere. Data Availability Statements are brief statements telling readers how they can access the data and other materials that would be necessary to replicate the findings of an article, in the interests of research transparency.
Acknowledgements
The authors thank Dr Chunlei Su for critically reviewing the manuscript for better interpretation of the virulence gene results. We are grateful to Dr Rafael Saavedra from the Instituto de Investigaciones Biomédicas-UNAM (México) for generously providing the RH T. gondii strain.
Author contributions
C.P.R.T., L.F.V.M., and H.C.O.: conception or design of this study. L.F.V.M. and H.C.O.: direction and supervision. C.P.R.T., L.F.V.M., and H.C.O.: data analysis and drafting the article. L.F.V.M., E.R.G., A.A.C.T., M.H.C., J.P.F., and F.G.L.: fieldwork, necropsies, and material acquisition. C.P.R.T., L.X.G., H.L.P., L.B.O.A., I.C.S. and C.C.P.: laboratory procedures performed. All the authors have read and approved the final manuscript.
Financial support
This work was partially supported by the Consejo Nacional de Humanidades, Ciencias y Tecnologías, CONHACyT, México (grant A1-S-21955) and by Programa E022, Investigación y Desarrollo Tecnológico en Salud (grant 2020/039) from Instituto Nacional de Pediatría.
Competing interests
None.
Ethical standards
This study was approved by the Institutional Review Board of the Instituto Nacional de Pediatría of the Ministry of Health of México (organization number IORG0011533), which includes the Research and Animal Care and Biosafety Committees (protocol number 2020/039).