The intestine is an important organ with nutritional, metabolic, immune, endocrine and barrier function. Intestinal barrier dysfunction is one of the main reasons for severe systemic infection such as sepsis and multiple organ dysfunction syndromes (MODS) in shock, burn and intensive care unit patients. The concept about the gut being the engine of MODS has been generally accepted, but the underlying mechanism is still uncertain and controversial(Reference Clark and Coopersmith1, Reference Leaphart and Tepas2). It was previously thought that in intestinal ischaemia–reperfusion (I/R) injury, normal intestinal bacteria are translocated to circulation due to intestinal barrier dysfunction. As a result, bacteria/endotoxin enter the hepatic portal venous system through the injured intestinal barrier and spread in the body, causing systemic inflammatory response syndrome, eventually leading to MODS(Reference Steinberg3–Reference Tsukamoto, Chanthaphavong and Pape5). Although some early experiments proved the association between bacterial translocation and MODS, the hypothesis has been questioned. More and more studies support the theory that the key mechanism of systemic inflammatory response and injury of distant organs caused by intestinal I/R may be due to the distribution of independent gut-derived factors into circulation through the ‘gut–lymph pathway’(Reference Senthil, Brown and Xu6–Reference Niu, Hou and Zhao9). Therefore, further research is necessary to clarify whether or not the bacteria/endotoxin are the only or major cause of MODS.
The high-mobility group protein box 1 (HMGB1), one of the Toll-like receptor (TLR) 4 endogenous ligands, is known as the key factor of inflammatory response caused by I/R injury(Reference Evankovich, Cho and Zhang10, Reference Xu, Yao and Su11), and binding with TLR4 is an important link in inflammatory response(Reference Levy, Mollen and Prince12, Reference Tsung, Klune and Zhang13). Breakthrough has been made in the research on microbe recognition of TLR and its products, but its role in non-infectious inflammation has not been cleared until recently, especially TLR4, which can be activated by many endogenous ligands to trigger inflammation. It has been recently discovered that in some systemic inflammatory response syndrome and distant organ damage induced by trauma or surgery, bacteria and endotoxin are not detected in the circulation, while TLR4 blockers are found to highly attenuate the inflammatory reaction and tissue damage. It is therefore possible that endogenous ligands, and not endotoxin, activate TLR4(Reference Marshall14, Reference Sodhi, Levy and Gill15). HMGB1 is released from necrotic or damaged cells, or secreted by activated immune cells and intestinal epithelial cells in intestinal I/R injury, reaching distant tissues via the intestinal lymph pathway and causing injury by activating TLR4(Reference Scaffidi, Misteli and Bianchi16, Reference Abreu, Fukata and Arditi17). More and more experiments prove that the activation of TLR4 by HMGB1 may be the most important trigger for the inflammatory response in I/R injury(Reference Kim, Lim and Yu18, Reference Liu, Mori and Takahashi19). Although the relationship between HMBG1 and TLR4 in I/R injury of other organs has been proved(Reference Shimamoto, Pohlman and Shomura20–Reference Kaczorowski, Nakao and Vallabhaneni22), it has seldom been reported in I/R injury of the intestine.
n-3 PUFA are a group of long-chain PUFA, mainly including EPA and DHA. n-3 PUFA can replace phospholipid arachidonic acid in cell membranes, inhibit cyclo-oxygenase and lipoxygenase, therefore reducing the inflammatory mediators derived from arachidonic acid, alleviating the inflammatory response, blocking the expression of adhesion molecules and increasing the synthesis of anti-inflammatory mediators. Recent studies have discovered that n-3 PUFA also inhibit the activation of TLR4-mediated signalling pathway and target gene expression(Reference Wong, Kwon and Choi23). The aim of this research was to study the role of TLR4 and HMGB1 in intestinal I/R in rats; and the impacts of intestinal lymph drainage and n-3 PUFA on systemic inflammatory response and distant organs.
Materials and methods
Animals
A total of forty-eight male specific pathogen-free Sprague–Dawley rats, weighing 280–320 g, were purchased from Beijing Vital River Laboratory Animal Technology Company, Limited (scxk 2007-0001). They were housed in a barrier system, kept at 25°C with 12 h light–12 h dark cycles. The rats had free access to water and chow for at least 5 d before the research. All rats were maintained in accordance with the recommendations of the guide for the care and use of laboratory animals. The research protocols were approved by Academic Committee, as well as Animal Care and Use Committee of PUMC & CAMS.
Gastrostomy and grouping
The forty-eight rats were randomly divided into three groups (n 16): normal diet (N), enteral nutrition (EN) and EN plus n-3 PUFA. Each group was further divided into lymph drainage (I/R+D) (n 8) and non-drainage (I/R) sub-groups (n 8). Before the gastrostomy, the rats were fasted for 12 h, but there was no limitation on water intake. The rats were anaesthetised by intraperitoneal injection of 1 % sodium pentobarbital (50 mg/kg). The silicone tube fixed into the stomach was drawn subcutaneously from the left abdomen to the opening at the back of the neck, and connected to the swivel rotary axis unit (Instech Laboratories, Inc.) through a protective spring. The clasp of the spring was sutured onto the skin and deep fascia of the back of the neck. The swivel unit was then connected to the syringe of a micro-infusion pump (B. Braun Company, Limited).
Nutrition schemes
Then, 2 h after the rats woke up following gastrostomy, EN was given to the EN and n-3 PUFA groups continuously for 5 d using a micro-infusion pump, started with half quantity in the first 12 h. The rats in the EN and n-3 PUFA groups received isonitrogenous (1·8 g N/kg per d) and isoenergetic intake (1046 kJ/kg per d). The EN group received the enteral nutritional emulsion (TP; Sino-Swed Pharmaceutical Corporation Limited). The n-3 PUFA group received the n-3 PUFA-rich enteral nutritional emulsion (TPF-T; Sino-Swed Pharmaceutical Corporation Limited, Table 1). The tube was washed with 1 ml normal saline every day when replacing the nutrient solution. The rats in the N group were given the normal diet. All rats had free access to water.
Table 1 Nutrition compositions in the enteral nutrition (EN) and n-3 PUFA groups (g/100 ml)*

* The 100 ml EN emulsion in the EN group added 2·0 g alanine and 8·0 ml 30 % Intralipid (Sino-Swed Pharmaceutical Corporation Limited), which reached the isonitrogenous and isoenergetic intake with the n-3 PUFA group.
Intestinal ischaemia–reperfusion and specimen collection
Intestinal ischaemia–reperfusion and lymphatic drainage
All surgical instruments, Nunc tubes (Nunc) for lymph collection, artery clamp and pipette tips were sterilised and ensured to be pyrogen-free in advance.
After 5 d with different nutrition schemes and 12 h fasting, the rats were anaesthetised again for intestinal I/R. Through an abdominal incision, the superior mesenteric artery was occluded for 60 min with artery clamp, followed by reperfusion of 120 min. In the rats in the I/R+D sub-groups, a small incision was made on the proximal end of the intestinal lymphatic trunk. A catheter (Jinan Medical Silicone Tube Plant) was inserted into the incision obliquely 3–5 mm towards the distal end. A small amount of medical adhesive (Beijing FuAiLe Science and Technology Development Company Limited) was smeared on the serosa adjacent to the right kidney to fix the catheter. Outflow of lymph from the catheter was collected in a sterile test-tube (Nunc) for 180 min.
Collection of specimens
After the operation, blood samples (5–6 ml) were collected from the inferior vena cava. The whole blood was allowed to stand at room temperature for 30 min and centrifuged at 4°C for 15 min (3000 rpm). The separated serum was frozen at − 80°C. The collected lymph was centrifuged at 4°C for 15 min (12 000 rpm), and the supernatant stored in a sterile Eppendorf tube (Eppendorf Company, Limited) at − 80°C.
After the rats were killed, a 3 cm proximal section of the jejunum and a 3 cm distal section of the ileum were cut out, rinsed in ice normal saline, dried on filter paper, and the liver and lung were put into the freezing tube and stored in a refrigerator at − 80°C.
Measurements
Cytokines, high-mobility group box 1, endotoxin and alanine aminotransferase in serum and lymph
The serum and lymph concentrations of TNF-α, IL-6, IL-1β and HMGB1 were determined using ELISA kits (Sun Biomedical Technology Company Limited).
A chromogenic limulus assay kit (Yi Hua Medical Technology Company Limited) was used for the quantitative detection of serum and lymph endotoxin.
Alanine aminotransferase (ALT) was detected with an Olympus automatic biochemical analyser (Olympus Corporation).
Immunohistochemical staining of high-mobility group box 1 in the jejunum and ileum
The jejunum and ileum of the rats were dipped in 50 ml of 10 % formalin. The fixed jejunum and ileum specimens were embedded in paraffin and used for histological examination. A mouse anti-HMGB1 primary antibody (Beijing Biosynthesis Biotechnology Company Limited) and biotinylated secondary antibody were used for immunohistochemical staining. Brownish-yellow parts were recognised as the sites with positive antigen expression.
High-mobility group box 1 protein expression in the jejunum, ileum, liver and lung (Western blot)
Total protein extract was prepared and separated using SDS polyacrylamide gels. Proteins were then transferred to nitrocellulose membranes overnight at room temperature and blocked for 8 h with 5 % bovine serum albumin. Following this, the membranes were incubated overnight in anti-HMGB1 primary antibody diluted in blocking solution (1:500; Beijing Biosynthesis Biotechnology Company Limited). The membranes were washed in Tris-buffered saline with Tween and incubated in horseradish peroxidase-conjugated mouse secondary antibodies in 5 % milk (1:3000; Santa Cruz, Inc.) for 1 h at room temperature. Protein bands were visualised by chemiluminescence.
Toll-like receptor 4 mRNA expression in the jejunum, ileum and liver (quantitative real-time PCR)
Total RNA was isolated from the liver, jejunum and ileum with TRIzol reagent (Invitrogen Corporation), followed by DNase digestion and repurification as recommended by the manufacturer. Complementary DNA was prepared from 5 μg of RNA using a Reverse Transcription System (Applied Biosystems) and subjected to quantitative real-time PCR analysis with TLR4 gene-specific primer. Real-time PCR data were processed using the 2− △△Ct method as described(Reference Livak and Schmittgen24).
NF-κB DNA-binding activity in the liver (electrophoretic mobility shift assay)
Nuclear proteins were isolated from the liver sample using the method as described(Reference Hua, Ha and Ma25). NF-κB binding activity was examined by electrophoretic mobility shift assay in a 15 μl binding reaction mixture containing 15 μg of nuclear proteins. The concentration of NF-κB oligonucleotide probe (5′-AGTTGAGGGGACTTTCCCAGGC-3′) was 500 fmol/μl. The activity of NF-κB was demonstrated by relative density on autoradiographic images.
Determination of myeloperoxidase, nitric oxide, total nitric oxide synthetase and inducible nitric oxide synthetase in the lung
Two pieces of lung tissues (weighing 100 and 200 mg, respectively) were mixed with the homogenate medium at the weight:volume ratio of 1:19 and 1:9. The myeloperoxidase (MPO), NO, total NO synthetase (tNOS) and inducible NO synthetase (iNOS) in the supernatant were determined using a test kit (Nanjing Jiancheng Bioengineering Institute).
Statistical analysis
Quantitative data were presented as mean values and standard deviations. Statistical software SPSS 17.0 was applied to test the homogeneity of variance. Multiple comparisons were performed with one-way ANOVA followed by the least-significant difference test. P < 0·05 was considered statistically significant.
Results
Inflammatory cytokines, high-mobility group box 1 and endotoxin in the lymph
The results of three lymph drainage sub-groups showed that the HMGB1, IL-6, TNF-α and endotoxin in the lymph in the n-3 PUFA (I/R+D) group were significantly reduced compared with the N (I/R+D) group (P < 0·05); HMGB1 and TNF-α in the n-3 PUFA (I/R+D) group were also lower than in the EN (I/R+D) group (P < 0·05). IL-6 in the EN (I/R+D) group was lower than that in the N (I/R+D) group (P < 0·05; Table 2).
Table 2 Concentrations of inflammatory cytokine, high-mobility group box 1 (HMGB1) and endotoxin in lymph
(Mean values and standard deviations, n 8)
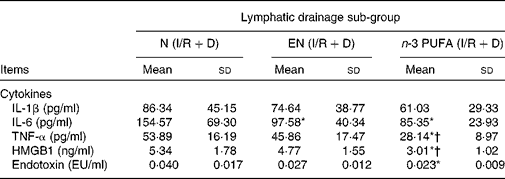
N, normal diet; EN, enteral nutrition; I/R, ischaemia–reperfusion; D, lymph drainage; EU, endotoxin units.
* Mean values were significantly different from those of the N (I/R+D) group (P < 0·05).
† Mean values were significantly different from those of the EN (I/R+D) group (P < 0·05).
Inflammatory cytokines, high-mobility group box 1, endotoxin and alanine aminotransferase in the serum
The serum levels of IL-1β, IL-6 and HMGB1 in the N (I/R+D) group were decreased significantly compared with the N (I/R) group (all P < 0·05); HMGB1 in the EN (I/R+D) group was lower than in the EN (I/R) group (P < 0·05). HMGB1 and TNF-α in the n-3 PUFA (I/R+D) group were reduced compared with the N (I/R+D) group (both P < 0·05). Also, HMGB1 and endotoxin were reduced in the n-3 PUFA (I/R+D) group compared with the n-3 PUFA (I/R) group (both P < 0·05; Table 3).
Table 3 Concentrations of inflammatory cytokines, high-mobility group box 1 (HMGB1) and endotoxin in serum
(Mean values and standard deviations, n 8)

N, normal diet; EN, enteral nutrition; I/R, ischaemia–reperfusion; D, lymph drainage; EU, endotoxin units; ALT, alanine aminotransferase.
* Mean values were significantly different from those of the N (I/R) group (P < 0·05).
† Mean values were significantly different from those of the N (I/R+D) group (P < 0·05).
‡ Mean values were significantly different from those of the EN (I/R) group (P < 0·05).
§ Mean values were significantly different from those of the n-3 PUFA (I/R) group (P < 0·05).
IL-1β, IL-6, HMGB1, TNF-α and endotoxin in the n-3 PUFA (I/R) group were significantly lower than in the N (I/R) group (all P < 0·05); endotoxin in the n-3 PUFA (I/R) group was also lower than in the EN (I/R) group (P < 0·05). HMGB1 was reduced in the EN (I/R) group compared with the N (I/R) group (P < 0·05; Table 3).
ALT in the n-3 PUFA and EN (I/R+D) groups were significantly lower than in the N (I/R) group (all P < 0·05). ALT in the EN (I/R) and N (I/R+D) groups were decreased compared with the N (I/R) group, but with no significant difference (Table 3).
Immunohistochemical staining of high-mobility group box 1
We observed that the mucosa of the jejunum and ileum were swollen, atrophic, erosive and even ruptured. Plenty of yellow staining was observed at the top of villi and the basement membrane in all groups. The injuries in three I/R+D groups were less severe, demonstrated as light swelling of the mucosa, a little exudate, less ulceration and no rupture; yet immunohistochemical staining showed that yellow staining was also noticed at the top of microvilli. Both HMGB1 staining and injury of the jejunum and ileum in the n-3 PUFA group were lighter compared with the other two groups (Fig. 1).

Fig. 1 Immunohistochemical staining of high-mobility group box 1 (HMGB1) in the (a) jejunum and (b) ileum ( × 200) (n 8). Immunohistochemical staining showed that there is a lot of yellow staining after ischaemia–reperfusion (I/R) injury. The injuries in three I/R+lymph drainage (D) groups were less severe, but yellow staining was also noticed at the top of microvilli. Both HMGB1 staining and injury of the jejunum and ileum in the n-3 PUFA group were lighter compared with the other two groups. N, normal diet; EN, enteral nutrition.
High-mobility group box 1 protein expression in the jejunum, ileum, lung and liver
High-mobility group box 1 protein expression in the jejunum and ileum
With intestinal I/R injury it was found that lymph drainage reduced the HMGB1 expression significantly in the jejunum and ileum as demonstrated by the results of Western blot (P < 0·05). In addition, the expression of HMGB1 in the n-3 PUFA group was lower than that in the other two groups (P < 0·05; Fig. 2).

Fig. 2 High-mobility group box 1 (HMGB1) protein expression in the (a) jejunum and (b) ileum (n 8). 1, Normal diet (N, ischaemia–reperfusion (I/R)); 2, enteral nutrition (EN, I/R); 3, n-3 PUFA (I/R); 4, N (I/R+lymph drainage (D)); 5, EN (I/R+D); 6, n-3 PUFA (I/R+D). The HMGB1/β actin grey scale value indicated that the lymph drainage reduced the HMGB1 expression significantly in the (c) jejunum and (d) ileum. * Mean values were significantly different from those of the N (I/R) group (P < 0·05). † Mean values were significantly different from those of the EN (I/R) group (P < 0·05). The expression of HMGB1 in the n-3 PUFA group was lower than that in the other two groups. ‡ Mean values were significantly different from those of the n-3 PUFA (I/R) group (P < 0·05).
High-mobility group box 1 protein expression in the lung and liver
The expression of HMGB1 in the liver and lung was increased after intestinal I/R, while lymph drainage reduced HMGB1 protein expression. The levels of HMGB1 in all the drainage groups were significantly lower than those in the corresponding non-drainage groups (all P < 0·05); HMGB1 in the lung of the n-3 PUFA (I/R) group was also significantly decreased compared with the N (I/R) group (P < 0·05; Fig. 3).

Fig. 3 High-mobility group box 1 (HMGB1) protein expression in the (a) lung and (b) liver (n 8). 1, normal diet (N, ischaemia–reperfusion (I/R)); 2, enteral nutrition (EN, I/R); 3, n-3 PUFA (I/R); 4, N (I/R+lymph drainage (D)); 5, EN (I/R+D); 6, n-3 PUFA (I/R+D). The grey scale value indicated that lymph drainage reduced HMGB1 protein expression in the (c) lung and (d) liver. The levels of HMGB1 in all the drainage groups were significantly lower than those of the corresponding non-drainage groups. * Mean values were significantly different from those of the N (I/R) group (P < 0·05). † Mean values were significantly different from those of the EN (I/R) group (P < 0·05). ‡Mean values were significantly different from those of the n-3 PUFA (I/R) group (P < 0·05). HMGB1 in the lung of the n-3 PUFA (I/R) group was also significantly decreased compared with the N (I/R) group.
Toll-like receptor 4 mRNA expression in the jejunum, ileum and liver
The levels of TLR4 mRNA expression in the jejunum in all lymph drainage groups were significantly lower than that in the N (I/R) group (all P < 0·05). The three drainage groups also demonstrated significantly lower TLR4 mRNA expression in the ileum compared with the N (I/R) group (all P < 0·05). TLR4 mRNA expression levels in both the jejunum and ileum in the EN (I/R+D) and n-3 PUFA (I/R+D) groups were decreased compared with the EN (I/R) group (all P < 0·05). The levels of TLR4 mRNA expression in the liver of drainage groups were significantly decreased compared with the corresponding non-drainage groups (all P < 0·05; Table 4).
Table 4 Toll like receptor 4 mRNA expression in the jejunum, ileum and liver (2−△△Ct)
(Mean values and standard deviations, n 8)

N, normal diet; EN, enteral nutrition; I/R, ischaemia–reperfusion; D, lymph drainage.
* Mean values were significantly different from those of the N (I/R) group (P < 0·05).
† Mean values were significantly different from those of the EN (I/R) group (P < 0·05).
‡ Mean values were significantly different from those of the n-3 PUFA (I/R) group (P < 0·05).
NF-κB activity in the liver
NF-κB activity in the liver of drainage groups was significantly lower than those in the corresponding non-drainage groups (all P < 0·05), consistent with the results of the expression of HMGB1, which is the endogenous ligand of TLR4. NF-κB activity in the n-3 PUFA group was also significantly decreased compared with the N (I/R) and EN (I/R) groups (P < 0·05) and the activity in the n-3 PUFA (I/R+D) group was significantly lower than that in the EN (I/R+D) group (P < 0·05; Fig. 4).

Fig. 4 (a) NF-κB activity in the liver (n 8). 1, Normal diet (N, ischaemia–reperfusion (I/R)); 2, N (I/R+D); 3, enteral nutrition (EN, I/R); 4, EN (I/R+ lymph drainage (D)); 5, n-3 PUFA (I/R); 6, n-3 PUFA (I/R+D); 7, negative control. (b) NF-κB ΔΦ grey scale value demonstrated that the NF-κB activity in the liver of the drainage groups was significantly lower than that of the corresponding non-drainage groups. * Mean value was significantly lower than that of the N (I/R) group (group 1; P < 0.05). † Mean value was significantly lower than that of the EN (I/R) group (group 3; P < 0.05). ‡ Mean value was significantly lower than that of the n-3 PUFA (I/R) group (group 5; P < 0.05). § Mean value was significantly lower than that of the EN (I/R+D) group (group 4; P < 0.05).
Myeloperoxidase, nitric oxide, total nitric oxide synthetase and inducible nitric oxide synthetase in the lung
MPO in the N (I/R+D) group was significantly decreased compared with the N (I/R) group (P < 0·05). The level of tNOS in the n-3 PUFA (I/R+D) group was significantly lower than that in the n-3 PUFA (I/R) group, and lower in the EN (I/R+D) group than that in the EN (I/R) group (both P < 0·05). The tNOS level in the n-3 PUFA (I/R) group was significantly lower than that in the N (I/R) group (P < 0·05). The levels of iNOS in the EN (I/R+D) and n-3 PUFA (I/R+D) groups were significantly reduced compared with the N (I/R) group (both P < 0·05). NO in the n-3 PUFA (I/R+D) group was decreased compared with the N (I/R+D) group (P < 0·05; Table 5).
Table 5 The concentrations of myeloperoxidase (MPO), nitric oxide, total nitric oxide synthetase (tNOS) and inducible nitric oxide synthetase (iNOS) in the lung
(Mean values and standard deviations, n 8)
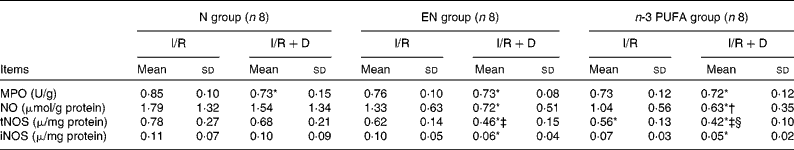
N, normal diet; EN, enteral nutrition; I/R, ischaemia–reperfusion; D, lymph drainage.
* Mean values were significantly different from those of the N (I/R) group (P < 0·05).
† Mean values were significantly different from those of the N (I/R+D) group (P < 0·05).
‡ Mean values were significantly different from those of the EN (I/R) group (P < 0·05).
§ Mean values were significantly different from those of the n-3 PUFA (I/R) group (P < 0·05).
Discussion
In the present study, we found that after the intestinal I/R injury, the results of HMGB1 immunohistochemistry showed that the injury of the I/R groups was more severe than that of the I/R+D groups. A large number of cells were stained in the I/R groups, which indicated that the expression of HMGB1 was increased. The intestinal lymph drainage could significantly reduce the serum levels of HMGB1, IL-1β, IL-6, TNF-α and ALT. It was also found that the intestinal lymph drainage could reduce the concentrations of MPO, NO, tNOS and iNOS in the lung. The expressions of TLR4 mRNA and HMGB1 in the gut and distant organs in drainage groups were decreased compared with non-drainage groups. The activity of the downstream NF-κB in drainage groups was also attenuated than that in non-drainage groups. The results of this study are consistent with our previous finding. Our previous studies showed that 60 min ischaemia followed by 120 min reperfusion plus lymphatic ligation in rats could cause intestinal barrier damage, intestinal permeability increase and bacteria/toxin translocation(Reference Dong, He and Cui26, Reference Cui, He and Dong27). Although the ligation of intestinal lymphatic trunk did not show a significant protective effect against intestinal morphologic changes, the bacteria detection rate in the ligation group was lower than that in the non-ligation group. In addition, lymphatic trunk ligation also reduced the serum levels of endotoxin, d-lactic acid and diamine oxidase obviously in intestinal I/R injury(Reference Cui, He and Dong27, Reference He, Dong and Cui28). The concentrations of MPO, NO and NOS in the lung tissue in I/R rats rose significantly, but the lung damage in the intestinal lymphatic trunk ligation group was obviously less severe compared with the non-ligation group, with the average number of alveolar epithelium apoptosis significantly lower in the ligation group(Reference He, Dong and Chen29).
Deitch et al. (Reference Deitch, Xu and Kaise7, Reference Deitch8) demonstrated that the intestinal lymph is a critical link between trauma haemorrhagic shock and MODS, and that bacteria and toxin may be translocated by lymph to cause lung injury. In the study by Cavriani et al. (Reference Cavriani, Domingos and Oliveira-Filho30), lymphatic ligation was observed to change the serum levels of IL-1β and IL-10, alleviating the inflammatory response and lung injury. Another research also revealed that intestinal lymph drainage in haemorrhagic shock could prevent lung injury and the activation of neutrophils(Reference Zallen, Moore and Johnson31). This study further supports the other researchers’ results and indicates that both lymph drainage and lymphatic ligation can block the ‘gut–lymph pathway’ to reduce the injury.
The combination of both the TLR4 and the participation of the endogenous ligand plays an important role in tissue injury and the innate immune response in I/R. The cells release a variety of endogenous ligands in the I/R early. The TLR4 recognises and binds the endogenous ligands (such as HMGB1), which may be the most important trigger of inflammatory response in I/R injury. Then the NF-κB signalling pathways are activated and certain proteins and enzymes and multiple cytokines are synthesised, leading to cascade reaction and tissue and organ damage(Reference Chen, He and Dong32, Reference Akira and Takeda33), therefore blocking the combination of TLR4 and its ligand may have a protective effect in I/R injury. Izuishi et al. (Reference Izuishi, Tsung and Jeyabalan34) and Tsung et al. (Reference Tsung, Sahai and Tanaka35) demonstrated that pretreatment of mice with HMGB1 significantly decreased the liver damage after I/R. HMGB1 preconditioning failed to provide the protection in TLR4 mutant (C3H/HeJ) mice, but successfully reduced the damage in TLR4 wild-type (C3H/HeOuj) mice. Liu et al. (Reference Liu, Mori and Takahashi19) showed that treatment with neutralising anti-HMGB1 monoclonal antibody ameliorated brain infarction induced by transient ischaemia in rats. These results indicate that HMGB1 plays a critical role in the I/R injury through the amplification of plural inflammatory responses. TLR4 mutations or neutralising antibodies of endogenous ligand (e.g. HMGB1) can significantly reduce inflammation and organ damage. In this study, intestinal lymph drainage can decrease the expressions of TLR4 mRNA and HMGB1 and inflammatory responses in I/R. For an understanding of this mechanism, there could be an outstandingly suitable target for future clinical treatment that provides a novel therapeutic strategy for I/R injury.
Our study demonstrated that n-3 PUFA could mitigate organ injury induced by intestinal I/R, as the expression of HMGB1 in the n-3 PUFA group decreased compared with the N and EN groups. The lymph and serum levels of inflammatory cytokines, HMGB1 and TLR4 mRNA in the n-3 PUFA group were significantly lower than those in the other two groups, indicating that n-3 PUFA could inhibit the HMGB1 and TLR4 signalling pathway and the release of downstream inflammatory factors in intestinal I/R injury. These results are consistent with other researchers’ findings. James et al. (Reference Lee, Bhora and Sun36) indicated that dietary flaxseed, a nutritional whole grain with a high content of n-3 fatty acids, has anti-inflammatory and antioxidant properties in a murine model of pulmonary I/R injury. Mice fed a n-3 fatty acids-supplemented diet had a significant improvement in both ****Pa (O2) and bronchoalveolar lavage protein and had less reactive oxygen species release from the vascular endothelium in lungs. Kielar et al. (Reference Kielar, Jeyarajah and Zhou37) demonstrated that renal ischaemic injury increased mRNA abundance for TNF-α and iNOS at 24 h. This increase was prevented by DHA administration. Deckelbaum et al. (Reference Deckelbaum, Worgall and Seo38) indicated that n-3 PUFA have distinct and important bioactive properties and reduce many risk factors associated with several diseases, such as CVD, diabetes and cancer. The mechanisms as to how n-3 PUFA affects gene expression are complex and involve multiple processes. For example, n-3 PUFAs regulate two groups of transcription factors, such as sterol-regulatory-element binding proteins and peroxisome proliferator-activated receptors.
It has been recently discovered that the anti-inflammatory effect of n-3 PUFA may be related to the inhibition of the signalling pathway and target gene expression activated by TLR4, suggesting a novel molecular mechanism of the anti-inflammatory effect of n-3 PUFA(Reference Musiek, Brooks and Joo39–Reference Adkins and Kelley41). A clear understanding of the mechanism of n-3 PUFA in the TLR4-mediated inflammatory response will provide an important theoretical basis for clinical application of n-3 PUFA.
In conclusion, we observed in this study the intestinal morphological changes in intestinal I/R injury. The nutrition with n-3 PUFA and the intestinal lymph drainage may both reduce inflammatory cytokine, endotoxin and HMGB1 in serum and lymph, and inhibit the expression and signal transmission of TLR4 mRNA, thereby alleviating intestinal I/R injury in rats. These data further support the important role of the ‘gut–lymph pathway’ in the transportation of gut-derived factors, absorption and translocation of bacteria/endotoxin in the development from intestinal I/R injury to MODS. This study demonstrates that n-3 PUFA and/or the ‘gut–lymph pathway’ blockade could be considered as the way to the treatment of diseases induced by intestinal I/R injury.
Acknowledgements
The contributions of the authors to the present study were as follows: G.-Z. H. designed the research and had primary responsibility for the final content; K.-G. Z. conducted the research and wrote the paper; R. Z. performed the animal experiment and research; X.-F. C. performed the animal experiment and analysed the data. All authors read and approved the final manuscript. This study was funded by National Natural Science Foundation of China (30940069) and the Natural Sciences Foundation of Beijing (7102127). The authors sincerely thank Mr. Dong Zhang, B.S., for his assistance with animal care and De-Tian Wang, technician, for help with the pathological section. The authors also express their thanks to Yun-Fei Xu, MMC and Xi-Zeng Cui, MMC. The authors declare that there are no conflicts of interest.










