Human breast milk is known as a rich source of antimicrobial substances, which can compensate for the naive state of adaptive immunity in suckling infants. These antimicrobial compounds have been proved to decrease the morbidity and mortality of breast-feeding infants( Reference Field 1 , Reference Murakami, Dorschner and Stern 2 ).
As a group of antimicrobial molecules, defensins are important components of the human innate immune system. Defensins are small, cationic, acid-stable, amphipathic peptides that contain eighteen to forty-five amino acid residues. All defensins have three pairs of conserved disulphide bonds between cysteine residues. Their three-dimensional structure possesses the same amphiphilic β-sheet structure, which plays an important role in antimicrobial potency. They have direct antimicrobial activity against a wide range of micro-organisms: Gram-positive and Gram-negative bacteria, viruses, fungi and some parasites in vitro ( Reference Seebah, Suresh and Zhuo 3 – Reference Schwaab, Hanseu and Pearson 5 ). Recently, increasing evidence has suggested that they interact with and modify adaptive immunity by attracting immature dendritic cells and monocytes, and act as immuoadjuvants( Reference Bowdish, Davidson and Hancock 6 – Reference Hazlett and Wu 8 ).
Human defensins are divided into two main classes, α-and β-defensins, according to their disulphide bond pairing pattern( Reference Pazgier, Hoover and Yang 9 ). Bensch et al. ( Reference Bensch, Raida and Mägert 10 ) first isolated and purified human β-defensin-1 (hBD-1) from the human blood filtrate in 1995. hBD-2 was first discovered and isolated from psoriatic scale extracts in 1997( Reference Harder, Bartels and Christophers 11 ). hBD-1 is abundantly expressed in epithelial cells and has been detected in the respiratory tract, kidneys, female reproductive tract and other tissues, whereas hBD-2 is widely expressed in the skin, lungs, trachea and salivary and urogenital glands( Reference Huttner and Bevins 12 ).
It has been reported that human mammary gland epithelia secrete β-defensins( Reference Tunzi, Harper and Bar-Oz 13 ). However, information about defensins in the breast milk of Chinese women is lacking. Also, factors that associate with defensin levels remain to be determined.
In the present study, we examined the concentrations of hBD-1 and hBD-2 in the human milk of healthy Han Chinese, and investigated the sociodemographic and clinical factors influencing hBD-1 and hBD-2 expression. We also assessed the potential protective effect of milk hBD-1 and hBD-2 on infants during the first 6 months of life.
Materials and methods
Subjects and procedures
From March to August 2012, 100 mother–infant pairs were recruited from the Department of Obstetrics, Shanghai First People's Hospital. Inclusion criteria were as follows: women with normal pregnancy, without any chronic diseases before pregnancy or any pregnancy complications during the gestation and lactation periods; babies delivered vaginally or with caesarean section without any complications. Exclusion criteria included acute infection, chronic infectious disease, autoimmune disease, immune deficiency, neuropsychiatric disease, malignant tumour, blood disease, heart disease, pre-eclampsia, breast problems and in vitro fertilisation. Exclusion criteria are based on clinical history in conjunction with laboratory data including blood tests, electrocardiogram and ultrasonography. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Ethics Committee of Xinhua Hospital affiliated to the Shanghai Jiao Tong University School of Medicine. Written informed consent was obtained from all subjects.
At the time of enrolment, a questionnaire was used to acquire information about mother and neonate characteristics, including the potential factors that might influence defensin levels. These mainly concerned sociodemographic characteristics (age of mother, place of residence, education level and smoking history) and clinical characteristics (mode of delivery, gestational age, reproductive history, allergy history and birth weight of infant). Data of BMI and serum total protein levels were also collected to assess the nutritious status of the mother.
Infants were followed up for a period of 6 months. On day 42 after birth, participants returned for the first follow-up visit. At the end of 3rd month and 6th month, an interview was conducted via telephone. During each follow-up visit, parents were asked about breast-feeding practices, the timing of solid food introduction, symptoms of respiratory and diarrhoea, and possible cause of these diseases. The diagnostic criteria of upper respiratory infection (URI)( Reference Hui, Li and Zeng 14 ) and diarrhoea( Reference Ye and Nie 15 ) were mainly based on symptoms. URI was diagnosed by a paediatrician if the infant had the following symptoms: sneezing, runny nose, nasal congestion, cough and fever, but breathing was within the normal range. Diarrhoea was diagnosed by a paediatrician according to the changes in stool consistency (watery stool, mushy stools or mucous pus) and stool frequency (more than usual). An episode was considered to be terminated on the last day of URI or diarrhoea followed by a 72 h symptom-free period. The outcome measures were incidence of URI and diarrhoea. The number of new episodes of URI or diarrhoea occurring during the follow-up was used as the numerator for incidence rate.
Sample collection and storage
Colostrum (1–3 ml) was obtained within 5 d postpartum, and mature milk (10–15 ml) was obtained on day 42 postpartum. All breast milk samples were collected by hand expression, as described previously( Reference Molinari, Casadio and Hartmann 16 ). The samples were centrifuged at 3000 g for 10 min at 4°C. The upper fat layer was discarded. The whey was stored at − 80°C until analysis. The cell pellet left at the bottom of the tube was transferred to a sterile 1·5 ml tube. TRIzol® reagent (Invitrogen) was added to the cell pellet immediately and stored at − 80°C until extraction.
Maternal blood was drawn in the morning shortly before the colostrum was collected. The blood sample (2 ml) was collected into a plain evacuated glass tube and clotted for 2 h at room temperature. Then, the samples were centrifuged at 1000 g for 20 min. Serum was divided into 200 μl aliquots and stored at − 80°C until analysis.
Measurement of milk human β-defensin-1/human β-defensin-2 and serum cortisol by ELISA
Breast milk concentrations of hBD-1 and hBD-2 were measured using commercially available ELISA kits (USCN Life Sciences). The kits are sandwich enzyme immunoassays for in vitro quantitative measurement. Serum cortisol levels were measured by a competitive ELISA kit (USCN Life Sciences). Standards, blank and samples were added into the appropriate wells according to the instruction. All wells were in a final volume of 100 μl/well in duplicate. Then, the plates were incubated and washed. Absorbance readings (450 nm) were taken using a microplate reader (Bio-Rad Laboratories). The amounts of targets in the samples were calculated with reference to the standard curve obtained with the known concentrations of standards. Processing was identical for all the samples, and concentrations were measured with clinical identification blinded.
Measurement of human β-defensin-1 and human β-defensin-2 mRNA by quantitative real-time PCR
The procedure for the extraction and purification of RNA from milk was carried out according to the methods published in the literature( Reference Gambichler, Skrygan and Tomi 17 , Reference Vordenbäumen, Pilic and Otte 18 ). Total RNA was extracted from the cell pellet of breast milk using TRIzol® reagent, according to the manufacturer's instructions. cDNA was synthesised using the PrimeScript® RT Master Mix (Perfect Real Time; Takara). Real-time PCR was performed using SYBR® Premix Ex TaqTM II (Takara) in the ABI PRISM® 7500 Real-Time PCR system (Applied Biosystems). The thermal cycler profile was 95°C for 30 s, and forty cycles of amplification with denaturation at 95°C for 5 s and a combined annealing and extension step at 58°C for 34 s. For the melting curve analysis, the profile was completed with warming to 95°C for 15 s, 58°C for 1 min and 95°C for 15 s. For the investigated transcripts, three biological replicates were performed. The reference gene glyceraldehyde 3-phosphate dehydrogenase was used as the internal control. Results were analysed using the comparative C T method. Primers were designed by Primer Express Software version 3.0. Primer sequences were as follows: hBD-1 – forward GTTCCTGAAATCCTGGGTGTTG and reverse GGCAGGCAGAATAGAGACATTG; hBD-2 – forward GCGATCCTGTTACCTGCCTTA and reverse TGAATCCGCATCAGCCACA; glyceraldehyde 3-phosphate dehydrogenase – forward GAAGGTGAAGGTCGGAGTC and reverse GAAGATGGTGATGGGATTTC. The expected product size was 182 bp for hBD-1, 155 bp for hBD-2 and 226 bp for glyceraldehyde 3-phosphate dehydrogenase.
Statistical analysis
After testing for normality using the Kolmogorov–Smirnov test, values are presented as medians and interquartile ranges (IQR) for binary data, or means with their standard errors for normally distributed data. Differences between two groups were assessed by the Mann–Whitney U test or t test. Differences in rate between groups were compared using the χ2 test. The relationships between two variables were determined by Spearman's correlation analysis. Based on the results of the Mann–Whitney U test and Spearman's correlation analysis, we introduced variables that may be significantly associated with dependent variables into the multiple linear regression models. The significance levels quoted were two-sided, and P values less than 0·05 were considered to be statistically significant. Statistical analysis was conducted using SPSS 17.0 for Windows (SPSS, Inc.).
Results
Characteristics of the participants
The sociodemographic and clinical characteristics of all the participants are shown in Table 1. A total of 100 mothers and their babies were screened for eligibility. The mothers were in good health throughout pregnancy and received no medication during pregnancy or lactation. Their mean age was 28·96 (se 0·40) years. All of them were of the Han race. In total, eighty mothers were primiparae. Of these, thirty-eight mothers had vaginal delivery and sixty-two underwent caesarean delivery. The mean serum cortisol levels of mothers were 1·46 (se 0·03) pg/ml. Serum cortisol levels were higher in the vaginal delivery group than in the caesarean section group (P= 0·015). Mean serum total protein levels were 58·85 (se 0·50) g/l.
Table 1 Characteristics of mothers and neonates (Mean values with their standard errors; number of participants)

All newborns established breathing rapidly after birth and had no diseases at the time of enrolment. Of these, sixty-six were boys and thirty-four were girls. Babies born at < 37 weeks' estimated gestational age were considered as premature (n 12) and those born at ≥ 37 weeks' estimated gestational age were considered to be full term (n 88). The mean birth weight of the babies was 3237·65 g. Overall, eighty-two (82 %) babies completed the 6-month follow-up evaluation.
Human β-defensin-1 and human β-defensin-2 concentrations and mRNA expression in human milk
Both hBD-1 and hBD-2 were found in all human milk samples. The concentration ranges of hBD-1 and hBD-2 in the colostrum were found to be 1·04–12·81 μg/ml and 0·31–19·12 ng/ml, respectively. In mature milk, the concentration ranges were 1·03–31·76 ng/ml for hBD-1 and 52·65–182·29 pg/ml for hBD-2. hBD-1 concentration was significantly higher than hBD-2 concentration both in the colostrum (P< 0·001) and in mature milk (P< 0·001).
In the colostrum, hBD-1 levels (median 1·43 (IQR 1·20–2·34) μg/ml) were significantly higher than those in mature milk (median 3·54 (IQR 1·82–8·15) ng/ml) (P< 0·001; Fig. 1). The levels of hBD-2 in the colostrum (median 1·06 (IQR 0·58–1·92) ng/ml) were significantly higher than those in mature milk (median 66·92 (IQR 60·47–86·72) pg/ml) (P< 0·001; Fig. 1).

Fig. 1 (a) Human β-defensin-1 (hBD-1) and (b) human β-defensin-2 (hBD-2) levels in the human colostrum (n 100) and mature milk (n 82). Values are medians. * Median values were significantly higher than those of mature milk (P< 0·05; Mann–Whitney U test).
The expression of mRNA for hBD-1 and hBD-2 was examined in the RNA samples obtained from the colostrum (n 88) and mature milk (n 76). Some of the mRNA transcripts were failed to be detected due to the limited amount of the cell pellet in the milk samples. The hBD-1 mRNA expression was higher than hBD-2 mRNA in both the colostrum (P< 0·001) and mature milk samples (P< 0·001) (Fig. 2).

Fig. 2 mRNA levels of human β-defensin-1 (hBD-1) and human β-defensin-2 (hBD-2) in the colostrum (n 88) and mature milk (n 76). The relative amounts of hBD-1/hBD-2 mRNA were estimated with the 2− ΔC T values, where 2− ΔC T = 2− (C T gene of interest − C T GAPDH). The reference gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the internal control. Magnification 10 000 × . Values are medians. * Median values were significantly higher than those of hBD-2 mRNA (P< 0·05; Mann–Whitney U test).
Relationship between independent variables and human β-defensin-1/human β-defensin-2 in human milk
By subgroup analysis, the effect of delivery mode and gestational age on hBD-1 and hBD-2 concentrations was evaluated using the Mann–Whitney U test. In the colostrum, the concentrations of hBD-1 and hBD-2 were significantly higher in the vaginal delivery group than those in the caesarean section group (hBD-1: median 1·79 v. 1·37 μg/ml, Z= − 2·401, P= 0·016; hBD-2: median 1·52 v. 0·74 ng/ml, Z= − 2·784, P= 0·005). In mature milk, higher concentrations of hBD-1 and hBD-2 were detected from the women who delivered prematurely when compared with those who delivered at term (hBD-1: median 9·95 v. 2·80 ng/ml, Z= − 2·095, P= 0·036; hBD-2: median 80·45 v. 65·36 pg/ml, Z= − 2·474, P= 0·013).
Spearman's correlation analysis results are shown in Table 2. The concentration of hBD-1 in the colostrum was significantly correlated with parity, serum total protein levels and pre-pregnancy BMI (P= 0·013, P= 0·001 and P< 0·001, respectively). The concentration of hBD-2 in the colostrum was positively associated with residence, education level and parity (P< 0·001, P= 0·003 and P= 0·018, respectively). The variables with P value less than 0·1 were introduced into the following multiple linear regression models. Age of mothers and BMI of mothers on day 42 postpartum were not significantly correlated with hBD-1 concentration in mature milk. However, based on our clinical knowledge, we considered these two factors to be important factors and introduced them into the multiple linear regression models.
Table 2 Results of Spearman's correlation analysis: correlates of human β-defensin-1 (hBD-1) and human β-defensin-2 (hBD-2) in human milk
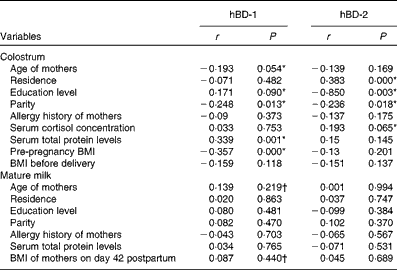
* Variables with P value less than 0·1 were introduced into the following multiple linear regression models.
† Variables were not significantly correlated with hBD-1 concentration in mature milk. However, based on our clinical knowledge, we considered these two factors to be important factors and introduced them into the following multiple linear regression models.
Multiple linear regression analysis results are presented in Table 3. Concentration of hBD-1 in the colostrum, concentration of hBD-2 in the colostrum and concentration of hBD-1 in mature milk were log-transformed into a normal distribution. LoghBD-2 in mature milk remained in a skewed distribution and was not analysed in multiple linear regression models. Pre-pregnancy BMI remained significantly associated with loghBD-1 in the colostrum (P= 0·001). Mode of delivery was significantly associated with loghBD-2 in the colostrum (P= 0·006). Gestational age was significantly associated with loghBD-1 in mature milk (P= 0·010).
Table 3 Results of multiple linear regression analysis: factors associated with loghBD-1 and loghBD-2 (β-Coefficients, standard errors and 95 % confidence intervals)
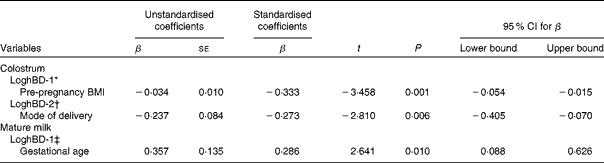
hBD-1, human β-defensin-1; hBD-2, human β-defensin-2.
* Variables introduced into the model included age of mothers, mode of delivery, education level, parity, serum total protein levels and pre-pregnancy BMI.
† Variables introduced into the model included mode of delivery, residence, education level, parity and serum cortisol levels.
‡ Variables introduced into the model included gestational age, age of mothers and BMI of mothers on day 42 postpartum.
Association of colostrum human β-defensin-1/human β-defensin-2 and infant diseases
Completed follow-up data were available for eighty-two infants. All of the followed-up infants had been fed with colostrum. Breast-feeding lasted for at least 1 month. The timing of the introduction of solid food was 4th month to 6th month. During the 6-month period of follow-up, URI had been diagnosed in nineteen infants (23·2 %) and diarrhoea in nine infants (11 %) by a paediatrician.
Choosing the 50th percentile of colostrum hBD-1 concentration as the boundary, we divided the infants into high-hBD-1 group and low-hBD-1 group. Using the χ2 test, we found that the incidence rate of URI during the first 6 months of life in the high-colostrum hBD-1 group was less than that in the low-colostrum hBD-1 group (χ2 4·995, P= 0·025; Table 4). The incidence rate of diarrhoea during the first 6 months of life did not differ significantly between the two groups (χ2 0·396, P= 0·529).
Table 4 Protective effect of colostrum human β-defensin-1 (hBD-1) against upper respiratory infection and diarrhoea during the first 6 months of life (Number of cases and percentages)
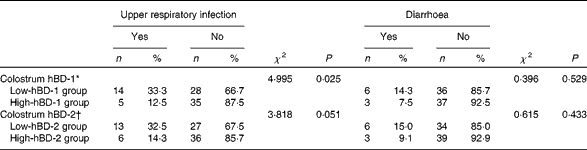
hBD-2, human β-defensin-2.
* Categorised by the 50th percentile of colostrum hBD-1 concentration.
† Categorised by the 50th percentile of colostrum hBD-2 concentration.
Choosing the 50th percentile of colostrum hBD-2 concentration as the boundary, we divided the infants into high-hBD-2 group and low-hBD-2 group. No significant difference in URI incidence or diarrhoea incidence was observed between the two groups (χ2 3·818, P= 0·051; χ2 0·615, P= 0·433, respectively).
Discussion
hBD-1 and hBD-2 are considered as two of the most important antimicrobial peptides in epithelial tissues. As short basic peptides, hBD-1 consists of thirty-six amino acid residues and hBD-2 of forty-one residues. They all contain six cysteines forming three intramolecular disulphide bonds( Reference Taylor, Barran and Dorin 19 , Reference Liu, Wang and Jia 20 ). Their gene expressions have been associated with many human diseases( Reference Prado-Montes de Oca 21 , Reference Gersemann, Wehkamp and Stange 22 ). They display a direct antimicrobial activity against a broad spectrum of bacteria and fungi. Furthermore, they play a role in linking innate and adaptive immunity through the chemotaxis of dendritic cells and T cells( Reference Röhrl, Yang and Oppenheim 23 – Reference Morgera, Pacor and Creatti 26 ).
In the colostrum from Chinese mothers, a high concentration of hBD-1 ranging between 1·04 and 12·81 μg/ml was detected, which is similar to that reported in previous studies. Jia et al. ( Reference Jia, Starner and Ackermann 27 ) estimated the quantity of hBD-1 in human breast milk to be 1 to 10 μg/ml. Armogida et al. ( Reference Armogida, Yannaras and Melton 28 ) found abundant hBD-1 in human milk with a range of 0–23 μg/ml by the ELISA method. The similarity of hBD-1 concentration in the colostrum between Armogida and the present study indicated that hBD-1 expression in the human colostrum was not influenced by races.
In the present study, hBD-2 concentration range was 0·31–19·12 ng/ml in the colostrum and 52·65–182·29 pg/ml in mature milk. Armogida et al. ( Reference Armogida, Yannaras and Melton 28 ) reported a range of 8·5–56 μg/ml for hBD-2 concentration, including all of their milk samples. Jia and his team failed to detect the presence of hBD-2 by Western immunoblotting( Reference Jia, Starner and Ackermann 27 ). This may be due to the limitation of their detection method.
Data from Armogida et al. ( Reference Armogida, Yannaras and Melton 28 ) showed a similar concentration of hBD-1 in the colostrum and mature milk. hBD-2 concentration was also similar between colostrum and mature milk. However, in the present study, the concentration of hBD-1 was high in the colostrum and fell rapidly to a relatively low level during the first 42 d of lactation. The similar reducing trend was found for hBD-2. This difference between Armogida and the present study may be caused by regional and race factors.
Previous studies have reported that hBD-1 had potent activity against Gram-negative bacteria. Starner et al. ( Reference Starner, Agerberth and Gudmundsson 29 ) showed that the in vitro minimal effective concentration of hBD-1 against E. coli was 2·9 μg/ml. Singh et al. ( Reference Singh, Jia and Wiles 30 ) demonstrated that, in vitro, the dose required to kill 50 % of Pseudomonas aeruginosa was 1 μg/ml for hBD-1. We considered that hBD-1 in the colostrum may kill harmful micro-organisms directly in the neonatal gastrointestinal tract, which will contribute to the establishment and development of the neonatal intestinal mucosal immune system.
Of note, ELISA was used as the method for the detection of hBD-1 and hBD-2 peptides, which cannot verify the protein identity by size. Hence, we cannot completely exclude the possibility of cross-reaction of the antibodies with other proteins. Future studies could incorporate Western blot analysis to confirm the present findings.
In the present study, hBD-1 concentration was significantly higher than hBD-2 concentration in both colostrum and mature milk. This difference was similar for their corresponding mRNA transcripts, demonstrated by quantitative real-time PCR. These results indicated that the expression of hBD-1 is rich in normal human milk, while that of hBD-2 is relatively low.
The mRNA expression of hBD-1 and hBD-2 was comparable between colostrum and mature milk. However, the protein level of hBD-1 and hBD-2 was significantly higher in the colostrum than in mature milk. There are two possibilities that may explain such discrepancy. First, it may be the dilution effect. A very small volume (approximately 30 ml/24 h) of the colostrum is available during the first 30–40 h after delivery( Reference Pang and Hartmann 31 ), while the daily mature milk volume increases substantially. In other words, colostrum is much more concentrated than mature milk. Second, post-transcriptional regulation may lead to the discrepancy of hBD-1 and hBD-2 translation at different milk secretion stages. Future studies may explore the mechanism.
In the milk cell pellet, we observed mammary epithelial cells, which naturally sloughed off from the lactating breast glands. Such cells could possibly be the source of hBD-1 and hBD-2. Tunzi et al. ( Reference Tunzi, Harper and Bar-Oz 13 ) demonstrated that hBD-1 expression is mainly localised in human mammary gland epithelia by immunohistochemistry. They found that blood leucocytes do not express the hBD-1 transcript. Therefore, it was suggested that the PCR signal of hBD-1 from milk is most probably derived from the sloughed-off epithelial cells. Due to the limited number cells in the milk, characterisation of such mammary epithelial cells was not conducted in the present study.
The present statistical results demonstrated that pre-pregnancy BMI was negatively associated with hBD-1 levels in the human colostrum. Women with a lower pre-pregnancy BMI showed a higher concentration of hBD-1 in the colostrum. Pre-pregnancy BMI, compared with height and weight, can more objectively reflect the basis of the nutritional status of women before pregnancy. Pre-pregnancy BMI has been reported as an important factor to affect pregnancy complications and pregnancy outcomes( Reference Jang, Jo and Lee 32 , Reference Morikawa, Yamada and Yamada 33 ). The present study indicated that pre-pregnancy BMI may regulate colostrum hBD-1 levels in Han Chinese women. The exact mechanism remains to be determined.
The results herein suggested that mode of delivery was a factor affecting hBD-2 levels in the colostrum. Colostrum hBD-2 concentration was significantly higher in the vaginally delivered group than that in the caesarean section group. That means the vaginal delivery mode can lead to an increased expression of colostrum hBD-2. What is the mechanism? Previously, Rae et al. ( Reference Rae, Hollebone and Chetty 34 ) declared that women who underwent spontaneous labour had a higher cortisol concentration than those who had caesarean section delivery. Yildiran et al. ( Reference Yildiran, Yurdakul and Guloglu 35 ) reported that serum cortisol levels were higher in women who had vaginal delivery than those who had caesarean section delivery. We also found higher serum cortisol levels among women who underwent vaginal delivery than those who had caesarean section delivery. Furthermore, Terai et al. ( Reference Terai, Sano and Kawasaki 36 ) demonstrated that hBD-2 mRNA expression can be up-regulated by dexamethasone. Therefore, we reckoned that higher serum cortisol level might be one of the critical factors that directly influences the transcription of hBD-2 mRNA in the mammary gland of women who had vaginal delivery. However, multiple linear regression analysis in the present study failed to verify a significant correlation between loghBD-2 and serum cortisol concentration. Its mechanism needs further elucidation in future research.
In the present study, gestational age was demonstrated as a factor associated with hBD-1 levels in mature human milk. As we know, for premature infants, immaturity of innate immunity results in increased susceptibility to infection and high mortality. Human milk can supply premature infants with particularly rich nutrients and immune components( Reference Molinari, Casadio and Hartmann 16 , Reference Newburg and Walker 37 ). The present data imply that mature human milk may provide premature infants with a relatively high concentration of hBD-1, which may protect them from infection and other diseases.
Many studies have supported the protective role of breast-feeding in decreasing the morbidity and mortality of infectious diseases in infants( Reference Kramer and Kakuma 38 , Reference Lamberti, Fischer Walker and Noiman 39 ). Roth et al. ( Reference Roth, Caulfield and Ezzati 40 ) declared that lack of exclusive breast-feeding in the first half of infancy is a risk factor for acute lower respiratory infection, its morbidity and death. We also followed up the babies in the present study for 6 months to determine whether abundant hBD-1 and hBD-2 in the colostrum can prevent URI and diarrhoea. We observed a tendency of decreased risk of URI in infants who received a relatively high concentration of hBD-1 from the colostrum. That means hBD-1 in the colostrum may have a protective function against URI in infants younger than 6 months. However, the incidence rate of diarrhoea during the follow-up period did not differ between the low-hBD-1 group and the high-hBD-1 group. No protective effect on URI incidence or diarrhoea incidence was observed for colostrum hBD-2.
The protective effect of colostrum hBD-1 may be explained by its function as a linkage between innate immunity and adaptive immunity. hBD-1 can enhance the adaptive immune response by chemoattracting immature dendritic cells to the neonatal gastrointestinal tract( Reference Oppenheim and Yang 41 ). Then, immature dendritic cells undergo a maturational process to become mature dendritic cells. These mature dendritic cells may migrate to secondary lymphoid organs to stimulate antigen-specific naive T lymphocytes( Reference Banchereau and Steinman 42 , Reference Palucka and Banchereau 43 ). Thus, colostrum hBD-1 can indirectly regulate adaptive immune responses in the upper respiratory tract. Further study is needed to explore the impact of breast milk hBD-1 on newborn adaptive immune systems.
Due to the limitation of the follow-up information in the present study, we cannot ascertain whether a high concentration of hBD-1 in the colostrum correlates with other immune factors in breast milk. Factors such as duration of breast-feeding and living environment may also influence infant health, and were not considered when assessing the role of defensins in breast milk on infant diseases. Further clinical studies are needed. If additional study also supports the protective role of breast milk hBD-1 in infants, supplementing infant formulas with hBD-1 may be beneficial for bottle-fed infants.
Conclusions
In summary, the present study measured the concentrations of hBD-1 and hBD-2 in breast milk from healthy mothers in Shanghai. The concentrations of both hBD-1 and hBD-2 in human milk were demonstrated to decrease over the course of lactation. Several factors that can regulate the levels of hBD-1 and hBD-2 in breast milk were identified. Furthermore, the study found that colostrum hBD-1 may play an important role in protecting infants younger than 6 months of age from URI. The present study sets a foundation for future research in this field.
Acknowledgements
The present study was supported by grants from Beingmate Group Company Limited (China). Beingmate Group Company Limited had no role in the design, analysis or writing of this article.
The contributions of the authors are as follows: R.-M. C. conceived the idea; T.-X. C. designed the study and reviewed the manuscript; S.-M. W. and J. L. raised the funds and contributed to the project design; X.-F. W. collected the samples, performed the laboratory work, analysed the data and wrote the manuscript; J. W. participated in the laboratory work.
The authors declare that there are no conflicts of interest.







