Introduction
Sleep disorders are highly prevalent in psychiatric populations and are increasingly recognized as core dimensions rather than secondary symptoms of psychiatric disorders [Reference Geoffroy and Gottlieb1–Reference Baglioni, Nanovska, Regen, Spiegelhalder, Feige and Nissen4]. Research has demonstrated bidirectional relationships between sleep disorders and psychiatric disorders [Reference Solelhac, Imler, Strippoli, Marchi, Berger and Haba-Rubio5–Reference Baglioni, Battagliese, Feige, Spiegelhalder, Nissen and Voderholzer7]. Additionally, sleep complaints – including insomnia, hypersomnia, sleepiness, and nightmares – have been shown to predict suicide attempts independently of any psychopathology [Reference Geoffroy, Oquendo, Courtet, Blanco, Olfson and Peyre8]. These sleep disturbances can exacerbate psychiatric symptoms, increase comorbidities, and elevate relapse risk [Reference Barbotin, Hoertel, Olfson, Blanco, Sanchez-Rico and Lejoyeux6, Reference Kallestad, Hansen, Langsrud, Ruud, Morken and Stiles9]. Sleep disorders are also among the earliest prodromal signs of psychiatric conditions. In the Adolescent Brain and Cognitive Development study, which followed over 11,000 children longitudinally, sleep disturbances emerged as the strongest predictor of mental health risk in adolescence – outweighing even adverse childhood experiences and family history of psychiatric illness [Reference Hill, Kashyap, Raffanello, Wang, Moffitt and Caspi10]. These alterations also appear to predict imminent mood relapse or recurrence. A key concept in understanding the progression of mood disorders is the “Chronos syndrome,” defined as “a clinical syndrome that reliably predicts an imminent transition to a mood episode” [Reference Geoffroy11]. In a recent study, 87.5% of patients reported sleep–wake disturbances in the days and weeks preceding a depressive episode, and 76.5% before a manic episode – further supporting the predictive value of sleep disorders in mood disorders [Reference Basquin, Maruani, Leseur, Mauries, Bazin and Pineau12]. Despite growing evidence, sleep disorders in psychiatric patients remain largely underdiagnosed and undertreated, particularly in inpatient settings.
Previous studies have documented high rates of insomnia, hypersomnia, parasomnias, and circadian rhythm disorders across various psychiatric disorders, including bipolar disorder [Reference Robillard, Naismith, Rogers, Ip, Hermens and Scott13–Reference Palagini, Miniati, Marazziti, Massa, Grassi and Geoffroy17], major depressive disorder [Reference Baglioni, Battagliese, Feige, Spiegelhalder, Nissen and Voderholzer7, Reference Geoffroy, Hoertel, Etain, Bellivier, Delorme and Limosin18, Reference Soehner, Kaplan and Harvey19], schizophrenia [Reference Wulff, Dijk, Middleton, Foster and Joyce3, Reference Akkaoui, Lejoyeux, d’Ortho and Geoffroy20], suicide [Reference Benard, Etain, Vaiva, Boudebesse, Yeim and Benizri21–Reference Geoffroy, Borand, Ambar Akkaoui, Yung, Atoui and Fontenoy25], and substance use disorders [Reference Meyrel, Rolland and Geoffroy26–Reference Mauries, Bertrand, Frija-Masson, Benzaquen, Kalamarides and Sauvage30]. Most of this work has focused on outpatients, specific diagnostic categories, or relatively small samples. Large-scale studies in psychiatric inpatients have also demonstrated a high prevalence of insomnia and other sleep problems, but often without addressing the persistence of symptoms or their clinical consequences. For instance, Talih et al. [Reference Talih, Ajaltouni, Ghandour, Abu-Mohammad and Kobeissy31] reported that 67.4% of hospitalized psychiatric patients (n = 203) screened positive for insomnia using the Insomnia Severity Index. Similarly, in a Dutch sample (n = 1082), Mijnster et al. [Reference Mijnster, Boersma, van Veen, Liemburg, Cath and Pijnenborg32] found that 46.2% of individuals with mental disorders scored above the cut-off for having a sleep disorder on the Holland Sleep Disorders Questionnaire. These studies, though informative, did not examine the longitudinal course of chronic sleep disorders (CSDs) or their broader implications across hospital stays [Reference Mao, Shalaby, Owusu, Elgendy, Agyapong and Eboreime33]. To date, no work has explored their impact on hospitalization burden, restraint use, and comorbidities, despite their high clinical and public health relevance. Large-scale, real-world studies in psychiatric hospitals are therefore crucial to better understand the prevalence and consequences of CSDs in this vulnerable population.
In this context, we aimed to systematically assess the prevalence and clinical characteristics of CSDs – defined as sleep disturbances affecting the patient during at least half of their hospital stays and not merely episodic – in a large cohort of adult psychiatric inpatients. Specifically, we sought to: (i) compare hospitalization characteristics (length of stay, restraint use, admission type) between patients with CSD and those without sleep disorders (NSD); (ii) examine diagnostic differences, particularly regarding mood, psychotic, addictive, developmental, eating, and personality disorders; and (iii) assess hypnotic medication use and prescribing patterns across diagnostic categories.
By leveraging data from the health data warehouse of the Paris Psychiatry Hospital Group, we hypothesize that this study will provide novel insights into the real-world impact of CSDs in psychiatric inpatient care.
Methods
Setting and study population
This retrospective study used routinely collected data extracted from the health data warehouse of the Paris Psychiatry Hospital Group (GHU Paris Psychiatrie & Neurosciences). This data warehouse is a secure, centralized repository that consolidates routinely collected health data of around 500,000 psychiatric patients. This data warehouse records all hospitalization information: administrative information (age, gender, type of admission, etc.) and medical information (primary diagnosis – the reason why the patient has been admitted and comorbidities, i.e., complications and morbidities that impact the course of hospitalization). The data warehouse also includes biological results, drug prescriptions, textual medical and imaging reports. It serves as a vital resource for researchers and healthcare professionals, facilitating data-driven insights and advancements in psychiatry.
General definition of CSDs
CSDs (CSD) were identified using a combined approach: ICD-10 diagnostic codes, hypnotic prescriptions, and textual mentions in electronic medical records. Patients were considered to have CSD when sleep disturbances were documented in more than half of their hospitalizations, according to our predefined Index of Length of Stays with Disorders (ILSD). This operationalization was chosen to reflect the persistence of symptoms over time, rather than transient sleep problems limited to one admission. Sleep diagnoses were captured from the entire hospitalization record, not solely at admission, and thus included both preexisting disorders and those documented during hospitalization. Hypnotic use was extracted from prescriptions during hospitalization; information about treatments initiated prior to admission was not systematically available. While this approach does not allow precise differentiation between insomnia, hypersomnia, and parasomnia, it increases ecological validity by capturing the broad spectrum of chronic sleep disturbances encountered in psychiatric inpatient settings.
Detailed operationalization
In more detail, we included all adult patients (aged 18 years and older at the time of admission) with at least one full-time psychiatric hospitalization from January 1, 2021 to December 31, 2023. Stays were flagged for sleep disorders if patients met at least one of the following three criteria:
-
- Diagnosis related to sleep disorders based on the International Classification of Diseases (ICD-10) diagnosis codes: F51 and G47.
-
- Drugs administered for sleep disorders (hypnotics): “Stilnox, Zolpidem, Imovane, Zopiclone, Circadin, Melatonin, Slenyto, or Theralene.”
-
- Textual evidence of sleep-related terms (e.g., insomnia, nightmares, drowsiness) within unstructured medical documents.
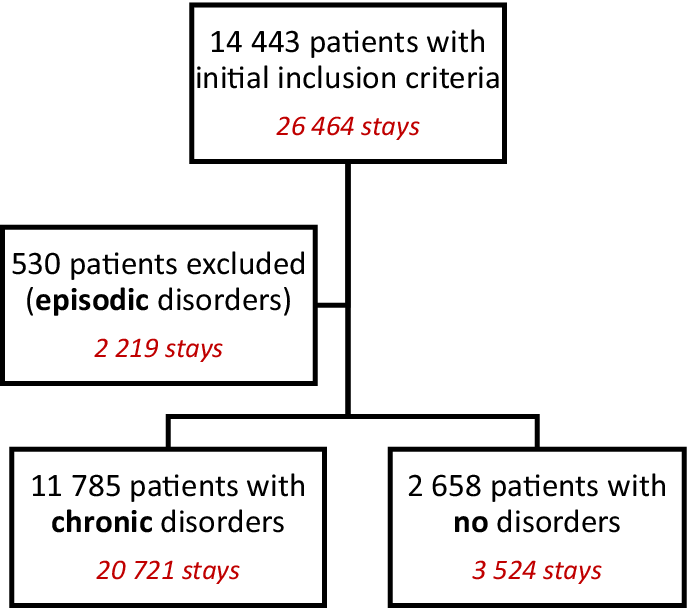
A total of 14,443 patients satisfying the criterion were included in this study (representing 26,464 stays). Among them, 11,785 patients presented with sleep disorders. The population with sleep disorders was finetuned by distinguishing CSDs from episodic ones. For that, we built a metric called the ILSD, defined as the ratio of the cumulative stay duration with sleep disorders to the cumulative duration of all stays over the study period. CSD patients corresponded to an ILSD greater than 0.5, whereas patients with no sleep disorders (NSDs) had an ILSD score of zero. Patients with episodic disorders were excluded from the analysis. The study flow chart is shown in Figure 1.
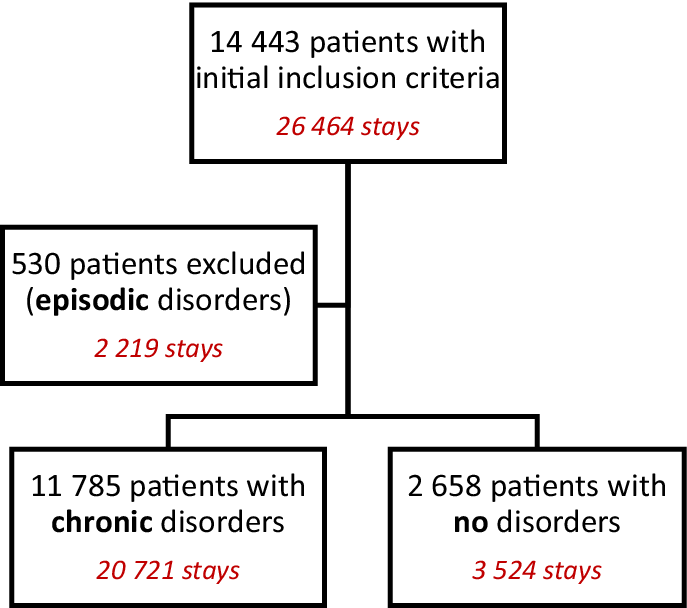
Figure 1. Study flow chart.
This study was approved by the Research Ethics Committee of the Paris Psychiatry Hospital Group (accreditation no. 2024-CER-D-004).
Variables
All data were extracted from the GHU health data warehouse. We selected specific variables among patient characteristics (gender and age, number of stays per patient) and stay characteristics (admission and discharge dates, type of admissions [involuntary admission or not]), and restraint practices such as seclusion and/or physical restraint measures, administration of hypnotic medications, primary diagnosis, and comorbidities (based on the 10th revision of ICD-10 diagnosis codes). Diagnoses were categorized according to medical similarities as assessed by medical professionals.
Statistical analysis
Quantitative variables were described using the mean and standard deviation. Qualitative variables were described as frequencies and percentages.
Comparisons between the NSD and CSD groups were conducted using Chi-square or Fisher’s exact tests for categorical variables and Student’s t tests for continuous variables, as appropriate.
All tests were two-sided, with a statistical significance level set at 0.05. All analyses were performed using Python (version 3.10.9) and Stata (version 18).
Results
Global analyses: Characteristics of patients and hospital stays
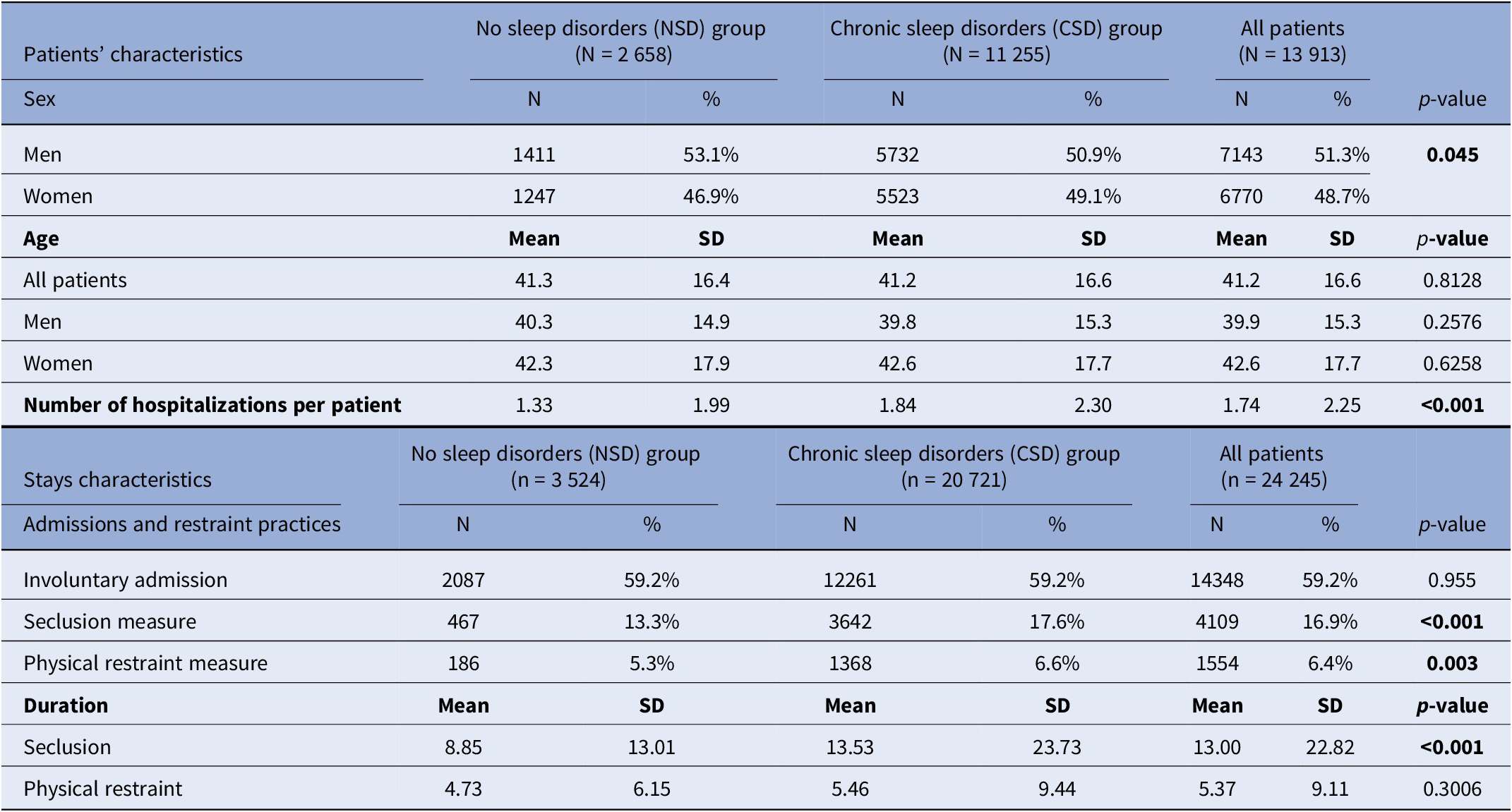
Our study included 13,913 patients, with 48.7% women and an average age of 41.2 years (Table 1). There were 2658 patients in the NSD group and 11,255 patients in the CSD group (CSDs), resulting in a CSD prevalence of 81% among hospitalized psychiatric patients. The proportion of women was significantly higher in the CSD group than in the NSD group (49.1% vs. 46.9%, p < 0.045). No significant age differences were observed between the two groups.
Table 1. Characteristics of patients and hospital stays

Note: The bold text represents the statistically significant results (p<0.05)
On average, patients in the NSD group had 1.33 hospital stays during the study period, compared to 1.84 stays in the CSD group (p < 0.001), leading to an analysis of 24,245 hospitalizations in total, with 3,524 stays in the NSD group (14.5%) and 20,721 in the CSD group (85.5%).
Over half of the stays were involuntary admissions (59.2%), with no difference between the groups. However, patients in the CSD group experienced seclusion more frequently during hospitalization than NSD patients (17.6% vs. 13.3%, p < 0.001), as well as physical restraint measures (6.6% vs. 5.3%, p = 0.003). In addition, the duration of seclusion was significantly longer for CSD patients (13.53 days) compared to NSD patients (8.85 days), while the length of physical restraint use did not differ between groups.
Clinical description of hospital stays
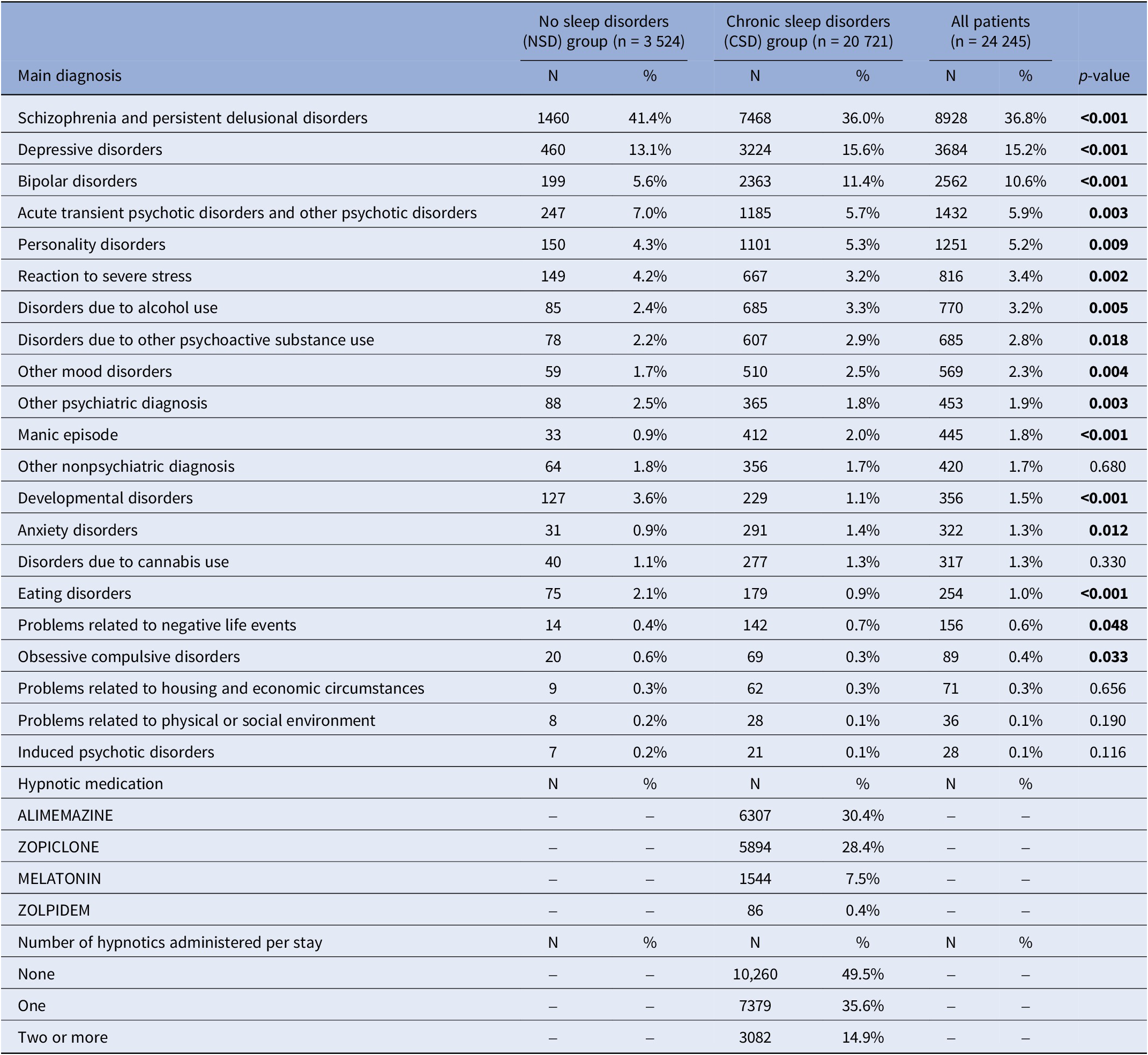
Regarding admission motives, there was more hospitalizations in the CSD than in the NSD group related to depressive disorders (15.6% vs. 13.1%, p < 0.001), bipolar disorders (11.4% vs. 5.6%, p < 0.001), personality disorders (5.3% vs. 4.3%, p = 0.009), alcohol abuse (3.3% vs. 2.4%, p = 0.005), substance use disorders (other than alcohol and cannabis) (2.9% vs. 2.2%, p = 0.018), manic episode (2.0% vs. 0.9%, p < 0.001), and anxiety disorders (1.4% vs. 0.9%, p = 0.012). In reverse, there was significantly more stays in the NSD group than in the CSD group for schizophrenia or persistent delusional disorders (41.4% vs. 36.0%, p < 0.001), transient psychotic disorders (7.0% vs. 5.7%, p = 0.003), reaction to severe stress (4.2% vs. 3.2%, p = 0.002), developmental disorders (3.6% vs. 1.1%, p < 0.001), or eating disorders (2.1% vs. 0.9%, p < 0.001) (Table 2).
Table 2. Clinical description of hospital stays
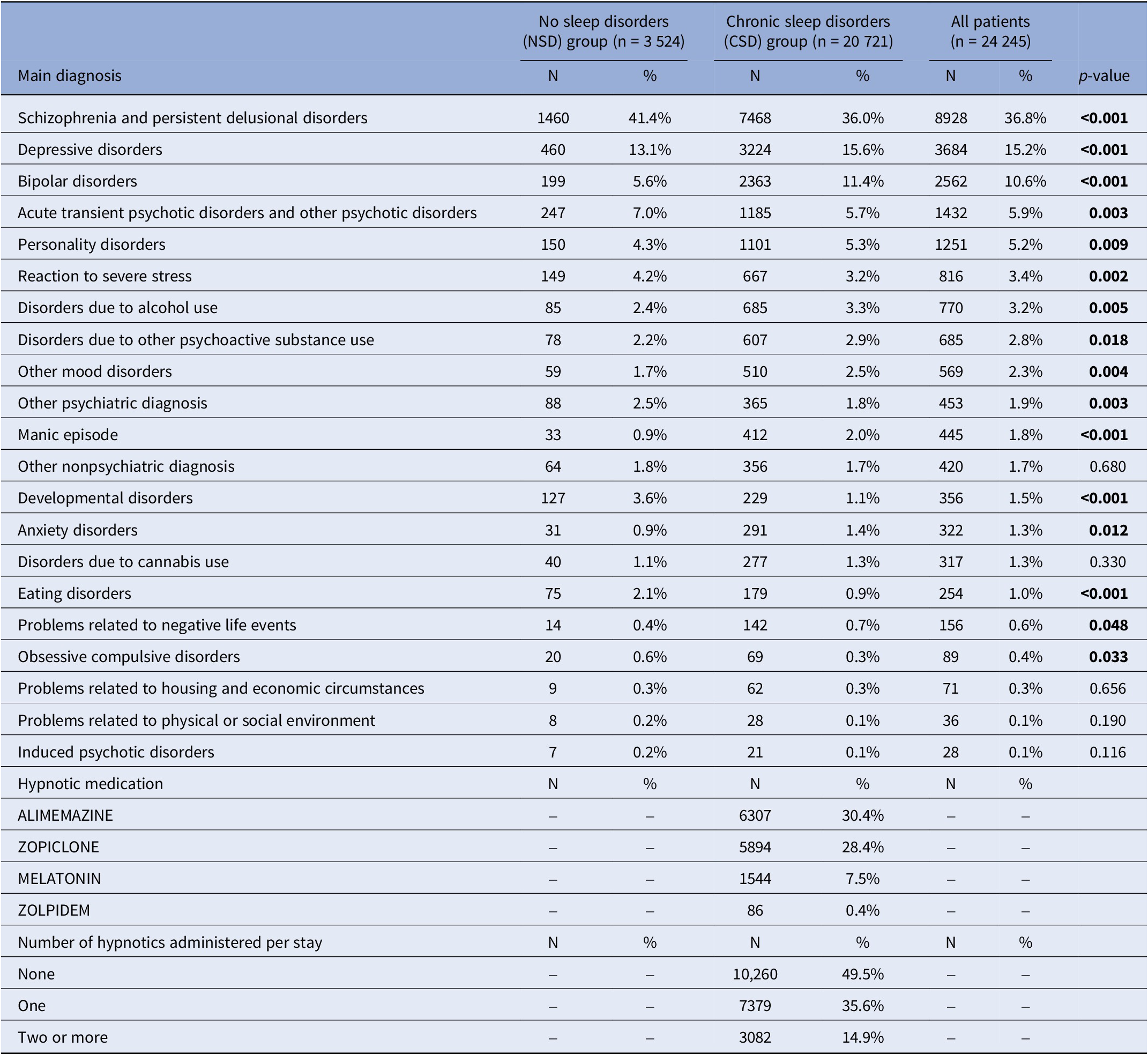
Note: The bold text represents the statistically significant results (p<0.05)
There was no significant difference between the two groups in the proportion of hospitalizations for nonpsychiatric causes, or related to social and economic difficulties. Notably, the prevalence of hospital stays related to cannabis use disorders was similar in both groups.
Hypnotic use
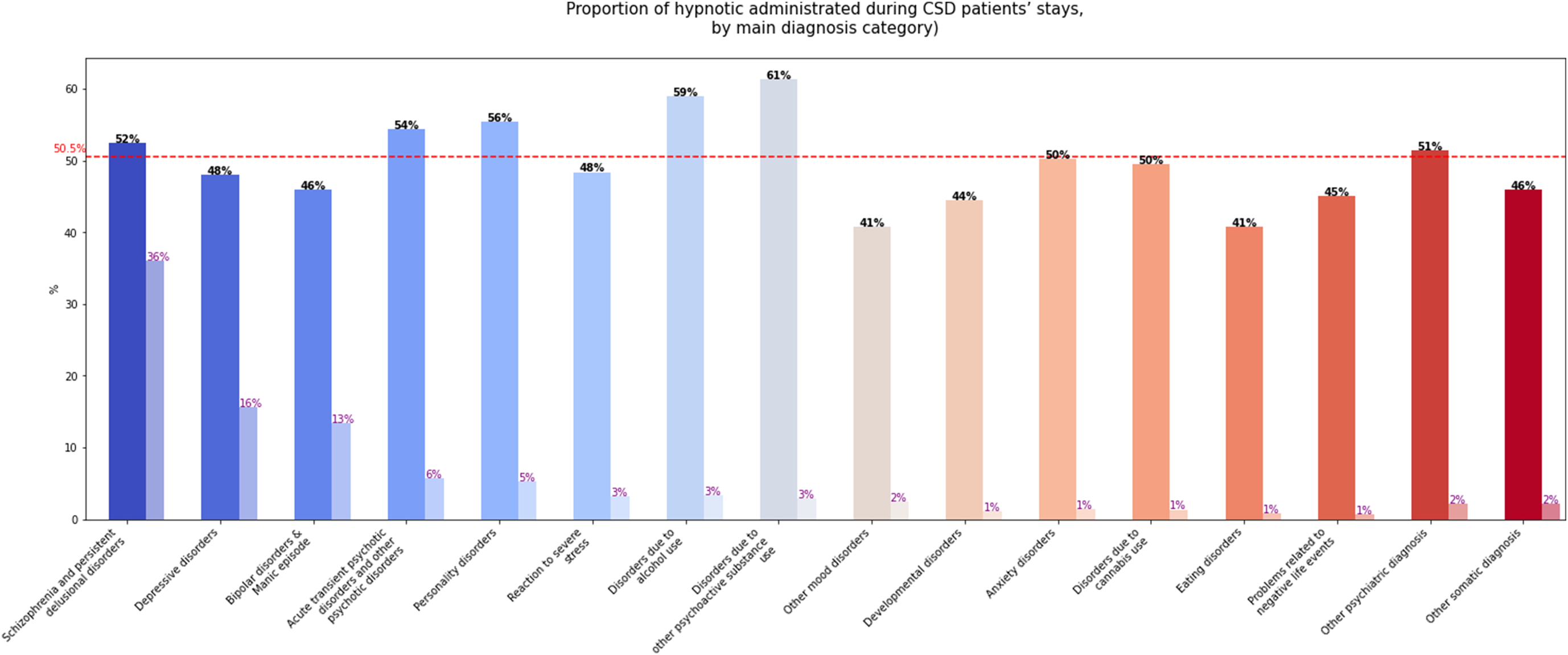
In the CSD group, a hypnotic medication was administered in 50.5% of hospital stays (Table 2). The most common molecule was alimemazine (30.4%), followed by zopiclone (28.4%), with more than one hypnotic administered in 14.9% of stays. Hypnotics were most frequently dispensed during stays related to psychoactive substance use disorders: 61% for substances other than alcohol or cannabis and 59% for alcohol use disorders. They were also administered in more than half of stays for personality disorders, acute and transient delusional disorders, and schizophrenia and persistent delusional disorders. In contrast, hypnotic prescriptions were lower for stays associated with mood and eating disorders (41% in each category). Figure 2 illustrates hypnotic use by primary diagnosis categories.
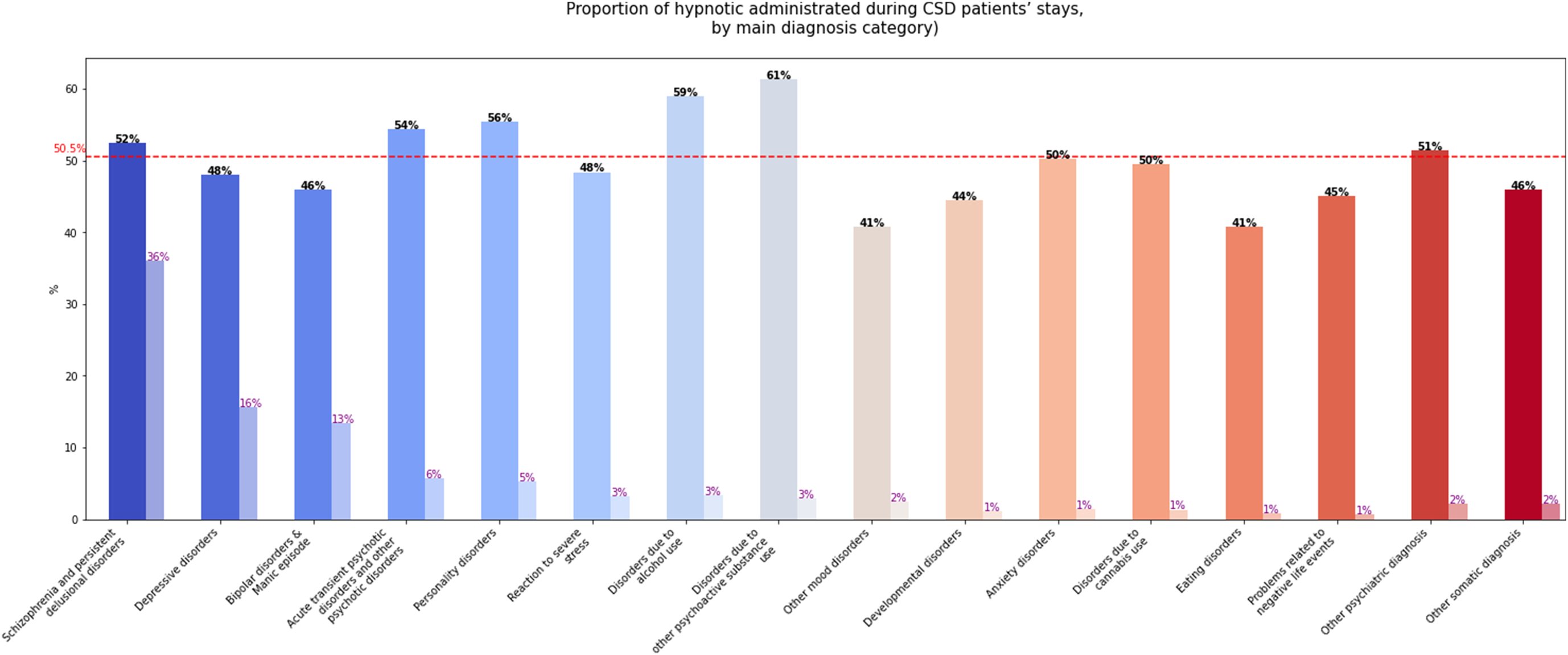
Figure 2. Proportion of hypnotic administered during stays of patients with chronic sleep disorders, by main psychiatric disorder.
Note: The red dashed line represents the average proportion of stays during which a hypnotic was administered. The first bar represents the proportion of stays in which a hypnotic was administered for each psychiatric disorder, and the second bar (lighter one) represents the proportion of stays related to each psychiatric disorder relative to the total of stays. For instance, a hypnotic drug was administered in 52% of stays for schizophrenia and persistent delusional disorders, and those disorders represented 36% of all stays.
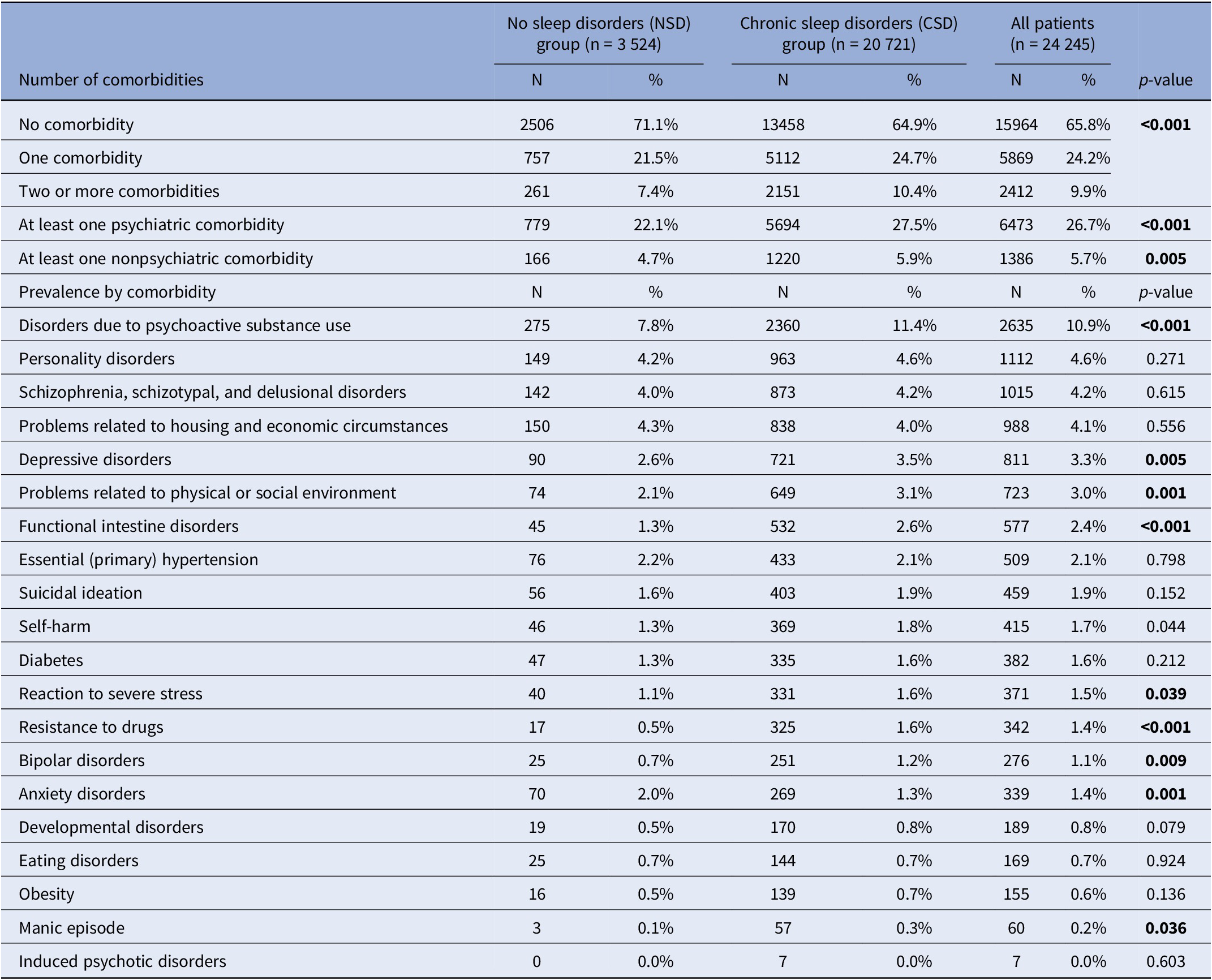
Comorbidities
Patients in the NSD group had fewer comorbidities than patients in CSD group, as no comorbidity was reported in 71.1% of stays in NSD patients and 64.9% in CSD patients (p < 0.001). Patients in the CSD group more frequently presented two or more comorbidities (10.4% vs. 7.4%), regarding both psychiatric and other medical comorbidities (Table 3). Compared to NSD patients, those in the CSD group presented more often disorders due to psychoactive substance use (11.4% vs. 7.8%, p < 0.001), depressive disorders (3.5% vs. 2.6%, p = 0.005), a history of self-harm (1.8% vs. 1.3%, p = 0.044), reactions to severe stress (1.6% vs. 1.1%, p = 0.039), bipolar disorders (1.2% vs. 0.7%, p = 0.009), manic episodes (0.3% vs. 0.1%, p = 0.036), environmental or psychosocial difficulties (3.1% vs. 2.1%, p = 0.001), and functional intestinal disorders (2.6% vs. 1.3%, p < 0.001). Conversely, anxiety disorders were significantly more prevalent in NSD patients than in CSD patients (2.0% vs. 1.3%, p = 0.001).
Table 3. Comorbidities
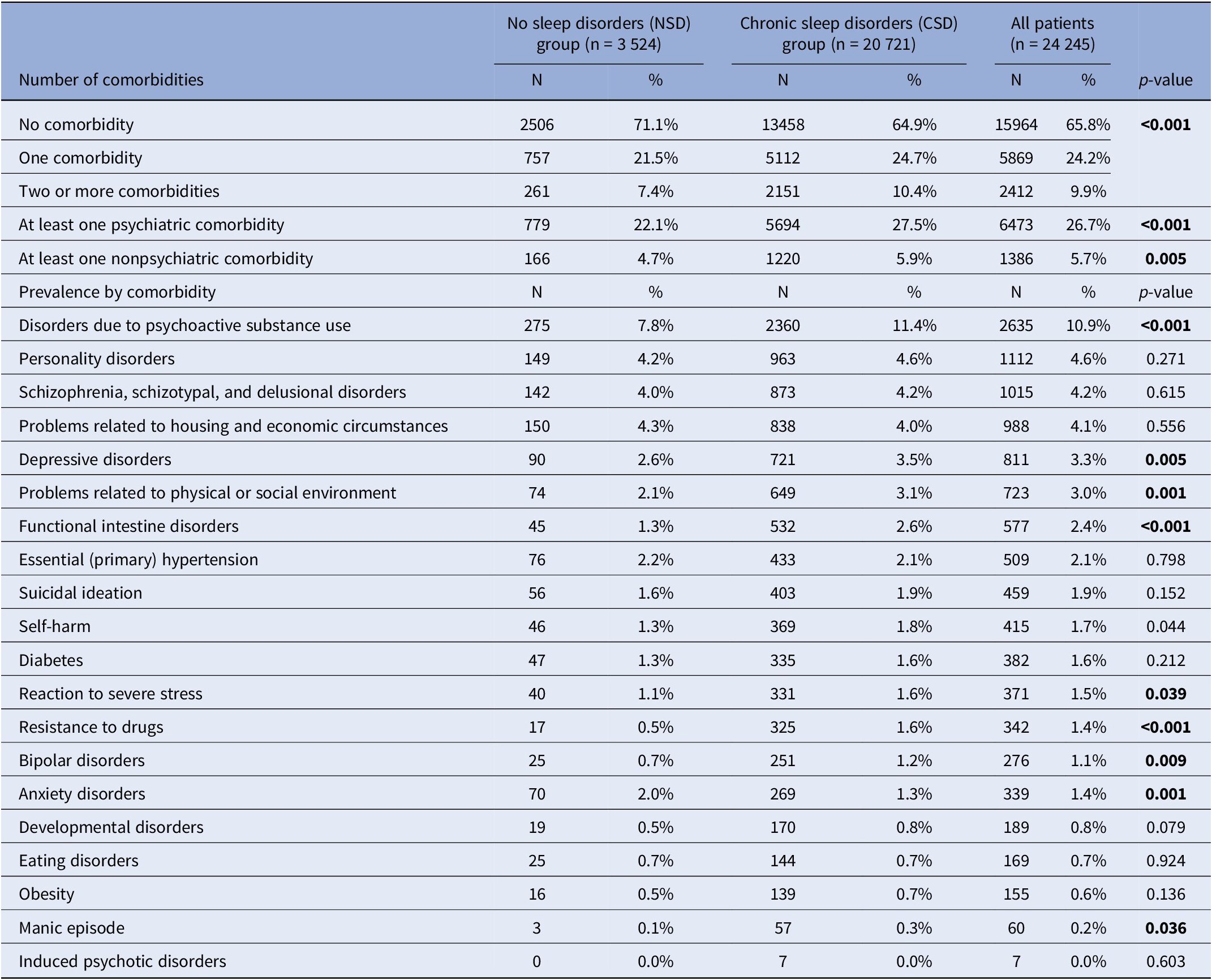
Note: The bold text represents the statistically significant results (p<0.05)
Discussion
This large-scale study of over 13,000 psychiatric inpatients reveals that CSD affects the vast majority (81%) of hospitalized patients, confirming their high prevalence in real-world psychiatric care. Importantly, CSD is not only common but also associated with more severe clinical profiles and a greater hospitalization burden, including increased frequency of hospitalizations, higher use and longer duration of seclusion, and more frequent physical restraint use. These findings highlight the considerable impact of sleep disturbances on inpatient care complexity and underscore the need to treat sleep not as a secondary symptom but as a core clinical dimension.
Patients with CSD had higher rates of depressive and bipolar disorders, personality disorders, and substance use disorders, especially those related to alcohol and other psychoactive substances. These associations align with existing literature linking sleep disruptions to affective instability, emotion dysregulation, and impulsivity [Reference Palagini, Miniati, Marazziti, Hickie, Crouse and Geoffroy16, Reference Garrivet, Gohier, Maruani, Ifrah, Trzepizur and Gagnadoux34, Reference Garrivet, Maruani, Mauries, Trzepizur, Lejoyeux and Gohier35]. Notably, hypnotics were prescribed in over half of the CSD-related stays, most commonly alimemazine and zopiclone, particularly in cases involving substance use and personality disorders. This pattern may reflect both the high clinical demand for sedation and the limited availability of nonpharmacological interventions such as cognitive behavioral therapy for insomnia (CBT-I) in inpatient settings, despite their efficacy as a first-line treatment, even during acute states [Reference Maruani, Stern, Boiret, Leseur, Romier and Lejoyeux36, Reference Riemann, Espie, Altena, Arnardottir, Baglioni and Bassetti37]. Beyond acute care, CBT-I has also been shown to reduce the risk of incident and recurrent depression. In a large randomized controlled trial, CBT-I decreased the risk of major depressive episodes over 36 months, particularly when sustained improvements in sleep were achieved [Reference Irwin, Carrillo, Sadeghi, Bjurstrom, Breen and Olmstead38]. These findings highlight the importance of addressing CSD not only to improve current symptoms but also to prevent relapse and recurrence of psychiatric disorders.
Patients with CSD also exhibited significantly more psychiatric and nonpsychiatric comorbidities, consistent with findings from previous studies, including prospective research [Reference Barbotin, Hoertel, Olfson, Blanco, Sanchez-Rico and Lejoyeux6]. The increased prevalence of conditions such as self-harm, depressive and bipolar comorbidities, stress-related disorders, environmental difficulties, and functional intestinal disorders points to a more complex and multidimensional clinical profile in patients with psychiatric disorders and comorbid CSD. Indeed, the dysregulation of sleep–wake rhythms and sleep disorders, beyond their negative impact on emotional regulation, can lead to dysfunction of the autonomic nervous system, impairment of the hypothalamic–pituitary–adrenal axis, and immune dysregulation [Reference Young, Bourgeois, Hilty and Hardin39]. These findings support the hypothesis that sleep disturbances are not isolated symptoms, but are integrated components of broader vulnerability profiles in psychiatric disorders.
Unexpectedly, some conditions – including schizophrenia, acute psychotic episodes, neurodevelopmental disorders, eating disorders, and anxiety disorders – were more common in patients without identified sleep disorders (NSD group). Several hypotheses may explain this counterintuitive finding. First, patients with severe cognitive or psychotic symptoms may be less likely to report sleep complaints, leading to underrecognition [Reference Demirlek and Bora40]. Second, clinicians may focus on acute psychotic symptoms during hospitalization, deprioritizing the assessment or documentation of sleep. Moreover, sleep disturbances are part of the diagnostic criteria for mood disorders, such as depression and bipolar disorder, but not for psychotic disorders. This raises the question of whether these symptoms are systematically assessed in patients with psychotic disorders. Also, sleep disorders may be underestimated in neurodevelopmental and eating disorder populations, where symptom expression is often atypical. Additionally, the CSD classification relied partly on documented evidence, such as medical record notes and hypnotic prescriptions, which may be less consistently recorded in certain diagnostic groups.
Also, previous studies have also highlighted the impact of hospitalization itself on sleep disturbances, both in terms of sleep quality and duration. Although these studies focused on hospitalizations for nonpsychiatric reasons, they emphasized that changes in daily rhythms and disruptive factors such as noise can impair sleep quality during the hospital stay – underlining the importance of addressing sleep-related symptoms in already vulnerable patients [Reference Wesselius, van den Ende, Alsma, ter Maaten, Schuit and Stassen41].
These results open important clinical perspectives. We anticipated a high prevalence of CSDs in psychiatric inpatients, but the magnitude observed (81%) and their strong associations with hospitalization burden and restraint use were greater than expected. These findings highlight the need for systematic and standardized assessment of sleep disorders in all hospitalized patients with psychiatric disorders, particularly in those with communication difficulties or during acute episodes. The high rate of hypnotic prescription – especially in cases involving sedative use for behavior management – also calls for careful consideration of pharmacological strategies, and where possible, greater integration of nondrug approaches, such as CBT-I or chronotherapy [Reference Maruani, Stern, Boiret, Leseur, Romier and Lejoyeux36, Reference Koffel, Bramoweth and Ulmer42, Reference Palagini, Aquino, Alfi, Massoni, Gambini and Miniati43]. Efforts such as the Delphi-based validation of a standardized assessment set for sleep, chronobiology, addictive, and psychiatric dimensions in the SoPsy-Depression French national cohort illustrate the feasibility and importance of implementing structured, consensus-based tools in clinical and research settings to better characterize and treat sleep disturbances in psychiatric populations [Reference Geoffroy, Schroder, Bourgin, Maruani, Lejoyeux and d’Ortho44]. Future research should validate our operational definition of CSD in independent cohorts, and develop standardized screening tools for routine practice. Interventional studies are also warranted to determine whether targeted sleep management can reduce hospitalization burden, improve quality of life, and lower relapse rates.
Several limitations should be acknowledged. First, the study relied on routinely collected clinical data, which may result in underreporting or inconsistent documentation of sleep complaints. Second, ICD-10 coding and free-text analyses, while comprehensive, may not fully capture sleep disorders diagnosed outside standardized classifications (e.g., insomnia symptoms not coded explicitly). Third, the population was limited to inpatients during acute episodes, which may restrict generalizability to community or outpatient settings. Finally, while the ILSD threshold (>0.5) provides an innovative operational definition of chronicity, it requires further validation in other cohorts.
Despite these limitations, this study offers important real-world insights into the clinical burden and characteristics of CSDs in psychiatric hospital settings. By demonstrating the links between CSD, severe psychiatric presentations, increased restraint use, and polypharmacy, the findings underscore the urgent need to integrate sleep-focused evaluation and interventions as part of comprehensive psychiatric care.
Conclusion
In conclusion, CSDs are not only highly prevalent in psychiatric inpatient care but are also associated with increased clinical burden and complex comorbidity profiles. Recognizing and treating sleep disturbances as core components of psychiatric disorders – not as secondary symptoms – is essential for improving patient outcomes and quality of care.
Data availability statement
Data supporting the findings of this study are derived from the Paris Psychiatry and Neurosciences Hospital Group health data warehouse. Due to French data protection regulations and patient confidentiality, individual-level data cannot be shared publicly. Access to aggregated or anonymized data may be granted upon reasonable request and with approval from the institutional Data Access Committee.
Acknowledgments
We are deeply grateful to all clinical teams and psychiatric sectors of GHU Paris Psychiatrie & Neurosciences for their daily commitment to patient care and for their contribution to the quality and completeness of the clinical data used in this study.
We also thank all members of the Data Science and Epidemiology Unit at GHU Paris Psychiatrie & Neurosciences for their assistance with data extraction and quality control, as well as the IT Department for maintaining the health data warehouse infrastructure used in this work.
This study used routinely collected data extracted from the health data warehouse of the Paris Psychiatry Hospital Group (GHU Paris Psychiatrie & Neurosciences). This data warehouse is a secure, centralized repository that consolidates routinely collected health data from around 500,000 psychiatric patients and was funded within the framework of France 2030 “Support for the Creation of Hospital Health Data Warehouses.”
Author contribution
PAG, JM, and SM designed the study. RR and AP performed data extraction and statistical analyses. PAG, JM, SM, and ML participated in the results interpretation. PAG, RR, and AP participated to the manuscript redaction and all authors approved the final version of the manuscript.
Financial support
This study was conducted without specific external funding. The research was supported by institutional resources from the Paris Psychiatry and Neurosciences Hospital Group (GHU Paris Psychiatrie & Neurosciences).
Use of AI-assisted technologies in the writing process
In the writing of this manuscript, the authors used ChatGPT 4.0 for English editing, given their non-native English-speaking background. The authors have reviewed the content and take full responsibility for the content of the publication.
Competing interests
The authors declare none.


Comments
No Comments have been published for this article.