Introduction
The migration of human beings from the tropics to the rest of the globe has been marked by variation in the production of the brown–black melanin pigment of the skin. While hominins near the equator developed dark photoprotective melanin-rich pigmentation due to high ultraviolet (UV) radiation exposure, those settling in low-UVB (λ = 280–315 nm) environments developed depigmented skin with facultative pigmentation (tanning) to sustain the photosynthesis of vitamin D3. Melanin is thus a key factor in one of the most noticeable human polymorphisms: skin color.[ Reference Jablonski and Chaplin 1 ]
Humans lacking melanocytes (melanin-producing cells) in the ear and animals with albinism (a deficit or absence of melanin) display hearing conditions as in domestic cats with white fur and blue eyes, thus suggesting a biofunctional role of melanin beyond photoprotection.[ Reference Murillo-cuesta, Contreras, Zurita, Cediel and Cantero 2 , Reference Borovansky 3 ]
In the melanin biopigments family, eumelanin is a brown–black type found in the human body, other mammals, reptiles, amphibians, and fishes as well as in invertebrates, such as cuttlefish and insects; Sepia melanin is a type of natural eumelanin extracted from the ink sac of cuttlefish. Pheomelanin is a yellowish-red melanin.[ Reference d'Ischia, Wakamatsu, Cicoira, Di Mauro, Garcia-Borron, Commo, Galván, Ghanem, Kenzo, Meredith, Pezzella, Santato, Sarna, Simon, Zecca, Zucca, Napolitano and Ito 4 , Reference Lamb 5 ]
Eumelanin has been intensively studied in recent decades for functional properties, such as UV–vis absorption, metal chelation, and free radical scavenging. It also features an antioxidant behavior. The limited solubility of eumelanin in most organic solvents has rendered challenging the understanding of its physicochemical properties.[ Reference d'Ischia, Wakamatsu, Cicoira, Di Mauro, Garcia-Borron, Commo, Galván, Ghanem, Kenzo, Meredith, Pezzella, Santato, Sarna, Simon, Zecca, Zucca, Napolitano and Ito 4 , Reference Prota 6 ]
Here, after a brief introduction of molecular structural aspects of eumelanin biopigments and their effect on the properties of the pigment, we critically review the binding properties of eumelanin toward metal cations and eumelanin–metal oxide interfaces. Eumelanin is involved in the accumulation and release of metal cations in the human body.[ Reference Panessa and Zadunaisky 7 ] It is also worthy of note that the interactions between iron and neuromelanin, a pigment made of eumelanin and pheomelanin present in the brain of humans and primates, have been related to Parkinson's disease.[ Reference Ben-Shachar and Youdim 8 ] The remarkable adhesion properties of melanin-like materials on surfaces, including metal oxides is the underpinning for emerging applications in the biomedical, water treatment, and energy fields.[ Reference Lee, Dellatore, Miller and Messersmith 9 , Reference Liu, Ai and Lu 10 ]
Molecular aspects of eumelanin: building blocks, free radicals, hierarchical development
Eumelanin consists of building blocks of 5,6-dihydroxyindole (DHI) and 5,6-dihydroxyindole-2-carboxylic acid (DHICA) (Fig. 1).[ Reference Prota 6 ] The building blocks can polymerize into eumelanin oligomers and polymers at different molecular sites of the monomers (indicated as 2, 3, 4, 7 in Fig. 1). The presence of two building blocks, different polymerization sites, and redox states [the reduced form is the hydroquinone (H2Q), the intermediate form is the semiquinone (SQ), and the oxidized form is the quinone Q and its tautomer (QI)] co-existing in the pigment result in the well-established chemical heterogeneity of eumelanin.[ Reference d'Ischia, Napolitano, Pezzella, Meredith and Sarna 11 ]

Figure 1. The 5,6-dihydroxyindole (DHI) and 5,6-dihydroxyindole-2-carboxylic acid (DHICA): R is –H in DHI, whereas it is the –COOH group in DHICA. The scheme also illustrates the redox forms of DHI and DHICA: hydroquinone (H2Q), semiquinone (SQ), and quinone (Q). The quinone imine form (QI) is the tautomer of Q.
Hierarchical development characterizes the formation of eumelanin-based materials, generated by supramolecular aggregation. Eumelanin planar oligomers can form from four or more DHI monomers (protomolecules),[ Reference d'Ischia, Napolitano, Pezzella, Meredith and Sarna 11 ] possibly stacking via π–π interactions (Fig. 2). Tetramers have been proposed as molecular units to explain a number of functional properties of eumelanin.[ Reference Meng, Kaxiras, Pezzella, Panzella, Natangelo, Arzillo, Napolitano and D'Ischia 13 – Reference Chen, Ball, De Almeida Gracio, Singh, Toniazzo, Ruch and Buehler 17 ] The π–π-stacked eumelanin protomolecules with different sizes, randomly oriented, leading to peculiar excitonic interactions among them, have been proposed to explain the broadband optical absorption of eumelanin (see “Photophysical behavior of eumelanin: absorption spectrum, chromophoric species” section and Fig. 3).[ Reference Chen, Chuang, Cao, Ball, Ruch and Buehler 18 ]
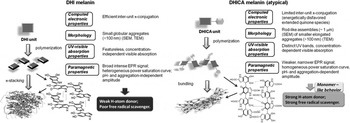
Figure 2. Formation of 5,6-dihydroxyindole (DHI)-melanin [solely composed of DHI monomers, (a)] and 5,6-dihydroxyindole-2-carboxylic acid (DHICA)-melanin [solely composed of DHICA monomers, (b)]. Adapted with permission from Ref. Reference Panzella, Gentile, D'Errico, Della Vecchia, Errico, Napolitano, Carfagna and D'Ischia12. Wiley–VCH, 2013.

Figure 3. Molecular models and equilibrium structures of small-scale systems in eumelanin. (a) Tetrameric model proposed by Kaxiras et al.;[ Reference Kaxiras, Tsolakidis, Zonios and Meng 14 ] (b) pentameric model, and (c) octameric models proposed by Chen et al. in Refs. Reference Sealy, Felix, Hyde, Swartz and Pryor21 and Reference Meredith and Sarna22 in Ref. Reference Chen, Chuang, Cao, Ball, Ruch and Buehler18 (d) monomeric model proposed by Dreyer et al. in Ref. Reference Amit, Appel, Cohen, Cheng, Hamley and Ashkenasy33 in Ref. Reference Chen, Chuang, Cao, Ball, Ruch and Buehler18. Reduced (I) and oxidized (II) forms of the 5,6-dihydroxyindole (DHI) monomer. Two-layer stacked structure of the (e) tetrameric model, (f) pentameric model, and (g) octameric model, respectively. (h) Two-set (two I monomers and two II monomers) stacked structure of the monomeric model. Three-layer stacked structure of the: (i) tetrameric model, (j) pentameric model, and (k) octameric model, respectively. (l) Three-set (three I monomers and three II monomers) stacked structure of the monomeric model. Reprinted with permission from Ref. Reference Chen, Chuang, Cao, Ball, Ruch and Buehler18. Nature Publishing Group, 2014.
The steric hindrance of the carboxyl groups on the DHICA building blocks causes DHICA to polymerize into non-planar structures, differently from the DHI case. The negative charge, possibly present in the deprotonated carboxyl groups, can induce a further twist in the polymer chain. This leads to rod-shaped assemblies in DHICA polymers (usually referred to as DHICA-melanin, Fig. 2). DHICA-melanin exhibits an absorption peak below 400 nm, which DHI-melanin (solely composed of DHI monomers) does not. In addition, DHICA-melanin has a lower optical absorption in the visible.[ Reference Panzella, Gentile, D'Errico, Della Vecchia, Errico, Napolitano, Carfagna and D'Ischia 12 ]
Melanin contains intrinsic stationary-free radicals (observed predominantly in the solid state and likely carbon-centered species associated with defects in the polymer backbone),[ Reference Seagle, Gasyna, Mieler and Norris 19 – Reference Sealy, Felix, Hyde, Swartz and Pryor 21 ] with additional extrinsic free radicals generated under UV or visible irradiation or in high hydration conditions.[ Reference Meredith and Sarna 22 – Reference Froncisz, Sarna and Hyde 25 ] The presence of intrinsic radicals is independent of the pH value.[ Reference d'Ischia, Napolitano, Pezzella, Meredith and Sarna 11 , Reference Pasenkiewicz-Gierula and Sealy 26 , Reference Nilges 27 ] DHICA-melanin exhibits hydroxyl radical-scavenging properties in the Fenton reaction, differently from DHI-melanin.[ Reference Panzella, Gentile, D'Errico, Della Vecchia, Errico, Napolitano, Carfagna and D'Ischia 12 , Reference Jiang, Liu, Dai, Zhou, Lei, Beermann, Wakamatsu and Xu 28 ] DHICA-melanin features relatively homogeneous free-radical species, i.e., spatially confined within restricted segments of the polymer, in contrast to the broader variety of free-radical species generated within the delocalized π-electron systems of the DHI-melanin.
Photophysical behavior of eumelanin: absorption spectrum, chromophoric species
Chemical disorder and geometric disorder models have been proposed to explain the broad optical absorption spectrum of eumelanin. The chemical disorder model posits that eumelanin is made of many chemically distinct species such that its broadband absorption spectrum results from the convolution over the spectra of these species.[ Reference Tran, Powell and Meredith 29 ] However, it has been reported that the spectrum is affected by interactions among eumelanin protomolecules.[ Reference Stark, Gallas, Zajac, Eisner and Golab 30 ] Recently, Buehler and co-workers, considering excitonic couplings between eumelanin oligomers (tetramers, pentamers, and octamers), found that the computational spectra with geometric (packing) disorder of a single species of DHI oligomers (Fig. 3) agree with experiments, and that excitonic couplings among eumelanin protomolecules have a considerable role in the increase of the probability of absorption toward the higher energy end of the spectrum.[ Reference Chen, Chuang, Cao, Ball, Ruch and Buehler 18 ]
Electrical response of eumelanin: amorphous semiconductor model, mixed ionic–electronic conduction, electrochemical interfacial processes, and energy storage
Biologic materials, such as proteins, peptides, and melanin, occur naturally in hydrated environments, such that their electrical response includes an important contribution from water-assisted proton transport.[ Reference Powell and Rosenberg 31 – Reference Hemmatian, Keene, Josberger, Miyake, Arboleda, Soto-Rodríguez, Baneyx and Rolandi 34 ] The electrical properties of eumelanin have fascinated scientists since the late 1960s. After the observation of a reversible resistive switching in eumelanin pellets reported in 1974 by McGinness et al.,[ Reference McGinness, Corry and Proctor 35 ] the amorphous semiconductor model was adopted to explain the strong hydration dependence of the conductivity. This model considered the increase of the dielectric constant of eumelanin pellets with the increase of the humidity level to explain the decreased activation energy for charge-carrier hopping. Recently, Mostert and co-workers demonstrated that the amorphous semiconductor model does not properly describe the hydration-dependent conductivity of eumelanin pellets. The presence of a comproportionation equilibrium between the reduced (hydroquinone) and oxidized (quinone) forms of eumelanin to give two semiquinones (intermediate redox species) (Fig. 1) would make eumelanin a mixed ionic–electronic material.[ Reference Sealy, Felix, Hyde, Swartz and Pryor 21 , Reference Mostert, Powell, Pratt, Hanson, Sarna, Gentle and Meredith 23 , Reference Rienecker, Mostert, Schenk, Hanson and Meredith 36 ] Wünsche et al. proposed an explanation of the charge-carrier transport properties of hydrated eumelanin thin films included between metal electrodes with an interplay between proton migration, interfacial metal electrode/eumelanin charge transfer (redox) processes, and electronic transport[ Reference Wünsche, Deng, Kumar, Di Mauro, Josberger, Sayago, Pezzella, Soavi, Cicoira, Rolandi and Santato 37 , Reference Wünsche, Cicoira, Graeff and Santato 38 ] (Fig. 4). The favorable proton conduction properties of eumelanin have been recently used to demonstrate melanin-based supercapacitors, working in slightly acidic aqueous media, in the absence of metal cations with a well-established affinity for melanin.[ Reference Kumar, Di Mauro, Zhang, Pezzella, Soavi, Santato and Cicoira 39 ]
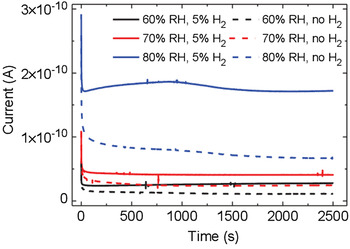
Figure 4. Transient current measurements of a eumelanin film (d = 50 nm) with Pd electrodes (electron injecting) and PdHx (proton and electron injecting) contacts (L = 9 µm, W = 20 µm) at 60, 70, and 80% relative humidity (RH). The applied bias is 0.5 V. Reprinted with permission from Ref. Reference Wünsche, Deng, Kumar, Di Mauro, Josberger, Sayago, Pezzella, Soavi, Cicoira, Rolandi and Santato37. American Chemical Society, 2015.
Eumelanin and metals: ion chelation in biologic systems, eumelanin/metal electrode interfaces for memory devices, self-assembly on metallic surfaces
The affinity of melanin with pharmaceutical organic compounds has been intensively studied in the past 60 years.[ Reference Borges, Roberts, Wilkins and Rollins 40 ] Its role in the accumulation and release of metal cations in vivo, such as calcium,[ Reference Panessa and Zadunaisky 7 , Reference Hong and Simon 41 ] copper,[ Reference Andrzejczak and Buszman 42 ] zinc,[ Reference Andrzejczak and Buszman 42 , Reference Borovanský 43 ] manganese,[ Reference Lydén, Larsson and Lindquist 44 ] iron, and other metals,[ Reference Andrzejczak and Buszman 42 , Reference Larsson and Tjälve 45 , Reference Saini and Melo 46 ] has received great attention, too. The uranyl oxycation has been used to reveal, by scanning electron microscopy (SEM), the presence of eumelanin deposited on carbon-based electrodes in supercapacitors.[ Reference Kumar, Di Mauro, Zhang, Pezzella, Soavi, Santato and Cicoira 39 ]
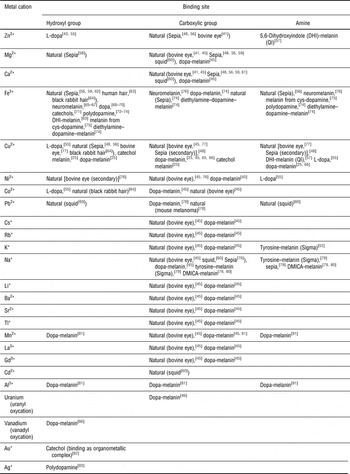
The primary binding site for a specific cation with respect to a building block is its most favorable group for binding; if a primary binding site is already occupied, binding will then take place at a secondary binding site.[ Reference Hong and Simon 47 ] Ion binding includes coulombic electrostatic interactions as well as coordination, possibly through chelation (multidentate binding, from Greek χηλή, chelè, meaning “claw”). The amine and catechol hydroxyl groups of DHI and DHICA as well as the carboxylic group of DHICA can work as primary or secondary binding sites for metal cations[ Reference d'Ischia, Napolitano, Pezzella, Meredith and Sarna 11 , Reference Hong and Simon 48 ]; factors determining the binding site for each cation are mainly the type of eumelanin (natural or synthetic) and the pH (Table I). Crucial features of melanogenesis, such as the rate of formation of eumelanin[ Reference Sutter and Birch 49 ] and the relative percentage of the two indolic building blocks present (DHI versus DHICA),[ Reference Palumbo, Solano, Misuraca, Aroca, Garcia Borron, Lozano and Prota 50 , Reference Palumbo, D'Ischia, Misuraca, Prota and Schultz 51 ] are influenced by the presence of metal ions. Whether their influence on self-assembly and structure is significant[ Reference Albano, Di Mauro, Kumar, Cicoira, Graeff and Santato 52 , Reference Gallas, Littrell, Seifert, Zajac and Thiyagarajan 53 ] or not[ Reference Liu and Simon 54 ] is still a matter of debate.
Table I. Molecular sites used by the indicated metal cations to bind to the melanin biopigments (synthetic and natural); dopa, 3,4-dihydroxyphenyl-alanine; 5,6-dimethoxyindole-2-carboxylic acid (DMICA)-melanin, synthetic melanin-like material from the oxidative polymerization of DMICA.
Neuromelanin, another class of melanins, is a black insoluble pigment found in some (but not all) dopaminergic neurons of three regions of the brain: the “substantia nigra” of the midbrain (where it derives from dopamine), the “locus coeruleus” in the pons and small regions of the “medulla oblongata” (where it derives from noradrenaline) (Fig. 5).[ Reference Schroeder, Double and Gerber 84 ] Neuromelanin has been reported to have a dichotomous role (adverse or protective): beneficial when it reduces the oxidative stress in the brain due to its ability to bind cations; detrimental when it exacerbates the oxidative stress releasing H2O2 or by reducing redox-active metals to a more reactive state.[ Reference Zucca, Segura-Aguilar, Ferrari, Muñoz, Paris, Sulzer, Sarna, Casella and Zecca 85 – Reference Sarna and Swartz 87 ] Particular efforts have been made in understanding the binding of iron to neuromelanin,[ Reference Larsson and Tjälve 76 , Reference Shima, Sarna, Swartz, Stroppolo, Gerbasi and Zecca 88 – Reference Zecca, Gallorini, Schünemann, Trautwein, Gerlach, Riederer, Vezzoni and Tampellini 95 ] as iron was reported in dopaminergic neurons of Parkinsonian brains.[ Reference Ben-Shachar and Youdim 8 ] Recently, Sepia melanin has been identified as a suitable model to describe the binding characteristics of neuromelanin.[ Reference Schroeder, Double and Gerber 84 ]
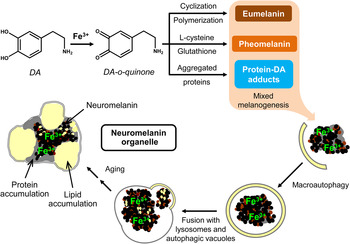
Figure 5. Possible neuromelanin formation mechanism. Reprinted with permission from Ref. Reference Zucca, Segura-Aguilar, Ferrari, Muñoz, Paris, Sulzer, Sarna, Casella and Zecca85. Elsevier, 2015.
Lately, some studies addressed the interactions between melanin and metal electrodes,[ Reference Wünsche, Cardenas, Rosei, Cicoira, Gauvin, Graeff, Poulin, Pezzella and Santato 96 , Reference Di Mauro, Carpentier, Yáñez Sánchez, Ignoumba Ignoumba, Lalancette-Jean, Lefebvre, Zhang, Graeff, Cicoira and Santato 97 ] after decades of focus on the melanin–metal cation affinity.[ Reference Hong and Simon 47 ] Hydrated eumelanin thin films proved able to promote the formation of tree-shaped electrically conductive bridges, dendrites, when in contact with gold electrodes, under electrical bias, in a planar configuration.[ Reference Wünsche, Cardenas, Rosei, Cicoira, Gauvin, Graeff, Poulin, Pezzella and Santato 96 ] Once the dendrites connected one electrode to the other, the resistivity plummeted: a resistive switch took place. The factors playing a determining role are: the chelating groups of indolic building blocks, the electrical bias, the amount of water, and chlorides, whose presence is highly likely in biomaterials. The amount of water in the thin films was proven to determine two types of switches, standard and hybrid, differing by the orders of magnitude of the current increase at the resistive switch as well as by the dendrite composition (pure gold versus gold–eumelanin complexes)[ Reference Di Mauro, Carpentier, Yáñez Sánchez, Ignoumba Ignoumba, Lalancette-Jean, Lefebvre, Zhang, Graeff, Cicoira and Santato 97 ] (Fig. 6).

Figure 6. Scanning electron microscopy (SEM) image of dendrites bridging one electrode to the other after 3 h of electrical bias at 1 V in a thin film of Sepia eumelanin (7 wt.% Cl−), hydrated for 1 h at 90% relative humidity (RH), deposited between Au electrodes (10 µm interelectrode distance). The resistive switch took place after 34 min. SEM voltage 5 kV. Reprinted with permission from Ref. Reference Di Mauro, Carpentier, Yáñez Sánchez, Ignoumba Ignoumba, Lalancette-Jean, Lefebvre, Zhang, Graeff, Cicoira and Santato97. Royal Society of Chemistry, 2016.
The mechanism of formation of the electrically conductive bridges in hydrated eumelanin thin films is strikingly similar to the working mechanism of resistive switching devices based on electrochemical metallization (electrochemical metallization memory cells).[ Reference Valov, Waser, Jameson and Kozicki 98 ] Here, melanin could be considered as biodegradable ion conductor,[ Reference Bettinger, Bruggeman, Misra, Borenstein and Langer 99 ] for the development of “green” non-volatile resistive switching memories.
The binding of the biopigment to monovalent and multivalent cations has been recently exploited in melanin-based electrochemical storage devices.[ Reference Kim, Wu, Chun, Whitacre and Bettinger 58 , Reference Kim, Wu, Chun, Whitacre and Bettinger 79 ]
Polydopamine, a synthetic melanin analog, has been used to selectively extract and separate metal cations from aqueous solutions, leveraging on the different binding affinities of the pigments toward different cations.[ Reference Park, Kim, Kwon, Klosterman and Bettinger 67 , Reference Yu, Liu, Liu and Zhou 100 , Reference Farnad, Farhadi and Voelcker 101 ] Films of polydopamine can reduce Ag+ cations into Ag nanoparticles, which impart antibacterial properties to the same films.[ Reference Ball, Nguyen, Haupt, Oehr, Arnoult, Toniazzo and Ruch 83 ]
Self-assembled molecular networks of indole-2-carboxylic acid (I2CA, i.e., melanin's monomer DHICA lacking the two hydroxyl phenolic groups) have been investigated on Au (111) surfaces: I2CA showed, both in ultrahigh vacuum and at liquid/solid interfaces, hydrogen-bonded assemblies from molecular dimers,[ Reference De Marchi, Cui, Lipton-Duffin, Santato, MacLeod and Rosei 102 ] the next step being the same study with DHI and DHICA. This study represents an important contribution in understanding, at the nanoscale, the process of formation of melanin, from the building blocks to the polymers.
Melanin/metal oxides interfaces: adhesion, biocompatibility, and photosensitization technologies
The underpinning of emerging technologies making use of interfaces between melanin and metal oxides is melanin biocompatibility together with its optical absorption and charge-carrier transport properties. Such interfaces are largely undiscovered since the poor processability of melanin thwarts contact between the pigment and the surface of the oxides. Nevertheless, promising results have been obtained with synthetic melanin-like polymers, e.g., polydopamine.[ Reference Liu, Ai and Lu 10 , Reference Nam, Kim, Ko, Jin, Kim and Jung 103 ]
Adhesive proteins (rich in dopa and lysine amino acids) present in Mytilus edulis (mussels) inspired a method of making polydopamine coatings, featuring catechol (ortho-hydroquinone of dopa) and amine (lysine) functions, through simple dip coating in aqueous solutions of dopamine on a wide range of materials, including metal oxides.[ Reference Lee, Dellatore, Miller and Messersmith 9 , Reference Lee, Scherer and Messersmith 104 ]
Based on its ion-chelating properties, polydopamine has been used as a template to assemble nanoarchitectures[ Reference Yan, Yang, Lin, Ma, Lu and Lee 105 , Reference Yao, Zhao, Kong, Wu, Zhou and Lu 106 ] and core (inorganic)-shell (polydopamine) nanoparticles.[ Reference Liu, Ai and Lu 10 , Reference Yue, Wang, Sun, Wang, Wang, Deng and Zhao 107 ] Fe3O4-polydopamine core–shell nanoparticles have been used for a wide range of technologic applications,[ Reference Si and Yang 108 , Reference Gu, Zhang, Sun, Song, Fu and Dong 109 ] including peroxidase-like catalysis for treatment of industrial waste and environmental monitoring.[ Reference Liu, Fu, Wang, Yan, Xin, Cai and Xu 110 ] Superhydrophobic and superoleophilic particles, obtained by modification with low surface energy materials of Fe3O4 on polydopamine particles have been used for oil/water separation.[ Reference Zhang, Li and Dang 111 ] Iminodiacetic acid-Cu functionalized core–satellite Fe3O4/polydopamine/Au magnetic nanocomposites have been used for protein detection.[ Reference Zhang, He, Chen and Zhang 112 ]
Multifunctional nanocomposites can integrate sensing, diagnostic, and therapeutic functions into a single nanostructure. Fe3O4–polydopamine core–shell nanocomposites have been fabricated through in situ polymerization. Their ability to act as theranostic agents for intracellular mRNA detection and multimodal imaging-guided photothermal therapy has been reported.[ Reference Lin, Cong, Cao, Ke, Peng, Gao, Yang, Liu and Chen 113 , Reference Ma, Ding, Yao and Jia 114 ] This work exploited polydopamine near-infrared absorption, high fluorescence quenching efficiency and the availability of a surface for further functionalization with biomolecules.
Graeff and co-workers prepared melanin-like/V2O5-layered hybrids for application in optoelectronics and electrochemistry, starting from dopa.[ Reference Oliveira, Graeff, Brunello and Guerra 115 ] The presence of melanin-like units induced the reduction of V5+ ions to V4+ ions. The melanin insertion was observed to increase the stability and reproducibility of Li+ electrochemical insertion/de-insertion.
Well-investigated metal oxide semiconductors, such as TiO2, have been coupled to melanin or melanin-like polymers for applications in solar energy conversion/storage. Purified natural melanin from squid ink and mussel-inspired synthetic polydopamine have been used as dyes in dye-sensitized solar cells.[ Reference Nam, Kim, Ko, Jin, Kim and Jung 103 , Reference Lee, Cho, Nakayama, Sekino, Tanaka, Minato, Ueno, Suzuki, Suematsu, Tokoi and Niihara 116 ] Polydopamine/TiO2 nanocomposites and plasmonic structures fabricated exploiting the reducing properties of polydopamine have been used for photocatalytic applications.[ Reference Feng, Zhang, Wang, Liao, Xi and Chen 117 – Reference Loget, Yoo, Mazare, Wang and Schmuki 119 ]
The biocompatibility and optical properties featured by TiO2 have been used for self-cleaning, self-sterilization, and photolithography purposes[ Reference Soliveri, Pifferi, Panzarasa, Ardizzone, Cappelletti, Meroni, Sparnacci and Falciola 120 , Reference Panzarasa, Soliveri, Sparnacci and Ardizzone 121 ] and also in fields such as cosmetics, where TiO2 has been used as filler and UV-filter.[ Reference Auffan, Pedeutour, Rose, Masion, Ziarelli, Borschneck, Chaneac, Botta, Chaurand, Labille and Bottero 122 ] To ensure transparency to cosmetic formulations, the average dimension of TiO2 particles has been typically of the order of 10–20 nm. Safety concerns about nano-TiO2-based products have been raised.[ Reference Serpone, Dondi and Albini 123 – Reference Carlotti, Ugazio, Sapino, Fenoglio, Greco and Fubini 125 ] Reports suggest that TiO2 nanoparticles do not penetrate the intact epidermal barrier,[ Reference Kiss, Bíró, Czifra, Tóth, Kertész, Szikszai, Kiss, Juhász, Zouboulis and Hunyadi 126 , Reference Senzui, Tamura, Miura, Ikarashi, Watanabe and Fujii 127 ] with no evidence of significant penetration beyond the stratum corneum.[ Reference Newman, Stotland and Ellis 128 ] Melanin is used in cosmetic products, sometimes in combination with photoactive oxides. Melanin (both synthetic and natural)–TiO2 interactions have been investigated,[ Reference Vitiello, Pezzella, Zanfardino, Varcamonti, Silvestri, Costantini, Branda and Luciani 129 – Reference Lee, Kang, Benayad, Lee, Ahn, Kim, Stefanovich, Park and Yoo 131 ] in particular the photocatalytic activity of TiO2 on the process of DHICA polymerization (Fig. 7).[ Reference Vitiello, Pezzella, Calcagno, Silvestri, Raiola, D'Errico, Costantini, Branda and Luciani 132 ]

Figure 7. Photocatalytic activity of TiO2 for 5,6-dihydroxyindole-2-carboxylic acid (DHICA) polymerization and formation of melanin–TiO2 hybrid nanostructures with biocide behavior. Adapted with permission from Ref. Reference Vitiello, Pezzella, Calcagno, Silvestri, Raiola, D'Errico, Costantini, Branda and Luciani132. American Chemical Society, 2016.
To conclude this Prospective article highlighting the exciting achievements reported in the field of melanin-based materials in recent years, we would like to emphasize the importance of an interdisciplinary approach for melanin research, where materials chemistry, physical chemistry, and materials physics all must be considered to effectively advance both the understanding of fundamental process in electrochemistry, photophysics, and transport physics, and the development of sustainable technologies. We believe this holistic approach will contribute to the advancement of knowledge about the functional properties of melanin-based materials in biologic systems.
At the fundamental level, exciting challenges loom for the melanin research community, such as (i) understanding the electron transfer processes in different electrolytes for sensing (and biosensing) as well as energy conversion/storage; (ii) advancing the knowledge of electron transport and proton transport in quasi-solid state for applications in (bio)electronics, e.g., protonic devices, edible devices, and transient electronics; (iii) gaining further insights into the nature of chromophoric units of melanin for photoprotection and solar energy conversion; (iv) establishing biocompatibility and biodegradability according to international standards; and (v) quantifying the physicochemical parameters defining ion binding for ion separation and water treatment.
Acknowledgments
The authors thank Mrs. J. Yelon, Mr. J. Villeneuve, Professors A. Yelon and F. Cicoira for valuable discussions. C.S. acknowledges financial support from FQRNT (Équipe), Québec MESI PSR-SIIRI as well as NSERC (D.G.). G.S. acknowledges financial support from FQRNT through a PBEEE Fellowship.











