Non-technical Summary
Fossil soft-bodied worms such as nematodes are rare, and, because they often have few features preserved, difficult to interpret. A number of worm-like specimens from the late Carboniferous Mazon Creek fossil site were originally identified as a species of free-living nematode, called Nemavermes mackeei, which was among the oldest and largest fossil free-living nematodes. Here we reinvestigate these specimens, and determine that they encompass multiple species, including worms (but not free-living nematode worms) and cyclostome fish. In particular, some of these specimens belong to a new species of cyclostome fish, which we name Squirmarius testai.
Introduction
Nemavermes mackeei Schram, Reference Schram1973 is an extinct, soft-bodied, vermiform animal that has been found in both the ~308.6–308.4 Ma (Montañez et al., Reference Montañez, McElwain, Poulsen, White, DiMichele, Wilson, Griggs and Hren2016) Mazon Creek fossil site in Illinois, USA (Schram, Reference Schram1973, Reference Schram and Nitecki1979) and the ~330.3–323.4 Ma (Grogan and Lund, Reference Grogan and Lund2002) Bear Gulch Limestone in Montana, USA (Schram, Reference Schram1977, Reference Schram and Nitecki1979). It varies widely in size, ranging from 40–140 mm long and 3–16 mm wide, and was described originally as having a sparse coating of hairs or setae and well-developed labial papillae (Schram, Reference Schram1973). Based on these features, N. mackeei was interpreted to be a free-living marine nematode (Schram, Reference Schram1973), making it one of the oldest and largest fossil free-living nematodes (Poinar, Reference Poinar2011).
Nemavermes mackeei is an element of a nearshore marine chronofauna with characteristic taxa and ecological structure that was relatively stable throughout the Carboniferous (Schram, Reference Schram and Nitecki1979) and might have continued into the Triassic (Briggs and Gall, Reference Briggs and Gall1990). This chronofauna is characterized by malacostracan crustaceans, worms, horseshoe crabs, and pelecypods (Johnson and Richardson, Reference Johnson and Richardson1966; Schram, Reference Schram and Nitecki1979; Baird and Maples, Reference Baird, Maples, Shabica and Hay1997; Grogan and Lund, Reference Grogan and Lund2002), and is distinct from the classic offshore Carboniferous marine fauna, which consists of shelly animals, e.g., corals, attached echinoderms, foraminiferans, ostracods, and stony bryozoans (Baird, Reference Baird, Shabica and Hay1997; Grogan and Lund, Reference Grogan and Lund2002; Clements et al., Reference Clements, Purnell and Gabbott2019). This nearshore marine chronofauna is preserved in Carboniferous fossil sites across North America and Europe, with some variability due to age (there is some faunal turnover) and preservational quality (Schram, Reference Schram and Nitecki1979; Baird et al., Reference Baird, Sroka, Shabica and Beard1985). The brackish-to-marine Essex assemblage from the Mazon Creek fossil site (which also preserves the freshwater/terrestrial Braidwood assemblage) and the Bear Gulch limestone—the two sites from which N. mackeei is known—exhibit some of the best preservation of this chronofauna, and therefore have some of the most diverse fossil assemblages (Schram, Reference Schram and Nitecki1979).
Here we reinvestigate the morphology of specimens identified as Nemavermes mackeei from the Mazon Creek fossil site. Based on the wide morphological variation, these specimens belong to multiple species, none of which is likely to have been a free-living marine nematode due to their large size and lack of nematode features.
Materials and methods
Specimens
Twelve specimens from the Invertebrate Paleontology collection at the Field Museum of Natural History in Chicago, Illinois, USA identified as Nemavermes mackeei were included in this study. These specimens were selected for the study because they either (1) exhibited diagnostic features from the original description of N. mackeei, (2) preserved distinctive features that would likely allow them to be identified, or (3) they cover the range of morphologies included in specimens identified as N. mackeei. During the course of the study, some of these specimens were identified as chordates (see Systematic paleontology and Results sections below) and were moved to the Vertebrate Paleontology collection at the Field Museum and given new numbers. Of these specimens, three were originally identified as N. mackeei in the original description of this species (Schram, Reference Schram and Nitecki1979), one was figured as N. mackeei in the ‘Richardson's Guide to The Fossil Fauna of Mazon Creek’ (Fitzhugh and Sroka, Reference Fitzhugh, Sroka, Shabica and Hay1997), and two were figured as N. mackeei in the book ‘The Mazon Creek Fossil Fauna’ (Wittry, Reference Wittry2012). The other six were presumably identified as N. mackeei by a curator or collections manager at the Field Museum.
One specimen from the Invertebrate Paleontology collection at the Royal Ontario Museum in Toronto, Ontario, Canada, presumably identified as a nematode by a collections manager or curator at the Royal Ontario Museum, was also included in this study. Nemavermes mackeei is the only nematode described from the Mazon Creek fossil site, and this specimen exhibited close morphological similarities to one specimen of N. mackeei from the Field Museum.
Two additional specimens were also included in this study. Both specimens were originally identified as Gilpichthys greenei Bardack and Richardson, Reference Bardack and Richardson1977 rather than Nemavermes mackeei, but they have a number of features that are very similar to those in specimens identified as N. mackeei from the Field Museum. One specimen was from the collection at the Lauer Foundation for Paleontology, Science, and Education in Wheaton, Illinois. The other specimen was from the David and Sandra Douglass collection and was generously donated to the Lauer Foundation for Paleontology, Science and Education in Wheaton, Illinois, so that it could be included in this study.
All specimen images are of dry specimens under normal light. Photographs of the whole concretions and entire specimens were mostly taken using the camera on a Samsung Galaxy S9®; two images (indicated in figure captions) were captured with a Nikon D60 camera. Key morphological features of each specimen were imaged using a Keyence VHX-7000 imaging microscope. Measurements for each specimen were taken from photographs using ImageJ (Schneider et al., Reference Schneider, Rasband and Eliceiri2012).
The specimens in this study were collected from various localities of the Mazon Creek fossil site: FMNH PE 21550 from the Peabody Coal Company, Northern Mine, Pit 11, coordinates 41.20119 and -88.215825; FMNH PF 17810 from Pit 11, coordinates 41.205305 and -88.239197; FMNH PE 93336, 93403, and 93402 from Pit 11, Peabody Coal Company Northern Mine NE 1/4 SW 1/4 SE 1/4, Sec. 5, T31N, R9E; FMNH PE 21551 from Kankakee county, Pit 11, Peabody Coal Company NE 1/4 of SW 1/4 of sec 8, T 31 N, R 9 E; FMNH PE 93404 from Pit 11, Peabody Coal Company Northern Mine; FMNH PF 17811, 17812, 17809, 17808, and ROM IP 47528 from Pit 11 with no further locality information; FMNH PE 24846 from the Peabody Coal Company, Northern Mine, Pit 1, coordinates 41.3174 and -88.267; LF 2101 from along the Mazon River, but with no more specific locality information; LF 5664 collected by David Douglass from the South Wilmington Sportsmen's Club, part of Pit 11, coordinates 41.20287300 and -88.20194900.
Scanning electron microscopy
Specimens FMNH PF 17809, 17810, 17811, 17808, and LF 2101 were analyzed using a JEOL JSM-6460LV scanning electron microscope (SEM) in the Advanced Analysis Facility at the University of Wisconsin-Milwaukee. Specimens were not coated. SEM imaging was conducted in BSE mode at 15 kV and in SE mode at 10 kV.
Repositories and institutional abbreviations
All but three specimens examined during this study are deposited in the invertebrate paleontology (PE) or vertebrate paleontology (PF) collections of the Field Museum of Natural History (FMNH), Chicago, Illinois, USA. One specimen is deposited in the invertebrate paleontology (IP) collection of the Royal Ontario Museum (ROM), Toronto, Ontario, Canada. Two specimens are deposited in the Lauer Foundation (LF) for Paleontology, Science, and Education, Wheaton, Illinois, USA.
Systematic paleontology
Vertebrata Linnaeus, Reference Linnaeus1758
Cyclostomi Duméril, Reference Duméril1806
Genus Squirmarius new genus
Type species
Squirmarius testai new genus new species, by monotypy.
Diagnosis
As for the type species, by monotypy.
Etymology
Masculine, meaning ‘one who squirms’; many specimens of this genus were collected by Tom Testa, who referred to them informally as ‘squirms.’
Occurrence
As for the type species, by monotypy.
Remarks
As for the type species, by monotypy.
Squirmarius testai new species
Figures 1–7; Table 1

Figure 1. Specimens of Squirmarius testai n. gen. n. sp.: (1) holotype, FMNH PF 17809; (2) FMNH PF 17810; (3) FMNH PF 17812; (4) FMNH PF 17811; (5) LF 2101; (6) LF 5664, which was collected by David Douglass and donated by the David and Sandra Douglass collection to the Lauer Foundation for Paleontology, Science, and Education in Wheaton Illinois for this study. Scale bars = 10 mm.

Figure 2. Detailed morphology of Squirmarius testai n. gen. n. sp., holotype, FMNH PF 17809: (1) whole body with boxes indicating regions that correspond to (2) box A, (3) box B, and (4) box C, and dots indicating the spots that correspond to (5) dot D and (6) dot E; also see Figure 1.1 for an image of this specimen without boxes and dots, photo taken with Nikon D60; (2) head, box A in (1), showing the blunt point at the anterior end of the body, with two eyes preserved as dark ovals with a white dot in the right eye that might represent a lens; (3) gut near the middle of the body, box B in (1), showing the flat kaolinite preservation; (4) tail, box C in (1), showing the narrow point at the posterior end of the body, the gut with dark preservation ending before the posterior point of the tail (white arrow), and the lack of a tail fin; (5) SEM image in SE mode of the leftmost eye, dot D in (1), showing ovoid melanosomes along with siderite crystals with molds of ovoid melanosomes; (6) SEM image in BSE mode of the gut with dark preservation in the tail, dot E in (1), with siderite crystals without melanosome molds, and abundant pyrite microcrystals and occasional pyrite framboids. Scale bars = 10 mm (1); 2 mm (2–4); 10 μm (6); 5 μm (5).

Figure 3. Detailed morphology of Squirmarius testai n. gen. n. sp., FMNH PF 17812: (1) whole body with boxes indicating the regions that correspond to (2) box A, (3) box B, (4) box C, and (5) box D; see also Figure 1.3 for this specimen without boxes; (2) head or anteriormost preserved part of the body, box A in (1), showing the mottled dark coating and two irregular, differently sized dark patches that are unlikely to be eyes; (3) triangular or C-shaped organ, box B in (1), directly anterior to gut; note also the dark mottled covering; (4) body region, box C in (1), showing dark mottled covering and 3D gut; (5) tail, box D in (1), showing the dark mottled covering, the gut ending before the tip of the tail (white arrow), and nothing that clearly represents a tail fin. Scale bars = 10 mm (1); 2 mm (2–5).

Figure 4. Detailed morphology of Squirmarius testai n. gen. n. sp., LF 2101: (1) whole specimen with boxes indicating the regions that correspond to (2) box A, with dot C indicating the spot that corresponds to (4) and (5); and (3, 4) box B; see Figure 1.5 for an image of this specimen without dots and boxes, image courtesy of the Lauer Foundation; note the very solid dark coating over almost all of the specimen and the gut indicated by a white arrow; (2) head, box A in (1); note the lighter inner circle in the rightmost eye that might represent a lens; (3) tail, box B in (1); (4) same image as in (3), with arrows indicating the faint annulations; (5) SEM of eye in BSE mode, dot C in (1), near its outer edge, showing siderite crystals with molds of ovoid melanosomes; (6) SEM of eye in SE mode, dot C in (1), near its center, showing preserved ovoid melanosomes. Scale bars = 10 mm (1); 2 mm (2–4); 5 μm (5); 1 μm (6).

Figure 5. Detailed morphology of Squirmarius testai n. gen. n. sp., FMNH PF 17810; note that the number visibly written on the specimen is an old number: (1) whole body with boxes indicating the regions that correspond to (2) box A, (3) box B, (4) box C, and a dot indicating the spot that corresponds to (4) and (5), and with white arrows indicating segment preservation; see also Figure 1.2 for this specimen without boxes and dot; (2) head, box A in (1), showing one eye preserved as a dark brown oval and a dark decay halo around the edges; (3) body, box B in (1), showing the triangular or C-shaped organ just anterior to the gut (black arrow); (4) detail of segment preservation, box C in (1), with white arrows indicating segments; (5) SEM of eye in SE mode, dot in (1), with melanosomes; (6) SEM of eye in SE mode, dot in (1), showing abundant molds of ovoid melanosome and a few melanosomes. Scale bars = 10 mm (1); 2 mm (2–4); 5 μm (5, 6).

Figure 6. Detailed morphology of Squirmarius testai n. gen. n. sp., LF 5664, collected by David Douglass and donated by the David and Sandra Douglass collection to the Lauer Foundation for Paleontology, Science, and Education for this study: (1) whole body with boxes indicating the regions that correspond to (2) box A, (3) box B, and (4) box C; see also Figure 1.6 for this specimen without the boxes; (2) head, box A in (1), showing one well-preserved eye and one poorly preserved eye; note also various dark and light patches and decay halo; (3) gut and segments (white arrows), box B in (1); (4) posteriormost preserved end of body, box C in (1), note that this specimen is likely missing at least the tip of the tail, but that the gut ends before the end of the body (white arrow). Scale bars = 10 mm (1); 2 mm (2–4).

Figure 7. Detailed morphology of Squirmarius testai n. gen. n. sp., FMNH PF 17811: (1) whole body with a box indicating the region that corresponds to (2) box A, and a dot indicating the spot that corresponds to (3) dot B; photo taken with Nikon D60; note the solid dark coating over large parts of the specimen, and the dark patches in the head (at left); see also Figure 1.4 for this specimen without boxes; (2) segments, box B in (1); (3) SEM of the dark coating in BSE mode, dot B in (1), showing abundant pyrite microcrystals and occasional pyrite framboids. Scale bars = 10 mm (1); 2 mm (2); 10 μm (3).
Holotype
FMNH PF 17809; Pennsylvanian (~308.6–308.4 Ma), Francis Creek Shale, Mazon Creek, Illinois, USA.
Diagnosis
Cyclostome with an elongate body (L/W > 20), small (~0.75 mm long, 0.5 mm wide) pigmented eyes, and no fins.
Occurrence
Pit 11, Francis Creek Shale, Pennsylvanian (~308.6–308.4 Ma), Mazon Creek fossil site, Illinois, USA. Preserved in siderite concretions in gray shale.
Description
Elongate (L/W > 20; Table 1) vermiform body with the soft tissues of the whole body preserved as a light-brown shape on the concretion (Fig. 1). In FMNH PF 17809 (Fig. 2), 17812 (Fig. 3), and LF 2101 (Fig. 4), it is apparent that the body tapers to a narrow point at the posterior end (Figs. 2.4, 3.5, 4.3). In FMNH PF 17809 (Fig. 2), LF 2101 (Fig. 4), FMNH PF 17810 (Fig. 5), and LF 5664 (Fig. 7), the body tapers to a blunter point at the anterior end (Figs. 2.2, 4.2, 5.2).
Table 1. Length and width measurements for specimens of Squirmarius testai n. gen. n. sp. NA = not available.

There are no fins preserved in any specimen. All specimens do include the regions of the body where paired fins would occur (Fig. 1), suggesting that Squirmarius testai n. gen. n. sp. did not have paired fins. FMNH PF 17809 (Fig. 2), 17812 (Fig. 3), and LF 2101 (Fig. 4) preserve the posteriormost end of the tail, and nothing that can definitively be identified as a tail fin is visible (Figs. 2.4, 3.5, 4.3). Another chordate from the Mazon Creek, the stem-hagfish (Miyashita et al., Reference Miyashita, Gess, Tietjen and Coates2021) Gilpichthys greenei, also has been described as having no fins (Bardack and Richardson, Reference Bardack and Richardson1977), indicating that it was either finless or does not commonly have fins preserved. In some species from the Mazon Creek that are known to have a tail fin, e.g., the lamprey Mayomyzon pieckoensis Bardack and Zangerl, Reference Bardack and Zangerl1968 and the hagfish Myxinikela siroka Bardack, Reference Bardack1991, the tail fin is not apparent in some specimens, typically those that are dorsoventrally flattened or that have a poorly preserved tail region (e.g., Miyashita, Reference Miyashita2020, fig. 3; Miyashita et al., Reference Miyashita, Gess, Tietjen and Coates2021, fig. 3). Most likely, S. testai n. gen. n. sp. had no tail fin, although we cannot rule out a small tail fin that did not preserve.
FMNH PF 17809 (Fig. 2), LF 2101 (Fig. 4), FMNH PF 17810 (Fig. 5), and LF 5664 (Fig. 6) have eyes preserved as dark brown-black ovals on both the part and counterpart (Figs. 1, 2.1, 2.2, 4.1, 4.2, 5.1, 5.2, 6.1, 6.2). FMNH PF 17809 has both eyes preserved, which are 0.73 and 0.83 mm in length and 0.52 mm in width (Fig. 2.2); one of these eyes has a circular spot in the middle infilled with white kaolinite that might represent a lens (Fig. 2.2) similar to that seen in Tullimonstrum gregarium Richardson, Reference Richardson1966 and the chondrichthyan Bandringa rayi Zangerl, Reference Zangerl1969 (Clements et al., Reference Clements, Dolocan, Martin, Purnell, Vinther and Gabbott2016). SEM analysis of the eyes revealed abundant spherical to ovoid microbodies and molds of microbodies, ~0.5-1 μm in diameter (Fig. 2.5). These microbodies are the size and shape of melanosomes, which fossilize readily and are commonly observed from a wide variety of fossil sites (Vinther, Reference Vinther2016). Specifically, these microbodies are the same size and shape as the oblate or elliptical melanosomes preserved in other Mazon Creek chordates, including the eyes of T. gregarium, Esconicthys apopyris Bardack, Reference Bardack1974, Elonichthys peltigerus Newberry, Reference Newberry1856, B. rayi, Mayomyzon pieckoensis, and Myxinikela siroka (Clements et al., Reference Clements, Dolocan, Martin, Purnell, Vinther and Gabbott2016; Gabbott et al., Reference Gabbott, Donoghue, Sansom, Vinther, Dolocan and Purnell2016), and the pigmented stripes along the body of Mayomyzon pieckoensi (see Gabbott et al., Reference Gabbott, Donoghue, Sansom, Vinther, Dolocan and Purnell2016), suggesting that these microbodies were in fact melanosomes. In these other Mazon Creek specimens, moldic melanosome preservation was not described; this has previously been reported at other sites (e.g., Li et al., Reference Li, Gao, Vinther, Shawkey, Clarke, D'Alba, Meng, Briggs and Prum2010). LF 2101 has both eyes preserved as dark brown ovals, 0.81 and 0.67 mm in length and 0.69 and 0.48 mm in width (Fig. 4.2). Both eyes have a raised spot in the center that might represent a lens (Fig. 4.2) similar to the raised dark spots interpreted as lenses in some specimens of Mayomyzon pieckoensis (see Gabbott et al., Reference Gabbott, Donoghue, Sansom, Vinther, Dolocan and Purnell2016), although they are not highlighted by kaolinite as in FMNH PF 17809 (Fig. 2.2). They have very similar positioning on the head as in FMNH PF 17809 (Figs. 2.2, 4.2). SEM analysis of this eye revealed melanosome molds around the outside of the eye (Fig. 4.5) and preserved melanosomes in the center of the eye (Fig. 4.6), all in the same size range (~0.5-1 μm) and shape (ovoid) as in FMNH PF 17809. FMNH PF 17810 has one eye preserved as a dark brown oval, 0.63 mm long and 0.39 mm wide (Fig. 5.2). Abundant melanosomes and melanosome molds were observed in this eye (Fig. 5.5, 5.6). LF 5664 has one well-preserved eye, a dark brown oval, 0.91 mm long and 0.70 mm wide, and possibly one poorly preserved eye, a dark-brown patch of similar size and shape (Fig 6.2). FMNH PF 17812 (Fig. 3) has dark, irregular spots near the anteriormost preserved region of the body (Fig. 3.2), but these are larger and closer to the edges of the body than the preserved eyes, suggesting that they are not eyes.
Some Mazon Creek cyclostomes have preserved otic capsules, which can look similar to eyes in that they are typically a pair of circles or ovals on the head of the fossil. However, otic capsules are rarely preserved as very dark brown or black material and are more typically a medium to light brown (see, e.g., the range of preservational variation of otic capsules figured by Gabbott et al., Reference Gabbott, Donoghue, Sansom, Vinther, Dolocan and Purnell2016 and Miyashita et al., Reference Miyashita, Gess, Tietjen and Coates2021). Moreover, SEM and Energy Dispersive X-ray Spectroscopy (EDS; Gabbott et al., Reference Gabbott, Donoghue, Sansom, Vinther, Dolocan and Purnell2016) analyses reveal that the otic capsules are pyritized. The dark-brown ovals on the head of Squirmarius testai n. gen. n. sp. are not pyritized (Figs. 2.5, 4.5, 4.6, 5.5, 5.6) and their very dark color is more consistent with typical eye preservation (see, e.g., the range of cyclostome eyes figured by Gabbott et al., Reference Gabbott, Donoghue, Sansom, Vinther, Dolocan and Purnell2016; Miyashita, Reference Miyashita2020; and Miyashita et al., Reference Miyashita, Gess, Tietjen and Coates2021). Otic capsules can contain preserved statoliths that occur as spherical to ellipsoidal microbodies, ~2.5-3.0 μm in diameter (Gabbot et al., Reference Gabbott, Donoghue, Sansom, Vinther, Dolocan and Purnell2016). The microbodies in the dark ovals on the heads of some specimens of S. testai n. gen. n. sp. are ~0.5–1 μm in size and are much smaller than the preserved statoliths. Therefore, these features are interpreted as eyes with preserved melanosomes rather than otic capsules with preserved statoliths.
FMNH PF 17811 (Fig. 7), 17810 (Fig. 5), and LF 5664 (Fig. 6) have segments (myomeres) preserved. In FMNH PF 17811, these are in a region of the body ~35 mm long, near one end of the specimen (Fig. 7.1, 7.2). In FMNH PF 17810, there are patches throughout the body that preserve a few segments (Fig 5.1). In LF 5664, these are in a region of the body ~5 mm long, near the anteriormost preserved region of the gut (Fig. 6.3). In all three specimens, these are slightly darker brown stripes (~0.15 mm wide) on the light-brown body, very similar in size and style of preservation to the myomeres in Mayomyzon pieckoensis, and Gilpichthys greenei (Bardack, Reference Bardack and Nitecki1979; Miyashita, Reference Miyashita2020, fig. 6). In LF 2102, the overall dark-brown color of the fossil makes it difficult to distinguish segmentation; however, there are faint annulations near the posterior tip of the body that might represent segments (Fig. 4.3, 4.4).
Five specimens have a prominent medial feature, which corresponds to the feature described by Schram (Reference Schram1973) in the original description of Nemavermes mackeei as a detritus-filled gut; these vary somewhat in size and preservation. In FMNH PF 17812 (Fig. 3) and 17810 (Fig. 5), the gut is not much different in color than the surrounding body, but has strong relief, and is ~1 mm wide (Figs. 3.1, 3.3–3.5, 5.1, 5.3). The posterior end of the body is not preserved in FMNH PF 17810, but in 17812, the gut does not continue to the very end of the body (Fig. 3.1, 3.5). In LF 2101 (Fig. 4), the gut is similarly wide, but is light-colored (similar to the surrounding concretion, and not the white of kaolinite), and with some relief. It is not preserved at the very end of the body. In FMNH PF 17809 (Fig. 2), the gut is a thin line, 0.1–0.2 mm wide, with little relief (Fig. 2.1, 2.3, 2.4). Near the middle of the body, it is preserved as a white kaolinite film (Fig. 2.3), and more posteriorly, it is dark black (Fig. 2.4). SEM analysis of the black posterior part of the gut in FMNH PF 17809 indicates that it is preserved due to authigenic mineralization, most notably of pyrite framboids and microcrystals (Fig. 2.6). At least in this specimen, the original gut contents are not preserved and thus it was not necessarily filled with detritus. Alternatively, the smaller size and different preservation of this feature in FMNH PF 17809 could suggest that it is not a gut, but something else; the flat, light-colored preservation near the middle of the body is similar to notochord preservation in Gilpichthys greenei, Pipiscius zangerli Bardack and Richardson, Reference Bardack and Richardson1977, Mayomyzon pieckoensis, and Tullimonstrum gregarium (Bardack and Richardson, Reference Bardack and Richardson1977; Bardack, Reference Bardack and Nitecki1979; Aldridge and Donoghue, Reference Aldridge, Donoghue, Jørgensen, Lomholt, Weber and Malte1998; McCoy et al., Reference McCoy, Saupe, Lamsdell, Tarhan and McMahon2016). Regardless, this feature in FMNH PF 17809 also does not continue to the end of the tail (Fig. 2.1, 2.4). In LF 5664 (Fig. 6), the gut is similarly thick as in FMNH PF 17812 (Fig. 3) and 17810 (Fig. 5) and is ~1 mm wide. In contrast to these other two specimens, the gut in LF 5664 is preserved as a dark line (Fig. 6.1, 6.3, 6.4). The posterior end of LF 5664 is not complete, but the gut does not continue to the posteriormost preserved end of the body (Fig. 6.4).
All specimens have a number of solid, medium-brown to dark-brown patches with clearly defined edges that might represent internal organs or other internal features (Figs. 1–7). In general, these dark patches are more common in the head region (Figs. 2.2, 3.2, 4.2, 5.2, 6.1, 6.2, 7.1), where they could represent the remains of internal cartilaginous supporting tissues. Internal organs and internal cartilaginous supports are commonly preserved in Mazon Creek cyclostomes as darker brown patches on the lighter brown fossil (Bardack and Zangerl, Reference Bardack and Zangerl1968; Bardack, Reference Bardack and Nitecki1979, Reference Bardack1991; Miyashita, Reference Miyashita2020; Miyashita et al., Reference Miyashita, Gess, Tietjen and Coates2021). Most of these patches do not show easily recognizable forms that repeat among the specimens. However, one structure that occurs in both FMNH PF 17810 (Fig. 5) and 17812 (Fig. 3) is a small (~1 mm) triangular or C-shaped structure, directly anterior to the gut, and a few centimeters posterior to the head, which is most likely an element of the digestive system (Figs. 3.1, 3.3, 5.1, 5.3). There is no evidence of mineralized internal structures, e.g., bones or mineralized teeth.
All six specimens have a dark line near the edges of some or all of the body, which most likely represents a decay halo (Figs. 1–7); similar decay halos are seen in other Mazon Creek chordates (e.g., Miyashita, Reference Miyashita2020, fig. 1). In three specimens, this decay halo is continuous with a dark brown to black coating over larger patches of the body. LF 2101 has a solid, dark-brown coating over all of the body except the head and gut (Fig. 4.1). FMNH PF 17811 (Fig. 7) has a near-solid dark brown to black coating over large areas of the body (Fig. 7.1); SEM analysis reveals dense accumulations of pyrite framboids and pyrite microcrystals in this coating (Fig. 7.3). FMNH PF 17812 has a less-solid, mottled, dark coating over some of its body (Fig. 3). Dark patches in Mayomyzon pieckoensis are the remains of original color patterns, as indicated by the fact that they occur in repeated broad stripes and that they preserve melanosomes (Gabbott et al., Reference Gabbott, Donoghue, Sansom, Vinther, Dolocan and Purnell2016). However, this interpretation is unlikely for these dark coatings in Squirmarius testai n. gen. n. sp. because they do not form patterns and there is no evidence of preserved melanosomes or melanosome molds.
Etymology
Named in honor of Tom Testa, who collected many of the specimens.
Materials
Specimens FMNH PF 17809–17812, and LF 2101 and 5664.
Remarks
Most specimens that are now assigned to this species were originally attributed to Nemavermes mackeei. However, FMNH PE 21551, the holotype of N. mackeei, is a proboscis of Tullimonstrum gregarium (see Results section below), making N. mackeei a junior synonym of T. gregarium. The specimens assigned here to Squirmarius testai n. gen. n. sp. are a complete animal, and have none of the features of T. gregarium, and so they belong to a new species.
Due to its large size and lack of nematode synapomorphies, Squirmarius testai n. gen. n. sp. is unlikely to be a free-living marine nematode (Tahseen, Reference Tahseen2012; Baliński et al., Reference Baliński, Sun and Dzik2013; Sperling, Reference Sperling2013). In addition, although some nematodes have pigmented eyespots, these are only found in free-living marine forms, not parasitic forms (Bollerup and Burr, Reference Bollerup and Burr1979), making S. testai n. gen. n. sp. unlikely to be a parasitic nematode. Moreover, nematodes lack melanosomes in their eyes (Kluessendorf and Doyle, Reference Kluessendorf and Doyle2000; Clements et al., Reference Clements, Dolocan, Martin, Purnell, Vinther and Gabbott2016). Therefore, despite these specimens originally being assigned to Nemavermes mackeei, originally interpreted as a nematode (Schram, Reference Schram1973), S. testai n. gen. n. sp. is not a nematode.
The style of eye preservation exhibited in Squirmarius testai n. gen. n. sp.—dark circles/ovals with a central white lens—is widespread among chordates in the Mazon Creek fossil site and occasionally observed in cephalopods from the Mazon Creek fossil site (Kluessendorf and Doyle, Reference Kluessendorf and Doyle2000; Clements et al., Reference Clements, Dolocan, Martin, Purnell, Vinther and Gabbott2016). The presence of spherical to ovoid melanosomes in the eyes is not diagnostic, becaise these occur in a few groups, including cubozoans, flatworms, cephalochordates, tunicates, and chordates (Vopalensky and Kozmik, Reference Vopalensky and Kozmik2009; Clements et al., Reference Clements, Dolocan, Martin, Purnell, Vinther and Gabbott2016). However, S. testai n. gen. n. sp. is segmented, and the only segmented phylum with melanosomes in their eyes is the chordates (Couso, Reference Couso2009). Among the other groups with melanosomes in their eyes, cubozoans have radial rather than bilateral symmetry and tentacles with stinging cells, so the vermiform body lacking tentacles makes a cubozoan identity for S. testai n. gen. n. sp. unlikely (Brusca and Brusca, Reference Brusca and Brusca2003; Kingsford and Mooney, Reference Kingsford, Mooney, Pitt and Lucas2014). Flatworms are typically either parasitic or microscopic, although some free-living forms can grow to be > 10 cm long (Noreña et al., Reference Noreña, Damborenea, Brusa, Thorp and Rogers2015; Collins, Reference Collins2017). The gut of flatworms consists of a blind intestinal sac that is typically very wide and often has branches, which looks very dissimilar to the gut preserved in S. testai n. gen. n. sp., making it unlikely that this new species is a flatworm (Noreña et al., Reference Noreña, Damborenea, Brusa, Thorp and Rogers2015). Tunicates and cephalochordates are typically considered to be subphyla within the chordates (Holland, Reference Holland2016). Therefore, S. testai n. gen. n. sp. was most likely a chordate.
Chordates, and particularly vertebrates, are notable for having not just spherical/ovoid melanosomes in their eyes, but also elongate cylindrical melanosomes (Clements et al., Reference Clements, Dolocan, Martin, Purnell, Vinther and Gabbott2016). No elongate cylindrical melanosomes were observed in Squirmarius testai n. gen. n. sp. Although most Mazon Creek chordates preserve both morphologies of melanosomes in their eyes, only ovoid melanosomes have been observed in the eyes of Bandringa rayi and Myxinikela siroka (Clements et al., Reference Clements, Dolocan, Martin, Purnell, Vinther and Gabbott2016; Gabbott et al., Reference Gabbott, Donoghue, Sansom, Vinther, Dolocan and Purnell2016). Moreover, although elongate cylindrical melanosomes were observed in the eyes of Mayomyzon pieckoensis, this was only true in some specimens and others only had ovoid melanosomes (Gabbott et al., Reference Gabbott, Donoghue, Sansom, Vinther, Dolocan and Purnell2016). The melanosomes in the vertebrate retina are arranged into distinct layers with different morphologies, so the layer at which the fossil splits controls which, if any, melanosome morphologies will be visible on the surface of the fossil (Gabbott et al., Reference Gabbott, Donoghue, Sansom, Vinther, Dolocan and Purnell2016).
As a chordate without mineralized structures or paired fins, Squirmarius testai n. gen. n. sp. was likely not a gnathostome. Mazon Creek is known to have a diverse cyclostome fauna, including Mayomyzon pieckoensis, Pipiscius zangerli, Myxinikela siroka, and Gilpichthys greenei (Bardack and Zangerl, Reference Bardack and Zangerl1968; Bardack, Reference Bardack and Nitecki1979, Reference Bardack1991; McCoy et al., Reference McCoy, Saupe, Lamsdell, Tarhan and McMahon2016, Reference McCoy, Wiemann, Lamsdell, Whalen, Lidgard, Mayer, Petermann and Briggs2020; Miyashita, Reference Miyashita2020; Miyashita et al., Reference Miyashita, Gess, Tietjen and Coates2021). Based on the similarity in morphology and preservation of S. testai n. gen. n. sp. to these, we interpret it as a cyclostome of uncertain affinity, albeit most likely a hagfish or stem hagfish due to its similarity to G. greenei. However, it could also be a stem vertebrate or nonvertebrate chordate.
Results
Nemavermes mackeei is a junior synonym of Tullimonstrum gregarium.—FMNH PE 21551, the holotype of Nemavermes mackeei, was originally interpreted as a free-living marine nematode with a vermiform body, labial papillae, and a sparse covering of hair-like structures (Schram, Reference Schram1973). However, none of these features, except possibly the worm-like shape, are apparent in FMNH PE 21551, the holotype of N. mackeei.
FMNH PE 21551 is mostly preserved as a light-brown outline of soft tissue on a siderite concretion (Fig. 8.1). There are three roughly straight sections of a uniform light-brown color, with sharp bends in between (Fig. 8.1, 8.2). The first of these sections is largely covered by the other end of the specimen, but is ~6.8 mm wide; the second is ~4.8 mm wide and the third is ~3.7 mm wide. There is a sharp increase in width where the narrowest of these straight sections connects to an expanded region, which is ~6.22 mm wide. The preservation of this expanded region differs from the rest of the specimen in that it is covered in irregular dark brown patches (Fig. 8.1). The end of this expanded portion is not well preserved.

Figure 8. Comparison of the holotype of Nemavermes mackeei Schram, Reference Schram1973 to the proboscis and buccal apparatus of Tullimonstrum gregarium Richardson, Reference Richardson1966; photos taken with Nikon D60: (1) holotype of N. mackeei, FMNH PE 21551; (2) line drawing of (1), showing in particular the projected shape of the bifurcate portion of the buccal apparatus; (3) T. gregarium proboscis with buccal apparatus, FMNH PE 39890, showing sharp bend in the proboscis, and the differential preservation between the proboscis and buccal apparatus; (4) T. gregarium proboscis with buccal apparatus, FMNH PE 39375, showing two sharp bends in the proboscis; (5, 6) T. gregarium buccal apparati, FMNH PE 28739 (5) and FMNH PE 31063 (6), showing the distinctive shape of the buccal apparatus and the preservational differences between the buccal apparatus and the rest of the proboscis. Scale bars = 10 mm.
The holotype of Nemavermes mackeei has no features to indicate that it is a complete animal (e.g., eyes, gut, etc.) and it bears a striking resemblance in size, shape, and preservation to the proboscis of Tullimonstrum gregarium (Fig. 8). The proboscis of T. gregarium is an anterior elongation of the body that has straight sections with two joints (Fig. 8.3, 8.4); the proboscis will commonly exhibit two sharp bends, similar to those in FMNH PE 21551 (McCoy et al., Reference McCoy, Saupe, Lamsdell, Tarhan and McMahon2016). The proboscis of T. gregarium ends in a bifurcate buccal apparatus that has a bulbous base, noticeably wider than the section of the proboscis to which it connects, supporting the bifurcate portion of the apparatus (Johnson and Richardson, Reference Johnson and Richardson1969; McCoy et al., Reference McCoy, Saupe, Lamsdell, Tarhan and McMahon2016). The shape of this bifurcate buccal apparatus (Fig. 8.3–8.6) is very similar to the expanded region of FMNH PE 21551 (Fig. 8.1), which has a bulbous base and possibly some evidence of a bifurcation past this base (Fig. 8.2). The bifurcate buccal apparatus in T. gregarium commonly exhibits different preservation than the rest of the proboscis (McCoy et al., Reference McCoy, Saupe, Lamsdell, Tarhan and McMahon2016), and often has patches of darker material (Fig. 8.3, 8.5, 8.6) similar to those seen in the wide portion of FMNH PE 21551 (Fig. 8.1). The buccal apparatus of T. gregarium does bear small triangular teeth that we did not observe in FMNH PE 21551. However, these teeth are rarely preserved in T. gregarium (see McCoy et al., Reference McCoy, Saupe, Lamsdell, Tarhan and McMahon2016), and the region of FMNH PE 21551 where the buccal apparatus splits into the bifurcate portion, where the teeth would be, is poorly preserved and possibly absent (Fig. 8.1, 8.2). The size and proportions of FMNH PE 21551 are very similar to that of a typical T. gregarium proboscis and buccal apparatus.
The holotype of Nemavermes mackeei is very likely a Tullimonstrum gregarium proboscis with a poorly preserved buccal apparatus, making N. mackeei a junior synonym of T. gregarium.
Some specimens previously identified as Nemavermes mackeei are a new species of chordate
Four specimens previously identified as Nemavermes mackeei (FMNH PF 17809–17812) are elongate, vermiform animals with segments and pigmented eyes, which often preserve elements of the digestive tract as well (Figs. 1–7). These specimens represent a chordate, most likely a cyclostome, and have been assigned to the new species Squirmarius testai n. gen. n. sp. In addition, two specimens previously identified as Gilpichthys greenei (LF 2101 and 5664) have a body shape and eye shape more similar to those of S. testai n. gen. n. sp. and have been reidentified as the latter species.
One specimen previously identified as Nemavermes mackeei is a small Gilpichthys greenei
FMNH PF17808 (Fig. 9) has an elongate body (Fig. 9.1) and eyes (Fig. 9.2–9.4) somewhat similar to those of Squirmarius testai n. gen. n. sp. However, the body is a little stouter than other specimens of S. testai n. gen. n. sp. (L/W = 15.77), and the eyes are rounder, closer together, and smaller (Fig. 9.2–9.4). However, the small (~0.3 mm wide), round, close-set eyes are very similar to those of Gilpichthys greenei. In addition, this specimen also has a dark line running down the middle of the head (Fig. 9.2) in front of the eyes, very similar to the dark patch that often preserves the pharynx of G. greenei (e.g., McCoy et al., Reference McCoy, Saupe, Lamsdell, Tarhan and McMahon2016, fig. 2b, d), although FMNH PF 17808 does not have the pharyngeal muscle blocks and teeth characteristic of G. greenei (e.g., McCoy et al., Reference McCoy, Saupe, Lamsdell, Tarhan and McMahon2016, fig. 3e). Based on these features and the small size, we tentatively identify this specimen as a juvenile G. greenei.

Figure 9. Gilpichthys greenei Bardack and Richardson, Reference Bardack and Richardson1977, juvenile, FMNH PF17808: (1) complete specimen with a box indicating the region of (2) and (3); (2) head, showing the eyes (black arrows) and dark pharynx (white arrow); (3) same as (2), with a box indicating the region corresponding to (4); (4) SEM of box in (3) in BSE mode, showing the small, round, close-set eyes. Scale bars = 10 mm (1); 2 mm (2, 3); 500 μm (4).
Diagnostic features in the original description of Nemavermes mackeei
Nemavermes mackeei was originally described with only a few diagnostic features (Schram, Reference Schram1973): (1) well-developed labial papillae, described in FMNH PE 21550 as well as a specimen (number T422 in the original description; Schram, Reference Schram1973) in a private collection that was not available for this study; (2) hair-like structures sparsely covering the body, described in FMNH PE 24846; and (3) a long and relatively stout vermiform body.
The labial papillae, which are fleshy extensions from the anterior end of the body, were described from FMNH PE 21550 (Schram, Reference Schram1973). However, in this specimen (Fig. 10), one end of the body comes to a narrow point with no evidence of papillae (Fig. 10.4) and the other end of the body is poorly preserved. At best, it shows a very vague impression of some projections (Fig. 10.2, 10.3) that do not look very similar to those in the reconstruction of Nemavermes mackeei (see Schram, Reference Schram1973, text-fig. 1B). Labial papillae were not observed in any other specimens during this study and cannot be confirmed to be present in this specimen.
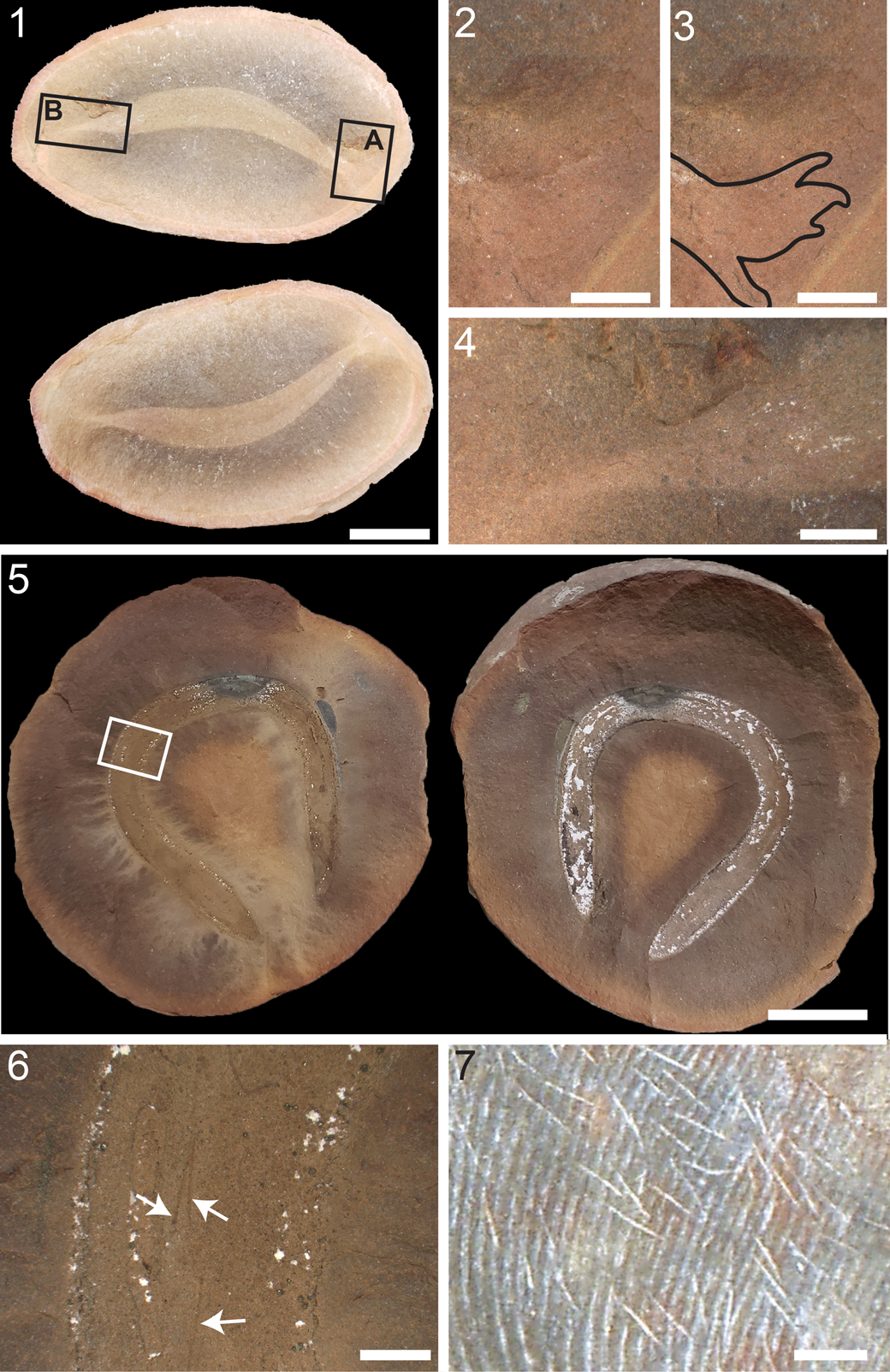
Figure 10. Specimens other than the holotype that were discussed in the original description of Nemavermes mackeei Schram, Reference Schram1973, FMNH PE 21550 and FMNH PE 24846: (1) complete specimen of FMNH PE 21550 with boxes indicating the regions corresponding to (2, 3) box A, and (4) box B; (2) one end of the body, box A in (1), showing poor preservation with possible labial papillae; (3) same as (2) with possible labial papillae outlined in black; (4) other end of the body, box B in (1), showing long narrow point; (5) complete specimen of FMNH PE 24846 with box corresponding to the region in (6); (6) body, box in (5), showing the hair-like structures. (7) Macroneuropteris scheuchzeri (Hoffmann, Reference Hoffmann1827) Cleal et al., Reference Cleal, Shute and Zodrow1990, FMNH P 31395, showing the hair-like waxy structures similar to the hair-like structures in (6). Scale bars = 10 mm (1, 5); 2 mm (2–4, 6, 7).
The original description of Nemavermes mackeei included hair-like structures, which were originally described in FMNH PE 24846 (Schram, Reference Schram1973; Fig. 10). Only a few of these structures are apparent, they are sparsely distributed, and they do not cover the whole body (Fig. 10.5, 10.6). They are fairly consistent in size, ~0.10 mm wide and ~2.47 mm long. We did not observe these hair-like structures in any of the other specimens included in this study, although for ROM IP 47528, we only had a photo, and were not able to carry out a microscopic investigation to assess it for the presence of hair-like structures. Possibly this specimen represents an isolated example of a vermiform animal with a sparse coating of hair-like structures as in the original N. mackeei reconstruction. Alternatively, these structures are very similar in size and shape to the hair-like waxy structures found on the lower surface of the leaves of Macroneuropteris scheuchzeri (Hoffmann, Reference Hoffmann1827) Cleal et al., Reference Cleal, Shute and Zodrow1990 (e.g., Wittry, Reference Wittry2020, p. 197, fig. 3; Fig. 10.7). Therefore, it is also possible that FMNH PE 24846 was not covered in hair-like structures but was just preserved along with shed plant defensive structures.
Other specimens previously identified as Nemavermes mackeei
The other specimens previously identified as Nemavermes mackeei investigated during this study are likely complete animals that show no resemblance to Tullimonstrum gregarium, and do not have the characteristic elongate body shape, pigmented eyes, segments, diffuse dark coating, or features of the digestive system seen in Squirmarius testai n. gen. n. sp. These can be roughly divided into two groups, although more work is needed to determine their specific taxonomic identity.
Three specimens that were originally attributed to Nemavermes mackeei (see Wittry, Reference Wittry2020) represent moderately sized, vermiform animals, and are the stoutest of the specimens originally assigned to N. mackeei, with L/W < 10 (Fig. 11). One end of the body narrows to a broad point and the other is rounded off (Fig. 11). All three specimens have a textured rather than smooth surface in the fossil (Fig. 11). They are covered in patches of a slightly darker material and the texture is further accentuated by white kaolinite precipitation; this is most pronounced in FMNH PE 93402. This material likely represents the remnants of some form of robust cuticle. The patchiness in the fossil might reflect a scaled or textured cuticle, or it might be due to partial decay or disintegration of the cuticle during fossilization. The presence of a robust external cuticle is also supported by the three-dimensional (3D) preservation of the fossils. FMNH PE 93336 has an internal medial structure preserved as a thin dark line that is most likely a gut (Fig. 11.3).

Figure 11. Squat, textured specimens: (1) FMNH PE 93402; (2) FMNH PE 93403; (3) FMNH PE 93336, indicating the gut (white arrow). Scale bars = 10 mm.
FMNH PE 93404 and ROM IP 47528 represent soft-bodied vermiform animals that are intermediate between Squirmarius testai n. gen. n. sp. and the three specimens discussed above in terms of stoutness, with L/W = ~15. Both ends of the body taper to blunt points that are very similar in shape to each other (Fig. 12). There is no evidence of a textured surface or cuticle. Both specimens preserve a 3D gut (Fig. 12). In FMNH PE 93404 (Fig. 12.1), the gut is preserved in patches, and the full extent of it is not clear. In ROM IP 47528, the gut is preserved continuously; it starts a few centimeters from the anterior end of the body and continues to within a millimeter or so of the posterior tip of the body (Fig. 12.2).

Figure 12. Moderately stout specimens: (1) FMNH PE 93404; note the 3D gut (white arrow); (2) ROM IP 47528; note the gut going to the very posterior end of the body (white arrow); image courtesy of the Royal Ontario Museum. Scale bars = 10 mm.
These five specimens, as part of Nemavermes mackeei, were initially identified as free-living marine nematodes. Although they lack preserved nematode synapomorphies, nematodes only have a few synapomorphies (e.g., amphids, specialized microscopic sensory structures; Tahseen, Reference Tahseen2012) and these are unlikely to preserve in fossils. Moreover, they are unusually large for free-living nematodes (Baliński et al., Reference Baliński, Sun and Dzik2013; Sperling, Reference Sperling2013). Nematodes rely on diffusion to circulate oxygen to their internal tissues, and therefore their size is limited by oxygen constraints (Soetaert et al., Reference Soetaert, Muthumbi and Heip2002; Heim et al., Reference Heim, Bakshi, Buu, Chen and Heh2020). Diffusion also limits the size of the pharyngeal muscles; the lack of a circulatory system means that diffusion is the only way by which the digestive glands, located in the pharynx, can uptake the components needed to synthesize digestive enzymes (Roggen, Reference Roggen1970). The maximum size of the pharyngeal muscles limits the amount of food that can be eaten and digested, and these nutrient limitations constrain the total body size of free-living nematodes (Kirchner et al., Reference Kirchner, Anderson and Ingham1980). Due to these physiological and environmental constraints, free-living nematodes today are all < 20 mm long and 2 mm in diameter (Kirchner et al., Reference Kirchner, Anderson and Ingham1980; Soetaert et al., Reference Soetaert, Muthumbi and Heip2002; Tahseen, Reference Tahseen2012). Parasitic nematodes can escape these constraints due to living in optimum conditions (Kirchner et al., Reference Kirchner, Anderson and Ingham1980); for example, the largest nematode, the whale parasite Placentonema gigantissima Gubanov, Reference Gubanov1951, grows to nearly 8 m long and 2.5 cm wide (Nielsen, Reference Nielsen1998; Tahseen, Reference Tahseen2012). There are a few larger nematodes, e.g., the benthimermithids and the marimermithids (Miljutin, Reference Miljutin and Schmidt-Rhaesa2014a, Reference Miljutin and Schmidt-Rhaesab), which are free-living as adults and parasitic as larvae and juveniles, during which time they achieve their large size. The benthimermithids do not feed as free-living adults, but rather subsist on nutrients stored during their parasitic phase, and therefore are not constrained by the size of pharyngeal muscles (Miljutin, Reference Miljutin and Schmidt-Rhaesa2014a). Marimermithids are poorly studied and assumed to have a very similar lifestyle to benthimermithids, although it is not yet known if they eat as free-living adults (Miljutin, Reference Miljutin and Schmidt-Rhaesa2014b). Therefore, the relatively large size of these specimens is not just outside the size range for modern free-living marine nematodes with no parasitic stage, it is inconsistent with the physiological constraints on the body size of free-living marine nematodes with no parasitic stage. Large nematodes, similar in size to the specimens considered in this study, are parasites for some or all of their life cycle. None of the specimens investigated during this study are likely to represent fully free-living marine nematodes with no parasitic stage.
Discussion
Animals with a vermiform body and few diagnostic characters are some of the most difficult to identify in the fossil record because this body plan is widespread across many animal phyla. It is particularly complicated at the Mazon Creek fossil site; although the fossils there exhibit exceptional soft-tissue preservation, soft-tissue structures are primarily preserved as smudges on the rock in various shades of brown, with extensive taphonomic variation (Baird et al., Reference Baird, Sroka, Shabica and Kuecher1986). Often multiple specimens of any species are needed to distinguish the true morphology amid the taphonomic variation and to correctly interpret the fossils. The specimens originally identified as Nemavermes mackeei are an example of this. Recent research on Tullimonstrum gregarium to clarify its morphology (McCoy et al., Reference McCoy, Saupe, Lamsdell, Tarhan and McMahon2016) made it possible to identify the holotype of N. mackeei as a specimen of T. gregarium. Moreover, new collections of material assigned to N. mackeei showed that this was not one species covering a continuous but very wide range of morphologies, but rather a collection of discrete subgroups. These represent species with similar but distinct morphologies, leading to the identification of the new species Squirmarius testai n. gen. n. sp. However, more research is needed to determine the identity of the other specimens originally assigned to N. mackeei, both from the Mazon Creek fossil site and the Bear Gulch Limestone.
Acknowledgments
We thank J. Lamsdell for helpful discussion. We thank A. Young and the David and Sandra Douglass collection for facilitating access to their specimens, and for donating the specimen important for this research to the Lauer Foundation for Paleontology, Science, and Education in Wheaton Illinois. We thank the Lauer Foundation for Paleontology, Science, and Education and the Field Museum of Natural History for facilitating access to specimens. We thank the Lauer Foundation for Paleontology, Science, and Education and the Royal Ontario Museum for photographs of specimens. This research was funded by a University of Wisconsin-Milwaukee start-up grant to VEM. We thank J. Vinther and L. Muir for helpful reviews.
Declaration of competing interests
The authors declare none.















