Introduction
Coronavirus disease 2019 (COVID-19) infection usually presents with fever, colds, throat pains, diarrhea, and respiratory symptoms. Reference Guan, Ni and Hu1 However, growing evidence show that the infection can present with atypical symptoms such as headache, dizziness, seizures, anosmia, stroke, and impaired consciousness. Reference Guan, Ni and Hu1,Reference Jiang, Deng, Zhang, Cai, Cheung and Xia2 In a case series in Wuhan, China, 36.4% presented with central nervous system (CNS) symptoms, 8.9% with peripheral nervous symptoms, and 10.7% with skeletal muscle symptoms. Reference Mao, Jin and Wang3
A previous systematic review on the CNS manifestations of COVID-19 has been published that involved six studies with primary data. Reference Asadi-Pooya and Simani4 However, decelerating the COVID-19 global pandemic and filling knowledge gaps necessitate pooling of new and emerging daily research output regarding the neurological involvement in COVID-19 infection. Reference Chahrour, Assi and Bejjani5 This current systematic review and meta-analysis aimed to determine the neurological manifestations and complications, including laboratory findings, and outcomes among patients with COVID-19 infection.
Methodology
This review complied with the Preferred Reporting Items for Systematic reviews and Meta-analyses. Reference Liberati, Altman and Tetzlaff6 The protocol was registered in PROSPERO (registration number: CRD42020180658).
Criteria for Selection of Studies
We considered studies with the following designs: cohort, case series/ reports. We considered studies that included a population of patients confirmed with COVID-19 infection according to the established diagnostic criteria. Reference Lu, Zhao and Li7,8 We included studies that enrolled a population of adult and pediatric patients with information on the frequencies and proportions of neurological symptoms/signs, new-onset neurological disorders/complications associated with COVID-19 infection and their outcomes (e.g., recovered/died). No restrictions were implemented on sex and ethnicity of the population. We excluded studies that were reported as abstract-only with no full texts available and non-English articles.
Search Methods for the Identification and Selection of Studies
Three investigators (MEVC, AIE, MCCS) searched the following major electronic healthcare databases until April 18, 2020 for relevant studies: PubMed by MEDLINE, Embase, Scopus, and the World Health Organization (WHO) database. We used the following general and MeSH terms: [“Coronavirus disease 2019” OR “Novel coronavirus” OR severe acute respiratory syndrome coronavirus 2 (“SARS-CoV-2”) OR “COVID-19” OR “2019-nCOV”] AND [“Brain” OR “Spine” OR “Meninges” OR “Central nervous system” OR “Peripheral nervous system” OR “Muscles” or “Neuromuscular junction” OR “Neurologic manifestations”]. To ensure literature saturation, we looked into the references of all identified relevant articles and narrative reviews/systematic reviews and/or meta-analysis for consideration to be included in this current review.
Three investigators (MEVC, AIE, and MCCS) screened for articles using predetermined screening criteria. Relevant articles were retrieved in full text and were subjected to the eligibility criteria. The studies that fulfilled the eligibility criteria were included in the qualitative and quantitative analyses. Any disagreements in the inclusion of the studies were resolved by consensus with the other two investigators (RDGJ and VMMA).
Methodological Assessment of Included Studies
The Murad tool was used to evaluate the risk of bias in noncomparative cohorts and case series/ reports. Reference Murad, Sultan, Haffar and Bazerbachi9 We considered “poor,” “moderate,” or “good” quality when 3 or fewer, 4, or 5 of the criteria were fulfilled, respectively. Three investigators (MEVC, VMMA, and RDGJ) evaluated the methodological quality of the included studies; discrepancies were resolved by consensus with the other two investigators (AIE and MCCS).
Data Collection
Three investigators (MEVC, MCCS, and AIE) extracted the data from the eligible studies, and any discrepancies were resolved by involvement of the other two investigators (VMMA and RDGJ). For each study, the following information was recorded: author and year, publication date, study design, study duration, setting, number of included patients, age, sex, disease severity, comorbidity, neurologic symptoms, neurologic disorders/complications and associated laboratory findings, and outcomes.
Data Analysis and Synthesis
We used frequencies and proportions for categorical variables and means (standard deviation) or median (range) for continuous variables. Data were pooled and expressed in 95% confidence intervals (CIs). The unit of analysis used was the individual patient.
All included studies were analyzed qualitatively. We performed the meta-analysis using the following R (Version 3.6.3) packages: metafor, meta, and weightr. Meta-analysis was conducted for studies with >5 included patients. The proportions were pooled by random-effects model estimated using the DerSimonian and Laird method. Heterogeneity was evaluated using chi-squared test (χ 2; p-value < 0.10 to detect significant heterogeneity) and I 2 tests with >25%, >50%, and >75% considered as low, moderate, or high degree of heterogeneity, respectively. Funnel plots were constructed for neurological symptoms with more than five study components to visually evaluate publication bias with Egger’s regression tests conducted to quantitatively assess this bias (p-value < 0.05 considered statistically significant). If publication bias was suspected, the trim-and-fill method was employed to adjust for potentially missing data in the funnel plot’s asymmetry.
Results
A total of 403 journal articles (MEDLINE by PubMed: 161; Scopus: 126; Embase: 30; WHO Database: 86) were identified using the search strategy. After duplicates were removed, 325 titles and abstracts were screened. After exclusion of 262 articles that did not fulfill the screening criteria, 63 full-text reports were assessed for eligibility. A total of 49 articles fulfilled our inclusion criteria. Among these studies, 35 articles were included in the quantitative synthesis. The diagrammatic flow of information for this review is shown in Figure 1.
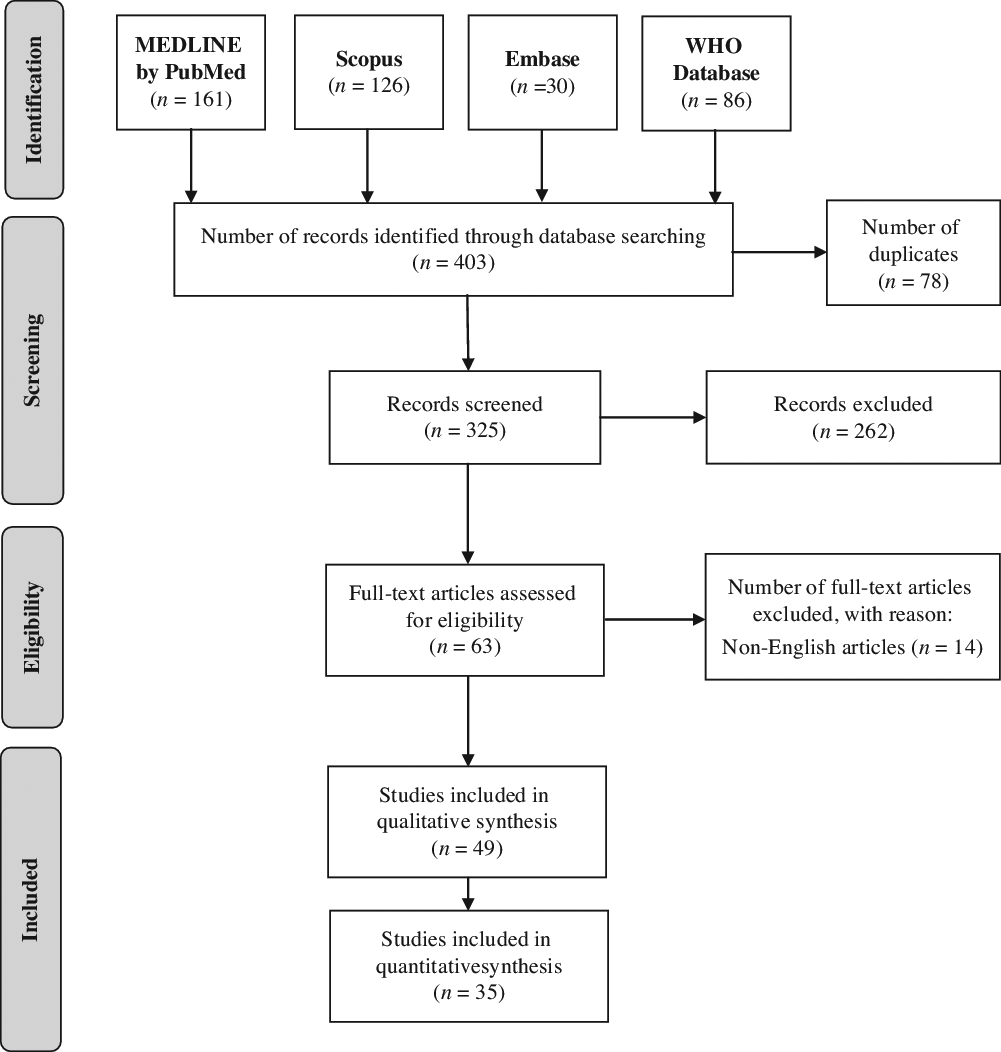
Figure 1: Flow diagram of information for the selection of relevant articles.
One prospective study Reference Du, Liang and Yang10 , 35 retrospective studies Reference Guan, Ni and Hu1,Reference Mao, Jin and Wang3,Reference Kim, Chin and Kang11–Reference Gupta, Agrawal and Ish43 , and 13 case report/series Reference Filatov, Sharma, Hindi and Espinosa44–Reference Gutiérrez-Ortiz, Méndez and Rodrigo-Rey56 with information from a total of 6,335 individuals with COVID-19 infection were involved in this review. Patient data from the included studies were obtained from various countries, as follows: China, France, India, Iran, Japan, South Korea, Spain, Thailand, and the USA. The sample size for each study ranged from 1 to 1099 patients. The features of the included studies and enrolled patients are presented in Table 1.
Table 1: Features of the included studies and population, reported neurologic symptoms, and quality assessment
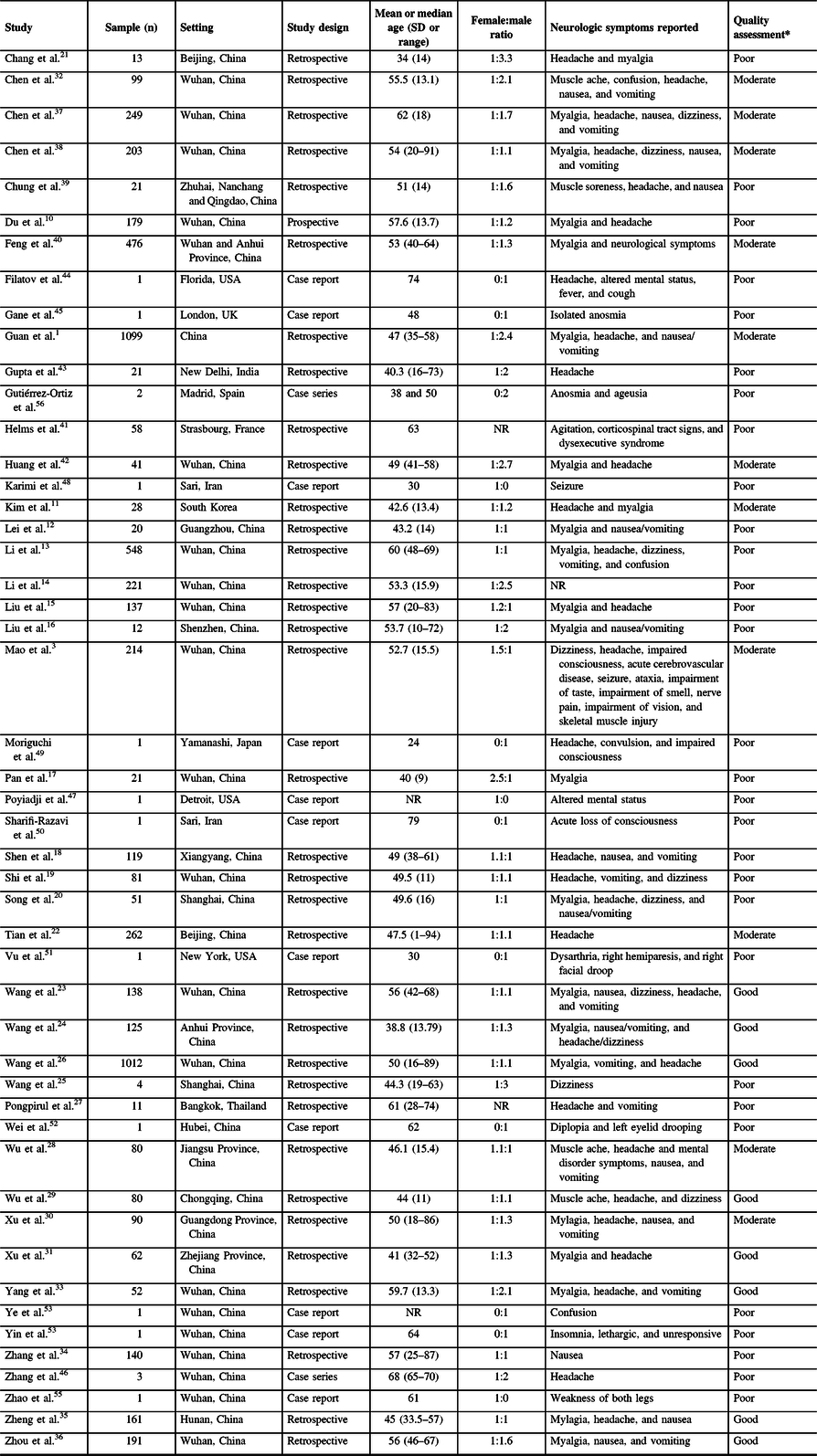
NR = not reported.
* Methodological quality assessment using the Murad tool: “Good” (5 points), “Moderate” (4 point), “Poor” (3 or lower points).
All studies enrolled patients confirmed with COVID-19 infection using clinical criteria corroborated with a positive test in SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) using sputum or nasopharynx swabs samples according to the recommendations of the WHO. The age of patients ranged from 1 to 94 years, with an overall female-to-male ratio of 1:1.2. The spectrum of COVID-19 disease severity was remarkably diverse and reported in varying definitions of categories of gravity of infection. All studies enrolled more stable/mild/ nonsevere cases with a proportion approximately ranging from 50 to 80%; with severe or critical cases varying from 5 to 50%. Ranges of proportions of described comorbidities in the studies were remarkably varied, as follows: hypertension (4.5%–3%), diabetes (2%–27%), chronic lung disease (1%–11%), cardiovascular and cerebrovascular diseases (1%–40%), gastrointestinal disease (1%–15%), liver disease (1%–11%), kidney disease (1%–17%), endocrine disease (5%–13%), malignancy (1%–7%), and others.
There were 8, 10, and 31 studies deemed with “good,” “moderate,” and “poor” methodological quality, respectively (see Table 1). Most studies had limitations due to deficient representativeness of the cases related to small sample size and uncertainties in attempts to exclude other diagnoses.
The random-effects modeling analysis for each neurological symptom reported in patients with COVID-19 infection showed the following overall point estimate of proportions with corresponding 95% CI, number of studies assessed, and the overall number patients with the neurological symptom evaluated divided by the total number of patients analyzed, respectively (see Table 2): “headache” (0.12, 95% CI 0.10–0.14; 24 studies; 586/4,882), “dizziness” (0.08, 95% CI 0.05–0.12; 6 studies; 132/1,458), “headache and dizziness” (0.09, 95% CI 0.06–0.13; 3 studies; 22/256), “nausea” (0.07, 95% CI 0.04–0.11; 7 studies; 77/1,026), “vomiting” (0.05, 95% CI 0.03–0.08; 9 studies; 116/2,409), “nausea and vomiting” (0.06, 95% CI 0.03–0.11; 9 studies; 100/1,796), “confusion” (0.05, 95% CI 0.02–0.14; 2 studies; 26/647), and “myalgia” (0.21, 95% CI 0.18–0.25; 25 studies; 952/5,155). The forest plots for all the reported neurological symptoms are shown in Figure 2. Other less commonly reported neurological symptoms are presented in Table 1.
Table 2: Pooled overall estimates of the proportions of various neurological symptoms in COVID-19 infection
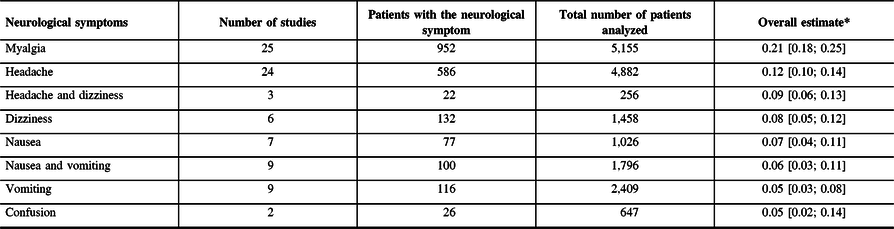
* Expressed as point estimates of the proportions [with 95% confidence intervals].
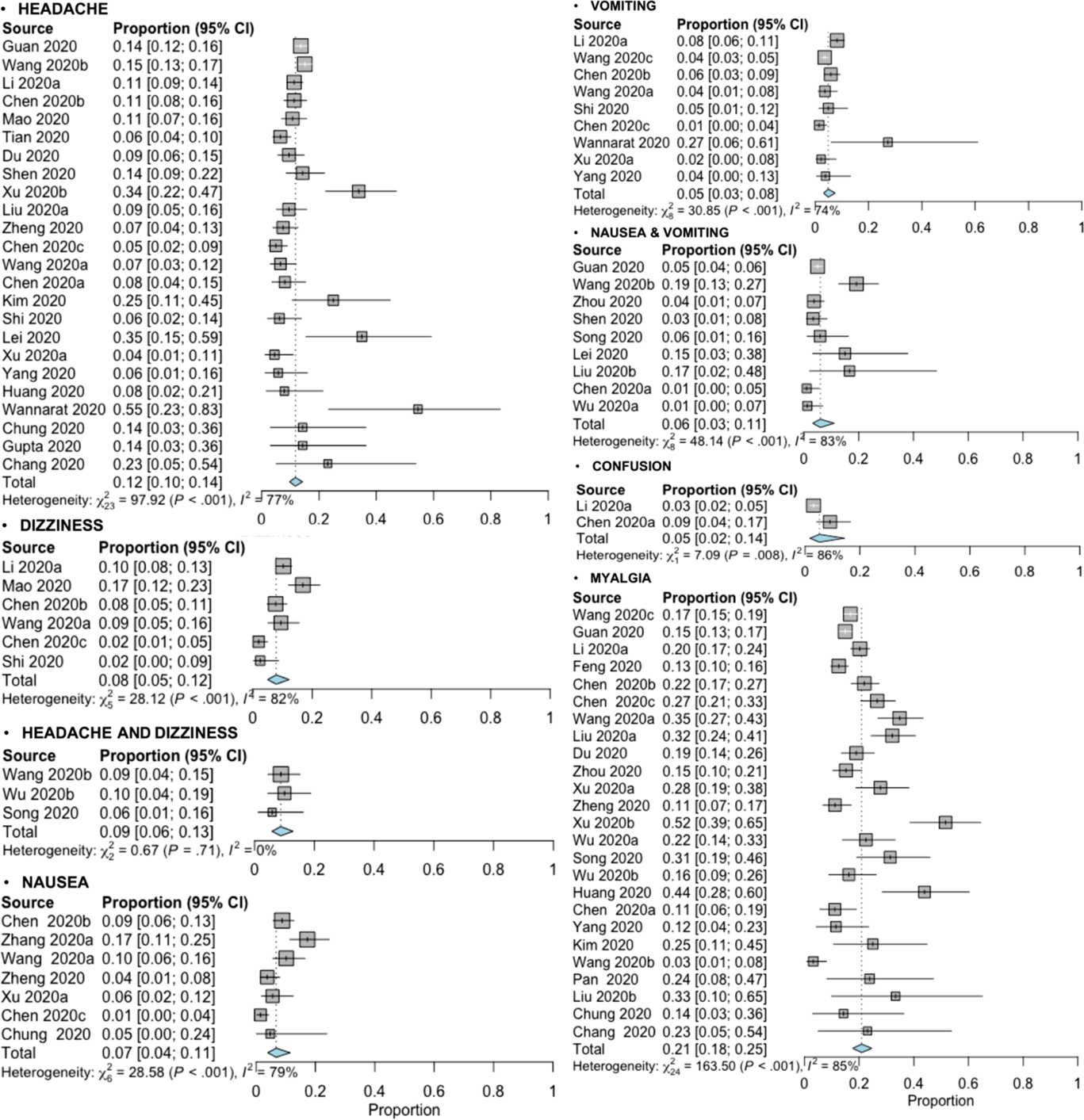
Figure 2: Forest plots of the meta-analysis of proportions for the reported neurological symptoms associated with COVID-19 infection from various study sources.
Nearly all symptom analyses detected statistically significant heterogeneity (p-value < 0.10) with a high degree of heterogeneity (I 2 > 75%) excluding the symptom of “headache and dizziness” (p-value = 0.71; I 2 = 0%) (see Figure 2). A subgroup analysis to explore possible heterogeneity source could not be done due to insufficient presented data in the included studies.
The funnel plots showed asymmetry in the “dizziness” and “nausea” symptoms and corroborated using Egger’s regression test (see Supplementary Material 1; funnel plot B: p-value = 0.0025; funnel plot C: p-value = 0.0227); all the other funnel plots and corresponding regression tests did not show statistically significant asymmetry (p-values > 0.05). Results from the trim-and-fold method demonstrated that two studies and three studies necessary were missed on the right side of the plots in the “dizziness” and “nausea” symptoms, respectively (see Supplementary Material 1G to H); the adjusted random-effects pooled proportion were 0.11 (95% CI 0.06–0.17) and 0.10 (95% CI 0.06–0.17) for “dizziness” and “nausea” symptoms, respectively.
A total of 14 studies with 33 patients were identified that described new-onset neurological disorders/complications linked to COVID-19 infection with some detail (see Table 3). Vascular disorders were noted to be most frequent (n = 23; 69.7%); these presented as acute cerebral infarction (n = 18), acute intracerebral hemorrhage (n = 4), and cerebral venous sinus thrombosis (n = 1); eight cases survived and five cases died. Reference Mao, Jin and Wang3,Reference Li, Wang and Zhou14,Reference Zhang, Xiao and Zhang46,Reference Sharifi-Razavi, Karimi and Rouhani50,Reference Vu, Ruggiero and Choi51 Four case reports described patients who manifested with encephalopathy/ encephalitis, with two patients recovering. Reference Filatov, Sharma, Hindi and Espinosa44,Reference Moriguchi, Harii and Goto49,Reference Ye, Ren and Lv53,Reference Yin, Feng and Wang54 A COVID-19 case with documented acute necrotizing hemorrhagic encephalopathy presented as altered mental status but the patient’s outcome was unclear. Reference Poyiadji, Shahin, Noujaim, Stone, Patel and Griffith47 Cranial and peripheral nerves were also deemed affected by COVID-19 infection as described in a number of patients who had isolated sudden-onset anosmia (n = 1), oculomotor nerve palsy (n = 1), Miller–Fisher syndrome (MFS; n = 2), and Guillain–Barre syndrome (GBS; n = 1). Reference Gane, Kelly and Hopkins45,Reference Wei, Yin, Huang and Guo52,Reference Zhao, Shen, Zhou, Liu and Chen55,Reference Gutiérrez-Ortiz, Méndez and Rodrigo-Rey56 Most patients with neurological complications survived (n = 14), a considerable number of patients died (n = 7) and the rest had unclear outcomes (n = 12). Overall, 28 patients (84.8%) and 5 patients (15.2%) had central and peripheral nervous system involvement, respectively.
Table 3: Neurological disorders or complications associated with COVID-19 infection (N = 33)
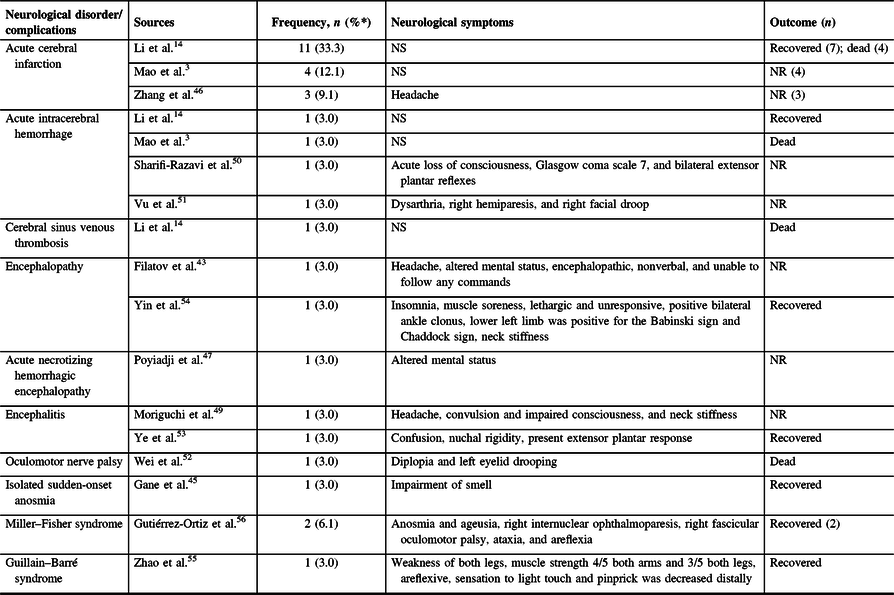
NR = not reported; NS = not specified.
* Percentages among patients with COVID-19 infection with neurological disorders/complications.
Neuroimaging of patients with COVID-19 infection and neurological manifestations using computed tomography or magnetic resonance interval may show leptomeningeal enhancement, localized decreased cerebral blood flow, both ischemic and hemorrhagic strokes, cerebral venous thrombosis, and hemorrhagic necrotizing encephalopathy. Reference Li, Wang and Zhou14,Reference Helms, Kremer and Merdji41,Reference Poyiadji, Shahin, Noujaim, Stone, Patel and Griffith47 Cerebral infarctions in both small and large vessels, including cardioembolic strokes, were also reported. Reference Li, Wang and Zhou14 Electroencephalography (EEG) showed only nonspecific changes. Reference Ye, Ren and Lv53 Cerebrospinal fluid (CSF) studies are often normal with some showing slight elevation in opening pressure and slight increase in mononuclear cells and protein. Reference Zhao, Shen, Zhou, Liu and Chen55 The result of CSF RT-PCR is not consistent and depends on the type of test kits and neurological complications. Reference Filatov, Sharma, Hindi and Espinosa44,Reference Moriguchi, Harii and Goto49,Reference Ye, Ren and Lv53,Reference Gutiérrez-Ortiz, Méndez and Rodrigo-Rey56 Only one of nine CSF tested was RT-PCR positive. Reference Helms, Kremer and Merdji41,Reference Moriguchi, Harii and Goto49,Reference Ye, Ren and Lv53 Meningoencephalitis may have a positive CSF RT-PCR, while cerebral infarction may have negative result. Reference Helms, Kremer and Merdji41,Reference Moriguchi, Harii and Goto49 Nerve conduction studies (NCS) done in one patient showed delayed distal latencies and absent F-waves, which may indicate a demyelinating neuropathy. Reference Zhao, Shen, Zhou, Liu and Chen55
Discussion
To the best of our knowledge, our review presents the most extensive evaluation of the neurological manifestations of COVID-19 infection with relevant information from 49 studies involving 6,335 patients. Our review provides the first large qualitative and quantitative analyses of neurological symptoms, complications, laboratory findings, and outcomes associated with this condition.
Pooled evidence showed that “myalgia” and “headache” were the most typical neurological symptoms of COVID-19, followed by “headache & dizziness,” “dizziness,” “nausea,” “nausea and vomiting,” “vomiting,” and “confusion.” Although neurological symptoms arise in COVID-19 infections, its occurrence may be considered low, that is, the upper limit of the 95% CI of “myalgia” symptom is 0.25 and the lower limit of the 95% CI of “confusion” is 0.02). The most common neurological complications associated with this infection were cerebrovascular disorders presenting as acute cerebral infarction or hemorrhage, or cerebral sinus venous thrombosis. Furthermore, other documented COVID-19-related cases were encephalopathy, encephalitis, oculomotor nerve palsy, isolated sudden-onset anosmia, and GBS which included MFS. The performance and need for neurological diagnostic tests may vary and may be determined by clinical examination. Brain imaging of these patients may provide evidence of infarction or hemorrhage in suspected patients with stroke-like syndrome. EEG may show nonspecific findings in patients with encephalitis. CSF studies may show normal parameters, slight opening pressure elevation, and small increase in mononuclear cells and protein, or may provide very low yield in testing for COVID-19 employing the current RT-PCR kits or protocols. A GBS case associated with COVID-19 showed an NCS finding consistent with a demyelinating neuropathy. Many COVID-19 patients with neurological conditions recovered but outcomes of the other reported cases were unclear.
Coronaviruses (CoVs) in general have been known to exhibit neurotropic properties and as such may cause neurological conditions. Reference Wu, Xu and Chen57 There are several possible mechanisms why CoV infections damage the CNS. First is that the genetic material and proteins of several viruses have been detected in the CSF or brain tissues. Reference Koyuncu, Hogue and Enquist58 A neuronal pathway is another possible route. The virus may lead to the disruption of the nasal epithelium and is released mostly on the apical as well as basolateral side of the epithelial cells and reach the bloodstream or lymph to reach other tissues, including the CNS. Reference Chilvers, McKean, Rutman, Myint, Silverman and O’Callaghan59,Reference Dijkman, Jebbink and Koekkoek60 The viruses can also migrate by infecting motor or sensory nerves, via neuronal transport. Reference Swanson and McGavern61 Alveolar gas exchange disorders are caused by viral proliferation in the lung tissue cells. This in turn causes CNS hypoxia, increasing anaerobic metabolism in the brain. Reference Wu, Xu and Chen57 Another mechanism would be immune-mediated. Reference Klein, Garber and Howard62 This is closely related to the development of systemic inflammatory response syndrome. CoV infections can infect macrophages, microglia, and astrocytes. It can activate glial cells and promote a proinflammatory state. Reference Li, Fu, Gonzales and Lavi63,Reference Desforges, Le Coupanec and Dubeau64 Cytokines can cross the blood–brain barrier and are associated with acute necrotizing encephalopathy. Reference Poyiadji, Shahin, Noujaim, Stone, Patel and Griffith47 The neurovirulence of CoV correlates with its ability to induce proinflammatory cytokine signals from astrocytes and microglia. Reference Li, Fu, Gonzales and Lavi63
The brain has been reported to express angiotensin-converting enzyme 2 (ACE2) receptors that have been detected over glial cells and neurons, which makes them a potential target of COVID-19. The spike protein S1 of the SARS-CoV virus enables its attachment to the cell membrane by interacting with host ACE2 receptor. Reference Baig, Khaleeq, Ali and Syeda65 By binding to ACE2 receptors, they may cause abnormally elevated blood pressure thereby increasing cerebral hemorrhage risk. The SARS-CoV-2 spike protein could interact with ACE2 expressed in the capillary endothelium, possibly causing damage to the blood–brain barrier and gaining entry into the CNS by attacking the vascular system. Reference Baig, Khaleeq, Ali and Syeda65 Moreover, a derangement in coagulation system causing D-dimer and platelet abnormalities are postulated to increase the risk of cerebrovascular diseases. Reference Jin, Hong and Chen66
The statistically significant heterogeneity found in the pooled proportional estimates of the neurological symptoms in this review may stem from clinical variations in age, disease severity, and underlying comorbidities that were difficult to subgroup due to limited information from the published studies. The age range of the population enrolled was wide, and this may lead to variances in the incidence of many neurological symptoms, that is, older individuals tend to have more underlying conditions and therefore, they have the proclivity to report more symptoms. Reference Mao, Jin and Wang3 Majority of the population included in the studies were more substantially represented by patients with stable/ mild/ nonsevere COVID-19 cases compared to severe/critical cases, which could explain the low occurrences of the reported neurological symptoms. It is theorized that individuals with severe COVID-19 infection may have increased probability of developing neurological manifestations such as disturbances in consciousness, skeletal muscle injury, including acute stroke. Reference Mao, Jin and Wang3 Many patients in several studies in this review had broad spectrum of comorbid conditions which may also affect the emergence of some neurological symptoms, complications, and outcomes. Reference Chen, Wu and Chen37 Furthermore, many studies exhibited “poor” methodological quality which may have contributed to the heterogeneity as well. Although heterogeneity may be evident, the authors agreed to pool the data since the main purpose of this review is to provide a reliable general approximation of the point estimates and 95% CI of the proportions of the neurological symptoms of all patients with COVID-19. These overall measurements reflect the best estimates of the frequencies of the neurological manifestations which are valuable pieces of information that can guide a clinician to appropriately recognize and diagnose COVID-19 infection in a suspected individual. Moreover, combining pertinent data made it feasible to increase the precision of our estimates by increasing the number of the included patients in the quantitative analysis.
The funnel asymmetry detected visually and quantitatively in the pooled analyses of “dizziness” and “nausea” symptoms may suggest the presence of publication bias. However, during this current COVID-19 crisis, any study related to this condition is preferably reviewed and published due to the recognized urgency to characterize and provide solutions to combat this pandemic. Reference Bedford, Enria and Giesecke67 While a skewed funnel plot may arise from publication bias, this phenomenon may also be explained by other reasons, such as exclusion of non-English articles (an acknowledged limitation of this review), clinical heterogeneity, poor methodological design, and chance. Reference Song, Parekh and Hooper68
While the evident advantage of this review lies in the inclusion of substantial quantity of studies and patients with COVID-19, its strength also depends on the rigor of the review process. Every step in the review process such as the systematic search for relevant trials, selection of studies, data extraction, data synthesis, and analysis involved at least three investigators; in consensus with the other two investigators, if there were discrepancies. This design was conducted to minimize unanticipated random or systematic error that could transpire if a single investigator managed a certain process in the review. Moreover, a sensitive approach in searching for significant articles was conducted to obtain greater yield records for evaluation. We employed broader, general terms including MeSH terms, and we handsearched the reference lists of relevant studies to ensure literature saturation. This strategy allows a more exploratory approach for discovery of significant studies to be included in this review.
However, this review could not capture information from gray literature or unpublished data that were not indexed in the major healthcare electronic databases and from non-English articles. Moreover, this review could only integrate available literature that were mostly retrospective in nature. These studies have inherent recording bias that may potentially obscure the validity of the pooled estimates.
Neurological symptoms such as headache, myalgia, dizziness, and nausea are nonspecific and may be present in other conditions. Thus, a thorough neurological examination and referral may be sought for early recognition, diagnosis, and treatment of neurological complications.
In summary, this systematic review and meta-analysis showed that neurological symptoms such as headache, myalgia, dizziness, and nausea are present in COVID-19; 1 in 10 patients may present with headache and 2 in 10 patients may present with myalgia. The most common neurological complication associated with this infection was cerebrovascular disease. In order to substantiate these findings, the performance of larger and ethically appropriate prospective studies must be undertaken; looking into these signs and symptoms early on, and carefully documenting their complications and outcomes over the full course of the illness.
Conflict of Interest
None.
Statement of Authorship
MEVC: Conceptualization, data curation, formal analysis, interpretation of data, writing original draft, writing review, and editing. AIE: Conceptualization, data curation, formal analysis, interpretation of data, writing original draft, writing review, and editing. MCCS: Conceptualization, data curation, formal analysis, interpretation of data, writing original draft, writing review, and editing. VMMA: Conceptualization, data curation, formal analysis, interpretation of data, writing original draft, writing review, and editing. RDGJ: Conceptualization, data curation, formal analysis, interpretation of data, writing original draft, writing review, and editing.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2020.146.






