Obesity is characterised by the accumulation of excessive body fat, which is associated with a range of major diseases including type 2 diabetes, hypertension, CVD and arthritis(Reference Low, Chin and Deurenberg1). Inhibition of adipogenesis through the ingestion of functional foods such as chitosan can reduce the deposition of excessive fat in adipose tissue(Reference Kumar2, Reference Cho, Rahman and Kim3). Chitosan is a polymer of β-(1 → 4)-2-acetamido-d-glucose and β-(1 → 4)-2-amino-d-glucose(Reference Kumar2, Reference Rinaudo4). It is synthesised through the chemical modification of chitin, which forms the hard shells of crustaceans such as crabs and shrimps(Reference Rinaudo4, Reference Kean and Thanou5). Chitosan is a non-toxic nutritional supplement generally regarded as a safe compound(Reference Chandy and Sharma6–Reference Chae, Jang and Nah8). Chitosan has been investigated for its therapeutic potential as an anti-obesity, anti-microbial and antioxidant agent(Reference Cho, Rahman and Kim3, Reference Fernandes, Tavaria and Soares9). Recent studies have indicated that chitosan can inhibit absorption of lipids in human subjects and animals, which is based on the fact that chitosan can readily bind dietary lipids in the proximal region of the small intestine of the mammalian gastrointestinal tract(Reference Cho, Rahman and Kim3, Reference Kaats, Michalek and Preuss10–Reference Zeng, Qin and Wang12).
Native chitosan polymers generally have a very high molecular weight (MW). It is associated with low solubility and high viscosity, which limits its cellular absorption(Reference Zeng, Qin and Wang12, Reference Mourya and Inamdar13). Hence, to enhance biological activity, chitosan is often modified to form chito-oligosaccharide, which has a lower MW, lower viscosity and higher solubility(Reference Kim and Rajapakse14, Reference Jin, Yumin and Hongbo15). Chito-oligosaccharide can be absorbed by mammalian cells and can participate in metabolic processes within a cell(Reference Chae, Jang and Nah8, Reference Zeng, Qin and Wang12, Reference Vinsova and Vavrikova16). However, the degree of absorption and intracellular activity of chito-oligosaccharide is highly dependent on the MW, solubility and deacetylation percentage(Reference Thanou, Verhoef and Junginger7, Reference Chae, Jang and Nah8, Reference Zeng, Qin and Wang12), with low-MW chito-oligosaccharide readily absorbed by intestinal cells in vitro and in vivo (Reference Chae, Jang and Nah8, Reference Zeng, Qin and Wang12).
The murine 3T3-L1 pre-adipocyte cell line has previously been used as an in vitro model system for investigating the molecular mechanisms of adipogenesis(Reference Green and Kehinde17–Reference Prokesch, Hackl and Hakim19). These fibroblast cells can be stimulated to differentiate into mature adipocytes, a process which mimics the maturation of adipose precursor cells and deposition of lipids during the development of obesity in humans(Reference Ntambi and Young20). In addition to playing a vital endocrine role in energy homeostasis, adipocytes are the primary sites of energy storage, where the excess energy is stored in the form of TAG(Reference Voshol, Rensen and van Dijk21). Recent studies have demonstrated that chito-oligosaccharides have the potential to alter the expression level of a number of genes involved in adipogenesis, including PPARγ, CCAAT/enhancer-binding protein (CEBPα), leptin, adiponectin (ADIPOQ) and resistin in adipocytes and mouse models(Reference Cho, Rahman and Kim3, Reference Kumar, Rahman and Lee22).
Adipogenesis occurs through the activation of biochemical pathways mediated through signalling molecules including cytokines, chemokines and adipokines(Reference Hoene and Weigert23, Reference Hirai, Takahashi and Goto24). One of the fundamental biochemical pathways vital to adipogenesis is the lipid metabolic pathway. The mode of action of chito-oligosaccharide as an anti-adipogenic compound has not yet been characterised. We hypothesised that anti-adipogenic chito-oligosaccharides will alter the expression of genes involved in the lipid metabolic pathway. Hence, the objectives of the present study were, first, to evaluate four chito-oligosaccharides for their concentration- and MW-dependent effects on the adipogenesis of mouse 3T3-L1 pre-adipocyte cells. Having identified the chito-oligosaccharide with the greatest anti-adipogenic potential, we subsequently evaluated its effect on the expression of a panel of genes involved in lipid metabolism and further validated the expression and regulation of the differentially expressed candidate gene at protein and gene promoter levels.
Materials and methods
Cell culture
Mouse 3T3-L1 pre-adipocytes were obtained from the American Type Culture Collection (Manassas, VA, USA). Cells were cultured in a Dulbecco's modified Eagle's medium (DMEM; Gibco, Invitrogen Corporation, San Diego, CA, USA) containing 10 % fetal calf serum (Gibco) and 1 % penistrep solution (Sigma-Aldrich Corporation, St Louis, MO, USA) in a 37°C humidified incubator with 5 % CO2. During the multiplication phase of the pre-adipocytes, the medium was changed every alternate day and the cells were trypsinised before reaching full confluence and were plated afresh.
Chito-oligosaccharides
A total of four chito-oligosaccharides of MW < 1000, 1000–3000 (1–3000), 3000–5000 (3–5000) and 5000–10 000 (5–10 000) Da were sourced from Kitto Life Company Limited (Kyungki-do, Seoul, Korea). Both the chito-oligosaccharide content and the deacetylation percentage of all of these chito-oligosaccharides were >70 %. For the cell culture experiment, chito-oligosaccharide stock solution (100 mg/ml) was made using DMEM basal medium and introduced at final concentrations of 600, 1200, 2400 and 4800 μg/ml. DMEM basal medium was used as a carrier control. This range of concentration was based on a previous study where a concentration of 4000 μg/ml chito-oligosaccharide was used in the mouse 3T3-L1 cells and no adverse cytotoxic effect has been reported(Reference Cho, Rahman and Kim3).
Cell viability and cytotoxicity test
Initially, cell viability and cytotoxicity of the mouse 3T3-L1 cells were assessed in response to a range of concentrations of the different MW chito-oligosaccharides. 3T3-L1 cells were plated in a ninety-six-well cell culture plate (Greiner Bio-One Gmbh, Frickenhausen, Germany) at an initial plating density of 6 × 104 cells/ml. First, the cells were allowed to reach full confluence and then kept at a fully confluent state for 24 h before treating with chito-oligosaccharide. On the day of treatment, cells were washed with sterile PBS (Sigma-Aldrich) and incubated with serum- and antibiotic-free medium, containing chito-oligosaccharide at final concentrations of 0, 600, 1200, 2400 and 4800 μg/ml, for 24 and 48 h. Cell viability test was performed on the cell monolayer, using the 3-(4,5-dimethyl-2-yl)-2,5-diphenyltetrazolium bromide (Sigma-Aldrich) test. Cell cytotoxicity was evaluated with 25 μl cell lysate, using the Tox-7 kit (Sigma-Aldrich).
Assessment of the anti-adipogenic potential of chito-oligosaccharides
Cells were cultured to full confluence and adipogenesis was induced in the presence of a range of concentrations of chito-oligosaccharide. Intracellular lipid accumulation was visualised, free glycerol was measured and the quantitative expression of four adipogenic marker genes was determined and compared with untreated cells, as described below.
Adipogenesis
Mouse 3T3-L1 cells (6 × 104 cells/ml) were cultured in DMEM containing 10 % fetal calf serum and 1 % penistrep solution in a twelve-well cell culture plate (Greiner Bio-One Gmbh) in a 37°C humidified incubator with 5 % CO2. Before induction of adipogenesis, the pre-adipocytes were allowed to reach full confluence and grown for 48 h. Adipogenesis was induced (day 0) by introducing the DMEM containing 10 % fetal bovine serum (Gibco), 0·5 mm-3-isobutyl-1-methylxanthine (Sigma-Aldrich), 0·25 μm-dexamethasone (Sigma-Aldrich) and bovine insulin (10 μg/ml; Sigma-Aldrich) and 1 % penistrep (differentiation induction medium). After 48 h of incubation with the induction medium (day 2), the medium was removed and post-differentiation medium (DMEM, 10 % fetal bovine serum, bovine insulin (5 μg/ml) and 1 % penistrep) was introduced and incubated for 48 h. Then (day 4), the post-differentiation medium was removed and the cells were grown in DMEM containing 10 % fetal bovine serum and 1 % penistrep, and the medium was changed after every 48 h until the cells were harvested at day 8 of induction of adipogenesis. During the process of adipogenesis, chito-oligosaccharides were added to the medium in every alternate day from day 0 to 8 at final concentrations of 0, 600, 1200, 2400 and 4800 μg/ml.
Determination of lipid accumulation
At day 8 of induction of adipogenesis, the medium was removed and the cells were washed using sterile PBS (Sigma-Aldrich) and then fixed by using 10 % formalin solution (Sigma-Aldrich). Oil Red O staining of the adipocytes was performed as described previously(Reference Hansen, Petersen and Larsen25). Counter staining was performed using 1 ml of haematoxylin (Sigma-Aldrich) solution per well. The adipocytes were evaluated under a microscope and the image captured were analysed using Image-Pro 5.0 software (Media Cybernetics, Inc., Bethesda, MD, USA). Six random microscopic fields per treatment were captured at a microscopic resolution of 10 × and the Oil Red O stain was quantified and is expressed as the ratio of the area stained with Oil Red O and the total area of the microscopic field.
Determination of free glycerol
Glycerol release by the adipocyte is an indirect reflection of the TAG status of the cell(Reference Otto, Taylor and York26, Reference Stinshoff, Weisshaar and Staehler27). Free glycerol estimation was performed on the medium harvested at day 8 of the induction of adipogenesis. Free glycerol was estimated on 5 μl of the cell lysate using the Enzychrom glycerol assay kit (BioAssay Systems, Hayward, CA, USA), following the manufacturer's instructions.
RNA extraction
To compare the expression of four adipogenic marker genes in the cells following exposure to the different MW chito-oligosaccharides, RNA was extracted from the cells, complementary DNA (cDNA) was synthesised and used for quantitative real-time PCR analyses. At day 8 of induction of adipogenesis, the medium was removed and the cell monolayer was washed with sterile PBS and the cells were collected in 1 ml TRI reagent (Applied Biosystems, Foster City, CA, USA). Total RNA was extracted using the Trizol method according to the manufacturer's instructions. RNA was dissolved in 20 μl of nuclease-free water and then subjected to deoxyribonuclease I (Sigma-Aldrich) treatment to eliminate the genomic DNA contamination. Column purification of RNA was performed using the GenElute mammalian total RNA miniprep kit (Sigma-Aldrich). RNA was finally eluted in 40 μl of nuclease-free water. The quality and quantity of total RNA were assayed by analysing 1 μl of total RNA on an Agilent 2100 Bioanalyser (Agilent Technologies, Inc., Santa Clara, CA, USA) using RNA Nano LabChips® (Caliper Technologies Corporation, Hopkinton, MA, USA). All RNA samples used for the gene expression study had an RNA integrity value ≥ 8·0.
Quantitative real-time PCR
cDNA synthesis was performed with 1 μg of total RNA, using the RevertAid H minus first-strand cDNA synthesis kit (Fermentas GmbH, St Leon-Rot, Germany) following the manufacturer's protocol. The quantitative expressions of four adipogenic marker genes (glyceraldehyde 3-phosphate dehydrogenase (GAPDH), PPARγ, CEBPα and ADIPOQ) and β-actin (ACTB) as a reference gene were evaluated using a 7300 Real-Time PCR System (Applied Biosystems). The PCR mixture (20 μl) contained 1 μl cDNA (after 1:5 dilution), 1 μl forward and reverse primer mix, 8 μ1 water and 10 μl SYBR green master mix (Applied Biosystems). The thermal cycle conditions were 94°C for 30 s, followed by 60°C for 1 min, for forty cycles. The mRNA abundances are expressed in C t values, the number of PCR cycles after which the PCR product crosses a threshold value. Each sample was run in triplicate and C t values < 35 were used for analysis.
Identification of molecular mechanisms of action of chito-oligosaccharides
Based on the results obtained on the anti-adipogenic potential of the chito-oligosaccharides, 3T3-L1 cells were treated with one chito-oligosaccharide (MW 5–10 000 Da) at a range of final concentrations of 0, 600, 2400 and 4800 μg/ml. The gene expression profile of forty-four genes involved in the mouse lipid metabolic pathway was assessed in these cells. As the IL-6 gene was identified as the most sensitive gene of the forty-four genes analysed, the IL-6 gene expression data were validated at the protein level, using an ELISA assay. An IL-6 promoter assay was also developed to determine whether the promoter activity would reflect the gene expression data. The details of these methods are outlined below.
Lipid metabolic pathway genes
The quantitative expression of a panel of genes involved in lipid metabolism in mice was evaluated using ‘TaqMan Array for Mouse Lipid Regulated Genes 96-Well Plate’ (Applied Biosystems) in a 7300 Real-Time PCR System (Applied Biosystems). This is a pre-customised array, which enables screening of forty-four different genes involved in mouse lipid metabolism. According to the manufacturer, the genes included in this array had either established or a potential role in lipid metabolism, and hence it was considered suitable to screen chito-oligosaccharide-mediated inhibition of adipogenesis. Real-time RT-PCR was performed in a 20 μl reaction mixture/well containing 1 μl cDNA (after 1:5 dilution), 9 μl water and 10 μl TaqMan gene expression master mix (Applied Biosystems). The thermal cycle conditions used were as stated above.
Normalisation of the expression of the target genes included in the PCR array was performed based on two reference genes (hypoxanthine guanine phosphoribosyl transferase 1 (HPRT1) and β-glucuronidase (GUSB) and following the 2–ΔΔC t method(Reference Livak and Schmittgen28). Briefly, average ΔC t was calculated as the difference of C t values of any target gene minus average of the C t value of the two reference genes. Then, fold change was calculated as 2( − average ΔC t target gene)/2( − average ΔC t reference gene).
Promoter activation assay
Promoter construct
A 1065 bp fragment of the regulatory region (stretching from − 1064 to +1 bp relative to the transcription start site) of the human IL-6 gene was amplified by PCR. The forward primer (5′-CCTTAGATCTCTGCGTCCGTAGTTTCCTTC-3′) was designed to include a Bgl II restriction site (italicised). The reverse primer (5′-GAGTAAGCTTTGAGCCTCAGACATCTCCAG-3′) incorporated a Hind III restriction enzyme site (italicised) plus a four-nucleotide anchor at the 5′ end. PCR amplification was performed in a PTC-225 DNA Engine Tetrad (MJ Research, Inc., Waltham, MA, USA) using 1X Platinum PCR SuperMix High fidelity (Invitrogen Corporation), 0·2 μm of each of the forward and reverse primers and 50 ng of human genomic DNA (Promega Corporation, Madison, WI, USA) in a total volume of 100 μl. The PCR cycle conditions were as follows: initial 94°C for 2 min followed by thirty-nine cycles of 94°C for 45 s, 60°C for 45 s and 70°C for 1 min followed by a final extension of 72°C for 10 min. The PCR product (1065 bp) was assessed on a 1·3 % agarose gel stained with ethidium bromide. Cloning of the PCR product into firefly luciferase expression vector pGL4.17 (Promega Corporation) was performed, as described previously(Reference Bahar, O'Halloran and Callanan29). Transfection-grade endotoxin-free plasmid construct containing the human IL-6 promoter insert was prepared using the EndoFree Plasmid Maxi kit (Qiagen, Chatsworth, CA, USA).
Cell culture and transient transfection assay
The day before the transfection assay, 3T3-L1 cells (6 × 104 cells/ml) were initially cultured in DMEM containing 10 % fetal calf serum in a twenty-four-well cell culture plate (Greiner Bio-One GmbH) in a 37°C humidified incubator with 5 % CO2. The transfection cocktail (for each well) contained 25 μl DMEM basal medium, 0·3 μl FuGENE HD transfection reagent (Roche Diagnostics GmbH, Mannheim, Germany) and 100 ng of IL-6 promoter construct DNA. Following incubation at room temperature for 15 min, this cocktail was introduced dropwise onto the cells. The cells were grown on a DMEM containing 10 % fetal calf serum until the fully confluent state was attained. After 48 h of attaining full confluence, the cells were treated with chito-oligosaccharide in (1) a serum-free basal medium or (2) a serum-containing differentiation induction medium, as described earlier.
Transiently transfected cells were allowed to grow for 24 h and luciferase assay was performed using the Luciferase Reporter Assay system (Promega Corporation). In brief, the medium was removed and the cells were washed with 500 μl PBS. Lysis of the cells was performed by adding 250 μl of passive lysis buffer (Promega Corporation), followed by incubation at 37°C in a shaking incubator for 30 min at 700 rpm. Firefly-relative luciferase unit activity was measured in 20 μl of the cell lysate in a 20/20n Single Tube Luminometer (Turner Biosystems, Inc., Sunnyvale, CA, USA).
IL-6 ELISA
To support the gene expression data, a time-course study was set up to measure the IL-6 protein levels in the medium over the course of the experiment. The cell culture medium was harvested at days 2, 4 and 8 of the induction of adipogenesis and stored at − 80°C until used for analysis. Abundance of IL-6 in the culture medium was measured using the mouse IL-6 duo set ELISA development system (R&D Systems Europe, Limited, Abingdon, UK), following the manufacturer's instructions. Absorbance was measured at 450 nm against 570 nm using the ELISA plate reader. The assay was performed in duplicate for each sample.
Statistical analysis
For the Oil Red O data, each data point of the three independent assays represents the mean of six random microscopic fields per treatment. The Oil Red O and free glycerol data are expressed relative to the control sample that contained no chito-oligosaccharide. Data for all the variables were checked for a normal distribution. Oil Red O and free glycerol data were analysed using two-way ANOVA and the statistical model included the effect of concentration, MW and the interaction between concentration and MW. Within each concentration or MW, the means were compared by Tukey's test. The adipogenic marker gene expression, ELISA and promoter assay data were analysed using one-way ANOVA and the means were compared by Tukey's test. Values are expressed as means with their standard errors or standard errors of the difference of the mean, as appropriate.
Results
Cell viability and cytotoxicity
The effects of the four chito-oligosaccharides on the viability and cytotoxicity of confluent 3T3-L1 pre-adipocyte in serum-free medium are presented in the supplementary material (available online at http://www.journals.cambridge.org/bjn). For the cell viability test, the interaction effect of concentration × MW was non-significant for both 24 and 48 h. After 24 h of incubation, concentration had a significant (P < 0·001) effect on cell viability, while cell viability dropped when treated with 4800 μg/ml of all chito-oligosaccharides. The MW had no effect at 24 h. After 48 h of incubation, the concentration and MW had significant effects (P < 0·001) on cell viability. Cell viability was most affected with 4800 μg/ml of oligosaccharide and least affected by the MW 5–10 000 Da after 48 h. For cell cytotoxicity, after 24 h of incubation with chito-oligosaccharide, neither concentration nor MW had any effect. However, after 48 h of incubation, the concentration and MW had significant effects (P < 0·001). Cell cytotoxicity was most affected with 4800 μg/ml of chito-oligosaccharide and least affected by the MW 5–10 000 Da after 48 h.
Inhibition of adipogenesis
The semi-quantitative data on Oil Red O staining (Fig. 1) suggested that chito-oligosaccharides had a concentration- and MW-dependent inhibitory effect (P < 0·001 and < 0·05) on lipid synthesis. Compared with the control sample (0 μg/ml chito-oligosaccharide), the mean percentage of lipid synthesis values at 600, 1200, 2400 and 4800 μg/ml of chito-oligosaccharides were 71·1, 66·1, 49·5 and 18·8 (sed 3·24), respectively. On the other hand, the mean percentage of lipid synthesis values for < 1000, 1–3000, 3–5000 and 5–10 000 Da chito-oligosaccharides were 53·3, 53·7, 52·7 and 45·0 (sed 3·22), respectively. Among the four chito-oligosaccharides evaluated, 5–10 000 Da had the highest inhibitory effect on lipid synthesis (Fig. 1). The interaction effect of concentration × MW on the relative Oil Red O stain was not significant. The concentration effect of one chito-oligosaccharide (5–10 000 Da) at the single-cell level (40 × resolution) and that of four chito-oligosaccharides, as observed in the microscopic field (10 × resolution), are presented in Fig. 2(a) and (b), respectively. It was evident that the control had a large number of intracellular lipid droplets, while those treated with 600–4800 μg/ml of various MW chito-oligosaccharides had a visible reduction in the number and size of the lipid droplets.

Fig. 1 Effects of chito-oligosaccharide on lipid synthesis in mouse 3T3-L1 adipocytes during adipogenesis. Cells were induced to differentiate in the presence of chito-oligosaccharide (molecular weight (MW) < 1000, 1000–3000, 3000–5000 and 5000–10 000 Da). Lipid synthesised during adipogenesis was stained with Oil Red O stain at day 8 of induction of differentiation. The data are expressed relative to the chito-oligosaccharide control (0 μg/ml) group (two-way ANOVA: concentration effect P < 0·001; MW P < 0·05). Values are means from three independent replications, with standard errors represented by vertical bars. a,b,c Mean values with the same letters are significantly different in concentrations of different MW chito-oligosaccharide (P < 0·05). □, Control; ![]() , 600;
, 600; ![]() , 1200;
, 1200; ![]() , 2400; ■, 4800.
, 2400; ■, 4800.

Fig. 2 (a) Effects of chito-oligosaccharide treatment on the adipogenesis of mouse 3T3-L1 pre-adipocytes at the single-cell level (40 × resolution). Cells were induced to differentiate in the presence of chito-oligosaccharide (molecular weight < 1000, 1–3000, 3–5000 and 5–10 000 Da). (b) Lipid synthesised during adipogenesis was stained with Oil Red O stain at day 8 of induction of differentiation (10 × resolution).
Free glycerol release
The effect of chito-oligosaccharide on free glycerol release during adipogenesis is presented in Fig. 3. Chito-oligosaccharides had a concentration-dependent inhibitory effect (P < 0·001) in free glycerol release, which was not affected by the MW. Compared with the control sample, the relative free glycerol release at 600, 1200, 2400 and 4800 μg/ml of chito-oligosaccharide was 76·0, 66·8, 52·6 and 41·8 % (sed 3·00), respectively. The interaction effect of concentration × MW on free glycerol release was not significant.

Fig. 3 Effects of chito-oligosaccharide on free glycerol release during adipogenesis. Mouse 3T3-L1 pre-adipocytes were induced to differentiate in the presence of four different chito-oligosaccharides (molecular weight (MW) < 1000, 1–3000, 3–5000 and 5–10 000 Da) (two-way ANOVA: concentration effect P < 0·001; MW P = NS). Free glycerol release in the medium was estimated at day 8 of induction of adipogenesis. The data are expressed relative to the chito-oligosaccharide control (0 μg/ml) group. Values are means from three independent replications, with standard errors represented by vertical bars. □, Control; ![]() , 600;
, 600; ![]() , 1200;
, 1200; ![]() , 2400; ■, 4800.
, 2400; ■, 4800.
Quantitative expression of adipogenic marker genes
The effect of chito-oligosaccharide (MW 5–10 000 Da) on the quantitative expression of the four adipogenic marker genes during adipogenesis is presented in Fig. 4. For all the four marker genes, the control (0 μg/ml chito-oligosaccharide) had the highest level of the abundance of gene transcripts, while treatment with chito-oligosaccharide (600, 1200, 2400 and 4800 μg/ml) resulted in a significant reduction in the transcript abundance level of all the four marker genes in a concentration-dependent manner.

Fig. 4 Effect of chito-oligosaccharide (molecular weight 5–10 000 Da) on the expression of adipogenic marker genes (a) glyceraldehyde 3-phosphate dehydrogenase; (b) CCAAT-enhancer-binding protein; (c) PPARG; (d) adiponectin. during adipogenesis. Mouse 3T3-L1 pre-adipocytes were induced to differentiate in the presence of chito-oligosaccharide. At day 8 of induction of differentiation, cells were harvested and total RNA was extracted and the relative abundance of the adipogenic marker genes were determined using quantitative real-time RT-PCR. Values are means from three independent replications, with standard errors represented by vertical bars. Mean values are significantly different compared with the chito-oligosaccharide control (0 μg/ml) group for each gene: *P < 0·05, **P < 0·01, ***P < 0·001.
Expression profiling of lipid metabolic genes
Based on the lipid synthesis inhibition profile of the four chito-oligosaccharides, the MW 5–10 000 Da was evaluated across the concentrations 600, 2400 and 4800 μg/ml for the regulation of gene expression. The quantitative expression of a total of forty-four genes involved in mouse lipid metabolism is presented in Fig. 5. These forty-four genes evaluated had either established or a potential role in lipid metabolism, and hence chosen for identifying the genes with modified expression during the chito-oligosaccharide-mediated inhibition of adipogenesis. Across the concentrations tested, the expression level of IL-6 and PTGS2 (also known as cyclo-oxygenase-2) genes had a concentration-dependent increase with chito-oligosaccharide. Compared with the control, at concentrations 600, 2400 and 4800 μg/ml, the expression level of IL-6 gene was up by 4·2, 7·3 and 21·2-fold, respectively (Table 1). This expression pattern of the IL-6 gene across the concentration of chito-oligosaccharides indicated an exponential increase (fold change = 3·149 e0·0004 (chito-oligosaccharide (μg/ml)), r 2 0·99). The expression level of PTGS2 was also up by 4·2- and 16·9-fold at the chito-oligosaccharide concentrations of 2400 and 4800 μg/ml, respectively (Table 1). Down-regulation in the expression of a total of twelve genes, mostly involved in lipid biosynthesis (Fig. 5), was only evident at the chito-oligosaccharide concentration of 4800 μg/ml.

Fig. 5 Differential expression of mouse genes involved in the lipid metabolic pathways as induced by the treatment of chito-oligosaccharide (molecular weight 5–10 000 Da) during adipogenesis. Mouse 3T3-L1 pre-adipocytes were induced to differentiate in the presence of chito-oligosaccharide. At day 8 of induction of differentiation, gene expression was analysed using the TaqMan PCR array. Relative abundances of complementary DNA in the samples treated with (a) 600, (b) 2400 (c) and 4800 μg/ml chito-oligosaccharide compared with the chito-oligosaccharide control group (0 μg/ml) are shown. 1, ATP-binding cassette, sub-family G (white), member 1; 2, 3-hydroxy-3-methylglutaryl-Coenzyme A synthase 1; 3, apolipoprotein E; 4, fatty acid binding protein 5; 5, nuclear receptor subfamily 1, group H, member 3; 6, hydroxyacyl-coenzyme A dehydrogenase/3-ketoacyl-coenzyme A thiolase/enoyl-coenzyme A hydratase (trifunctional protein); 7, leukotriene C4 synthase; 8, PPARG; 9, acyl-coenzyme A dehydrogenase, very long chain; 10, stearoyl-coenzyme A desaturase 1; 11, CD36 antigen; 12, fatty acid binding protein 4.
Table 1 Regulation of the expression of lipid metabolic genes in mouse 3T3-L1 pre-adipocytes during adipogenesis as induced by chito-oligosaccharide (molecular weight 5–10 000 Da)
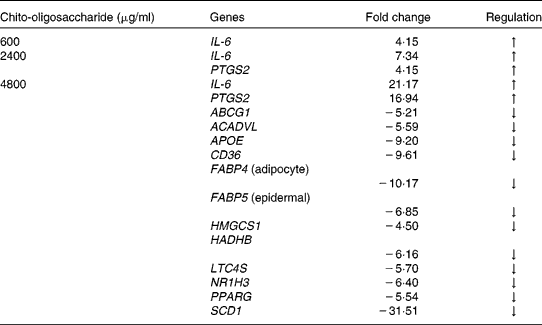
PTGS2, prostaglandin-endoperoxide synthase-2; ABCG1, ATP-binding cassette, sub-family G (white), member 1; ACADVL, acyl-coenzyme A dehydrogenase, very long chain; APOE, apolipoprotein E; CD36, CD36 antigen; FABP4, fatty acid binding protein 4; FABP5, fatty acid binding protein 5; HMGCS1, 3-hydroxy-3-methylglutaryl-coenzyme A synthase 1; HADHB, hydroxyacyl-coenzyme A dehydrogenase/3-ketoacyl-coenzyme A thiolase/enoyl-coenzyme A hydratase (trifunctional protein), β-subunit; LTC4S, leukotriene C4 synthase; NR1H3, nuclear receptor subfamily 1, group H, member 3; SCD1, stearoyl-coenzyme A desaturase 1; ↑ , up-regulation; ↓ , down-regulation.
IL-6 promoter transcriptional activity
The stimulatory effect of chito-oligosaccharide on IL-6 gene promoter activity in transiently transfected mouse 3T3-L1 cells (at a post-confluent state) is presented in Fig. 6. IL-6 promoter transcriptional activity increased (P < 0·001) due to the chito-oligosaccharide treatment in both serum-containing and serum-free cellular environments. Higher levels of IL-6 promoter activity were evident in the serum-containing differentiation medium than in the serum-free medium.

Fig. 6 Effects of chito-oligosaccharide on IL-6 promoter activity; expressed as relative luciferase units (RLU). Mouse 3T3-L1 pre-adipocytes were transiently transfected with the human IL-6 gene promoter construct and treated with chito-oligosaccharide (molecular weight 5–10 000 Da) in (a) serum-free medium or (b) serum-containing differentiation medium. Values are means from three independent replications, with standard errors represented by vertical bars. Mean values were significantly different in comparison with the chito-oligosaccharide control (0 μg/ml) group: **P < 0·01, ***P < 0·001.
IL-6 ELISA
The abundance of IL-6 at day 8 post-induction of differentiation in the presence of chito-oligosaccharide (5–10 000 Da) is presented in Fig. 7. The presence of chito-oligosaccharide resulted in the secretion of IL-6 in the cell culture medium in a concentration-dependent manner. While the cells treated with 0 μg/ml of chito-oligosaccharide had only 1·69 (sem 0·39) pg/ml of IL-6 in the medium, those treated with 600, 1200, 2400 and 4800 μg/ml of chito-oligosaccharide had 26·39 (sem 2·41), 25·78 (sem 2·54), 33·13 (sem 1·38) and 63·28 (sem 51·6) pg/ml of IL-6 (P < 0·001). Secretion of IL-6 over time by the differentiating 3T3-L1 cells in the presence of chito-oligosaccharide (2400 μg/ml) is presented in Fig. 8. At 0–48 h post-induction of adipogenesis, chito-oligosaccharide caused a sixteen-fold increase in IL-6 secretion compared with the control. During the 48–96 and 96–144 h periods, although IL-6 secretion was relatively small compared with 0–48 h post-induction of differentiation, the level of IL-6 secretion was considerably high relative to the control samples.

Fig. 7 Effects of chito-oligosaccharide on the secretion of IL-6 protein during adipogenesis. Mouse 3T3-L1 pre-adipocytes were induced to differentiate in the presence of chito-oligosaccharide (molecular weight 5–10 000 Da). At day 8 of induction of adipogenesis, IL-6 protein in the cell culture medium was quantified using ELISA. Values are means from three independent replications, with standard errors represented by vertical bars. Mean values are significantly different in comparison with the chito-oligosaccharide control (0 μg/ml) group: ***P < 0·001.

Fig. 8 Temporal effects of chito-oligosaccharide treatment (0 μg/ml (□) and 2400 μg/ml (■)) on the secretion of IL-6 protein during adipogenesis. Mouse 3T3-L1 pre-adipocytes were induced to differentiate in the presence of chito-oligosaccharide (molecular weight 5–10 000 Da). The cell lysate was harvested at 48, 96 and 144 h post-induction of adipogenesis and IL-6 protein was quantified using ELISA. Values are means from three independent replications, with standard errors represented by vertical bars.
Discussion
Adipogenesis is a vital physiological process, with excessive adipogenesis contributing to the development of obesity. In the present study, all four chito-oligosaccharides examined had concentration-dependent anti-adipogenic activities, with the 5–10 000 Da chito-oligosaccharide having the most pronounced inhibitory effect on lipid accumulation. To understand the cell-signalling events of the chito-oligosaccharide-mediated inhibition of adipogenesis, the expression of a panel of genes in the lipid metabolic pathway was evaluated. These data suggest that during the chito-oligosaccharide-mediated inhibition of adipogenesis, up-regulation of the IL-6 gene, and at higher chito-oligosaccharide concentrations, the PTGS2 gene, occurred in a concentration-dependent manner. Stimulatory activity of chito-oligosaccharide on the regulation of the IL-6 gene was also evident at transcription and translation levels. The results of the present study suggest that elevated expression levels of the pro-inflammatory cytokine IL-6, as well as PTGS2, are among the signalling molecules likely to have a fundamental role in the chito-oligosaccharide-mediated inhibition of adipogenesis.
The anti-adipogenic potential of chito-oligosaccharide was found to be influenced by the MW, where the 5–10 000 Da chito-oligosaccharide had the most pronounced inhibitory effect on lipid accumulation. It also had a better cell viability and cytotoxicity profile in comparison with the other MW chito-oligosaccharide. With regard to the anti-adipogenic potential of different MW chito-oligosaccharides, two previous studies have reported varying information. Similar to the present results, Qin et al. reported 5–10 000 Da to be more effective in weight reduction in mice compared with low ( < 1000 Da) and medium (1–3 000 Da) MW chito-oligosaccharides. In contrast, 1–3000 Da was found to be the most effective in inhibiting TAG release during adipogenesis in 3T3-L1 cells when 1–3000, 3–5000, 5–10 000 Da and their mixture were compared(Reference Cho, Rahman and Kim3). All four chito-oligosaccharides had concentration-dependent anti-adipogenic activities in the mouse 3T3-L1 pre-adipocytes, as reflected by the lipid accumulation (Oil Red O) and free glycerol release data. The anti-adipogenic potential of chito-oligosaccharide (5–10 000 Da) was further verified through quantifying the expression levels of four adipogenic marker genes (GAPDH, CEBPα, PPARγ and ADIPOQ), where the expression levels of these genes were down-regulated by the chito-oligosaccharide treatment. Profiling of mouse 3T3-L1 adipocytes treated with chito-oligosaccharide previously revealed that the inhibitory effect of chito-oligosaccharide on lipid synthesis is mediated by the modulation of adipokines and adipose tissue-specific genes such as CEBPα and PPARγ (Reference Rahman, Kumar and Kim30).
To identify the potential candidate genes involved in the chito-oligosaccharide-mediated inhibition of adipogenesis in the mouse 3T3-L1 adipocytes, and to ascertain the key signalling pathways involved, we have evaluated the quantitative expression of a panel of lipid metabolic pathway genes. This panel included representation of genes for various pathways, including lipid biosynthesis and breakdown, cytokine, chemokines, adipokines and their receptors. The genes included in this array had either an established or a potential role in lipid metabolism – a vital metabolic process reportedly affected by chito-oligosaccharides(Reference Cho, Rahman and Kim3). This screening procedure enabled the identification of a total of fourteen differentially expressed genes. This included two cytokine genes (IL-6 and PTGS2) that were up-regulated and twelve genes, mostly involved in lipid breakdown, that were down-regulated.
While IL-6 is commonly known for its dual pro- and anti-inflammatory functions, it clearly acts as an inhibitor of lipid accumulation and an inducer of fat lipolysis and oxidation in vivo (Reference Wallenius, Wallenius and Ahrén31–Reference van Hall, Steensberg and Sacchetti33) and in vitro (Reference Gustafson and Smith34). Transgenic mice deficient in the IL-6 gene were found to develop obesity and reverted back to normal once treated with IL-6(Reference Wallenius, Wallenius and Ahrén31). The anti-obesity action of IL-6 was reportedly mediated through an increase in the energy expenditure and breakdown of lipids(Reference Wallenius, Wallenius and Ahrén31). Therefore, it may be likely that the high level of IL-6 gene expression in differentiating 3T3-L1 cells treated with chito-oligosaccharide is actually responsible for the inhibition of lipid synthesis. Chito-oligosaccharide-mediated inhibition of lipid synthesis both in vitro and in vivo has been reported previously(Reference Cho, Rahman and Kim3, Reference Qin, Du and Xiao35), and the novel finding of the present study is that this inhibition is mostly probably mediated through the IL-6 signalling pathway.
The exact molecular mode of action of IL-6 during the differentiation of pre-adipocytes is not well understood because of the fact that the previous studies on IL-6 used mature adipocytes. IL-6 can directly down-regulate the expression of a number of adipogenic markers, including PPARγ, CEBPα and ADIPOQ in adipocytes, which results in decreased adipocyte differentiation and an attenuation of lipid accumulation(Reference Fasshauer, Kralisch and Klier36, Reference Moon, Lee and Seo37). This IL-6-mediated suppression of key adipogenic marker genes was reportedly mediated through the mitogen-activated protein kinase extracellular signal-related kinases signalling pathway, which can lead to a reduction in TAG synthesis in adipocytes(Reference Moon, Lee and Seo37). Therefore, in the present study, it is possible that the inhibitory role of chito-oligosaccharide on the expression of key adipogenic markers was, at least, partly due to a high level of IL-6 secretion by the 3T3-L1 cells in response to the chito-oligosaccharide treatment during the differentiation process.
In the present study, treatment of differentiating 3T3-L1 cells with chito-oligosaccharide (MW 5–10 000 Da) resulted in an increase in the IL-6 gene expression and IL-6 secretion in the medium across the concentrations tested (600–4800 μg/ml). Although a concentration of 4800 μg/ml may be considered too high to attain, under physiological conditions, a concentration-dependent increase in the IL-6 gene and protein was evident even at low chito-oligosaccharide concentrations (600 and 2400 μg/ml). Since the 5–10 000 Da-treated cells had high cell viability and low cytotoxicity profiles, it is unlikely that the gene expression, ELISA and promoter activity results were a manifestation of cell cytotoxicity caused by chito-oligosaccharide.
Another interesting gene, whose expression level was up-regulated by chito-oligosaccharide, was the PTGS2 gene. An up-regulation of the PTGS2 gene was only evident at 2400 and 4800 μg/ml of chito-oligosaccharide, and hence this gene is not considered to be as sensitive as IL-6. In adipose cells, PG endoperoxidase synthase is involved in the conversion of arachidonic acid, which is derived from the membrane phospholipids, to PG(Reference Harris, Padilla and Koumas38). In the 3T3-L1 cells, PGE2 was found to be abundantly produced at the rapidly growing sub-confluent state and its level gradually dropped when the cell reached the confluent state followed by a substantial reduction during the differentiation phase(Reference Hyman, Stoll and Spector39). The authors have suggested that cessation of cell growth at the confluent state and differentiation phases were associated with the inhibition of PGE2 production. PGE2 has also been reported to be a suppressor of the differentiation of 3T3-L1 pre-adipocyte(Reference Tsuboi, Sugimoto and Kainoh40). In diet-induced obesity of mice, PGE2 was less abundant in the tissue obtained from obese mice compared with lean mice(Reference Hetu and Riendeau41). In obese human subjects, PGE2 signalling in white adipose tissue was impaired(Reference Fain, Madan and Hiler42). In line with the previous reports, the findings of the present study suggest that chito-oligosaccharide-mediated inhibition of adipogenesis involved increased PTGS2 gene expression, which is linked to a pro-inflammatory response.
While the present study does not explore the interactions between IL-6 and PTGS2, it can be hypothesised that the elevated concentrations of IL-6 may influence the expression of the PTGS2 gene. While IL-6 expression was dependent on the concentration of chito-oligosaccharide and expressed at the lowest levels, the PTGS2 expression was only elevated at the higher chito-oligosaccharide concentrations. The promoter region of the PTGS2 gene has two binding sites for CCAAT/enhancer-binding protein-β (CEBPβ, also known as NF-IL-6)(Reference Reddy, Wadleigh and Herschman43). The CEBPβ transcription factor complex is an IL-6-dependent DNA-binding protein, hence one could expect its transcriptional activity level to increase with increased intracellular abundance of IL-6. In support of this concept, two CEBPβ sites present in the promoter region of the PTGS2 gene were important for the higher expression of the PTGS2 gene in activated murine mast cell 34(Reference Reddy, Wadleigh and Herschman43).
Among the genes whose expression was down-regulated due to the chito-oligosaccharide treatment included many genes involved in the lipid biosynthesis process. The down-regulation of the PPARγ gene has also been reported to be mediated through the action of PGE2, since the latter had an inhibitory role on the expression of PPARγ (Reference Reginato, Krakow and Bailey44). PPARγ is a potent transcription factor, which is a major activator of the differentiation process that ultimately leads to lipid accumulation in adipocytes(Reference Berger45). Our data support a previous report on the inhibition of PPARγ expression at protein and mRNA levels by chito-oligosaccharides(Reference Cho, Rahman and Kim3). A reduction in the expression of FABP4 and FABP5 genes indicated that chito-oligosaccharide-mediated inhibition of adipogenesis may have occurred during the process of fatty acid uptake, transport and metabolism(Reference Maeda, Cao and Kono46). In adipocytes, the SCD is an important enzyme required for lipid metabolism(Reference Jones, Standridge and Claycombe47). It regulates the synthesis of SFA, and a dramatic reduction in the SCD gene expression due to the chito-oligosaccharide treatment indicated a reduction in the synthesis of SFA(Reference Jones, Standridge and Claycombe47).
Surprisingly, the genes whose expression was down-regulated were conspicuous only when a high concentration of chito-oligosaccharides (4800 μg/ml) was used. Although the cell viability and cytotoxicity data indicate that chito-oligosaccharide at 4800 μg/ml after 48 h of incubation may affect cell health adversely, for the adipogenesis study, it is unlikely that the down-regulation of the genes was a manifestation of the cytotoxicity effect of chito-oligosaccharide. This is primarily because the chito-oligosaccharides in the serum-containing medium used during adipogenesis would likely to have only minimal effect on the cell health compared with the serum-free medium used for the cell viability and cytotoxicity tests(Reference Seibert, Mörchel and Gülden48, Reference Gülden, Mörchel and Seibert49). Second, during the adipogenesis process, fresh medium was introduced in every alternate day, which is likely to further minimise the cytotoxic effect due to nutrient depletion in the medium. Third, most of the marker genes of the PCR array had established their roles in the lipid metabolism process and their alteration in expression level was as expected. The exact physiological concentration of chito-oligosaccharide, which can be attainable in human subjects without adverse health effect, is presently unknown, although it is suggested that toxic side effects have not yet been reported in human subjects after oral administration of a dose as high as 3 g/d(Reference Sumiyoshi and Kimura50). Similarly, no side effects have been reported in mice after oral administration of a dose of 200–600 mg/kg per d(Reference Sumiyoshi and Kimura50, Reference Rahman, Kumar and Yun51).
In conclusion, we have identified that IL-6, and PTGS2 to a lesser extent, are among the vital signalling molecules in the chito-oligosaccharide-mediated inhibition of adipogenesis in the 3T3-L1 cells. The IL-6 produced by chito-oligosaccharide can cause a breakdown of lipid molecules as well as the prevention of lipid accumulation during adipogenesis, while the high level of PTGS2 gene expression may contribute to the PG-mediated inhibition of the differentiation of pre-adipocytes to adipocytes. Since chito-oligosaccharide has the potential to regulate the expression of IL-6 and PTGS2 genes that may inhibit adipogenesis, further in vivo and in vitro studies would be useful to explore its potential use as a functional food to reduce adipogenesis in human subjects.
Acknowledgements
The present study was funded jointly by the Department of Agriculture, Food and Fisheries, Ireland and Marine Institute under the Marine Functional Food Research Initiatives (NutraMara consortium). The authors would like to thank Dr Sam Maher and Dr Jason McMorrow of the Veterinary Sciences Centre for their technical assistance in the cell viability and cytotoxicity experiments and promoter assay experiments, respectively. B. B. contributed to the study design and laboratory work, T. S. contributed to the study design and data collection and J. V. O. D. contributed to the data analysis. All the authors contributed to the writing of the manuscript and interpretation of the results, reviewing its content and approved the final version submitted for publication. None of the authors had a financial or personal conflict of interest in relation to the present study.










