The role of bacteria in maintaining homeostasis in the human gut and throughout the body has been promoted widely in both the research community and the lay press( Reference Pollan 1 ). As this interest continues to grow, there is a greater need for clinical studies designed to establish the role of probiotics in the microbiome and its therapeutic effects on human gastrointestinal function and health( Reference Quigley 2 ). Probiotics, defined by the WHO as ‘live microorganisms which when administered in adequate amounts confer health benefits to the host’, are commercially available but differ in strain, dose and delivery method. Probiotic studies have targeted functional gastrointestinal disorders such as irritable bowel syndrome, traveller's diarrhoea and inflammatory bowel disease as well as ‘improving’ non-optimal bowel function in healthy populations, but their effectiveness is dependent on strain, dose, formulation and treatment duration( Reference Heizer, Southern and McGovern 3 ).
Bifidobacterium spp., the natural inhabitants of the colon, have been shown to survive in the human digestive tract when consumed in the form of either fermented or non-fermented dairy products as the vehicle, which strengthens their attractiveness as a potential treatment for gastrointestinal disorders and as a dietary adjunct to help maintain a healthy gastrointestinal function( Reference Kullen and Bettler 4 – Reference Fukushima, Li and Hara 8 ). For this reason, bifidobacteria have emerged as a popular probiotic. Several different species and strains have been assessed in multiple human and animal trials to investigate their potential role in improving gastrointestinal function, such as regulating transit time, inhibiting the growth of pathogenic bacteria, regulating cell growth and differentiation of gut epithelial cells and increasing immune system responsiveness( Reference Picard, Fioramonti and Francois 9 ).
However, the question remains whether consuming other Bifidobacterium animalis ssp. lactis strains might also be beneficial. The health effects of probiotics are purported to be strain specific( Reference Picard, Fioramonti and Francois 9 ). However, genome sequencing has shown that commercially available B. lactis strains are very closely related, to the point of being indistinguishable by many classic DNA analysis methods( Reference Briczinski, Loquasto and Barrangou 10 , Reference Barrangou, Briczinski and Traeger 11 ). B. animalis ssp. lactis Bf-6 (LMG 24 384) (Bf-6) has been shown to be indistinguishable from other commercially available B. lactis strains by repetitive extragenic palindromic PCR. The close relationship between Bf-6 and other B. lactis strains has been confirmed by SNP analysis and whole-genome analysis (R. Roberts, unpublished results). Therefore, the purpose of the present study was to determine whether this strain might also have similar beneficial effects on human gastrointestinal health, specifically gut function.
Randomised cross-over trials have been recommended for treatments with short-lived and reversible effects, and are appealing as each subject serves as their own control, resulting in fully powered studies with a reduced sample size( Reference Cleophas 12 – Reference Cleophas 15 ). The literature on ingestion of probiotics demonstrates that once the probiotic is stopped, biological and clinical changes generally return to baseline within 2 to 3 weeks, with a maximum demonstrated effect being observed at 6 weeks( Reference Meance, Cayuela and Raimondi 16 ). Colonic transit times (CTT) are approximately symmetric and are likely to be constant over a 12-week period, both of which are ideal conditions for a cross-over trial( Reference Fleiss 17 – Reference Senn 19 ). The present randomised, placebo-controlled cross-over trial was designed to investigate the effect of Bf-6-supplemented yogurt on CTT.
Experimental methods
Study design
A triple-blinded, placebo-controlled, two-period cross-over trial was conducted. Participants were initially randomised to either a 2-week intervention of yogurt (control) or a Bf-6 yogurt (active). Participants started consuming the first yogurt (period 1) after an initial run-in period. At the end of the run-in period, the participants completed a 2-week intervention, followed by a 6-week washout period and then finished with a 2-week intervention (period 2).
A protocol was developed a priori for subject recruitment, randomisation, and collecting follow-up primary and secondary endpoints. An independent Data and Safety Monitoring Board reviewed data and all adverse events at four a priori determined milestones: before study initiation and 33, 50 and 66 % data completion. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Georgetown University Institutional Review Board. The present trial was registered at www.clinicaltrials.gov (registration no. NCT01203462). Written informed consent was obtained from all subjects before randomisation. Appropriate measures were taken to ensure allocation concealment, and the analysis was performed according to the intention-to-treat principle.
Randomisation
All participants received two X-rays in both periods at the end of the 14 d intervention. The first cohort (n 35) was started in the middle of January, the second cohort (n 25) began in the beginning of February and the final cohort (n 8) started in the beginning of March 2011 (all groups 4 weeks apart).
For each cohort, participants were randomised in a 1:1 ratio, so that within each cohort/period, half of the subjects would receive the probiotic yogurt and half would receive the placebo yogurt. For each of the three cohorts, a separate randomisation code was developed. In order to aid in blinding and allocation concealment, the yogurt was packaged into six bins labelled with unique, randomly derived three-digit codes and accompanying colour (three codes each for ‘A’- and ‘B’-type yogurts). The three bins of each type were all made from the same batch of either the placebo or intervention yogurt. Participants were randomised in block sizes of six using permuted blocks.
Participants
Healthy adult women aged between 18 and 65 years were recruited in the Washington, DC area. Inclusion criteria were predefined as follows: ability to speak and write in English or Spanish; history of straining during bowel movements or hard or lumpy stools in the past 2 years; willingness to refrain from a list of prebiotic- and probiotic-supplemented products and certain yogurts (those containing any B. animalis ssp. lactis strain) during the 12-week trial; access to a telephone, refrigerator and freezer. Exclusion criteria were predefined as follows: allergies to any ingredients in the yogurts; BMI ≥ 40 kg/m2; history of inflammatory bowel disease; history of malabsorption syndrome; history of immunodeficiency; current chemotherapy; diabetes; use of medication to treat, prevent or cure diarrhoea or constipation within 1 month before the start of the trial; history of gastric, small bowel or colonic resection; history of gastric-emptying disorder or thyroid disorder; consumption of narcotics, antipsychotics or verapamil within 1 month before the start of the trial; known pelvic outlet obstruction; consumption of antibiotics within 2 weeks before the start of the trial; pregnancy. Participants were screened during the months of September 2010 up to March 2011 and commenced study initiation in three different cohorts. Participants were recruited from the community around the metropolitan Washington, DC area.
Interventions
Both the placebo and active interventions comprised vanilla-flavoured yogurts manufactured in a pilot facility located at Cargill, Inc. Texturizing Solutions Dairy Applications Center. Both yogurts were produced using the same standard yogurt formula and the same starter cultures of Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus, but the active probiotic yogurt was additionally inoculated with Bf-6. The dose of Bf-6 in each batch of yogurt was measured at the beginning and end of each intervention period by plating onto selective Reinforced Clostridial Agar. A fresh supply of yogurt was produced for each cohort, so no yogurt was consumed more than 4 weeks after production. The goal was to maintain a minimum dose of 1·0 × 1010 colony-forming units/serving, which was considered sufficient to have impact throughout the trial. The dose of Bf-6 ranged from 5·6 × 1010 colony-forming units/serving at the beginning of the intervention period to 2·0 × 1010 colony-forming units/serving at the end of the intervention period, a relatively small change over the 4 weeks. Participants were provided with fourteen individual four-ounce (113 g) containers of yogurts and instructed to take one per d for 2 weeks, during both periods.
Outcomes
The primary objective was to measure the effect of Bf-6-supplemented yogurt v. placebo on regulating CTT by a simplified segmental colonic transit technique( Reference Metcalf, Phillips and Zinsmeister 20 ). Starting on the 8th day of the intervention period, participants ingested a once-daily series of three distinctive SITZMARKS® capsules (Konsyl Pharmaceuticals, Inc.) at the same time each day for three consecutive days. Each capsule contained twenty-four radiopaque makers of a one shape; the O-ring marker was taken on day 1, the Double D marker was taken on day 2 and the Tri-Chamber marker was taken on day 3. Participants then received abdominal X-rays 24 and 96 h after the ingestion of the final capsule. The CTT was calculated as the sum of the markers detected on both X-rays.
Secondary outcomes were determined a priori and recorded during the registration of the trial. A modified Rome criteria questionnaire for determining irritable bowel syndrome was collected for each participant( 21 ). The Rome score is a validated instrument referring to irritable bowel syndrome symptoms for ‘the last 12 weeks’; for the purposes of the present study, the instrument was modified for ‘the last 2 weeks’. Other secondary outcomes were measured using the Gastrointestinal Symptom Rating Scale Quality of Life( Reference Svedlund, Sjodin and Dotevall 22 ) and the Bristol Stool Chart( Reference Lewis and Heaton 23 ) for the number of bowel movements/week and of those bowel movements, the number constipated. To determine nutrient intake, four separate 2 d diet recalls were collected. Participants were asked to maintain a similar diet to their baseline diet throughout the 12-week study. The diet recalls were analysed for total energy, protein, carbohydrates, fat and fibre.
Participant compliance was measured through follow-up questionnaires every 2 weeks and daily stool diaries recorded during the intervention periods. Stool samples were collected four times during the study: during the run-in period; during both intervention periods; at the end of the 6-week washout period. Stool samples were immediately frozen by the subject in a normal commercial freezer. Samples were collected by the study staff within 24 h and stored at − 80°C until DNA extraction. DNA was extracted from stool samples using the QIAamp DNA Stool Isolation Mini Kit (Qiagen)( Reference Ventura, Reniero and Zink 24 ). After extraction, the DNA was analysed by PCR using primers for subspecies-specific identification of B. animalis ssp. lactis: Bflact2 5′-GTG GAG ACA CGG TTT CCC-3′ and Bflact5 5′-CAC ACC ACA CAA TCC AAT AC-3′(24).
Adverse event data were collected at all regularly scheduled follow-ups, and participants were provided with 24 h emergency contact numbers for immediate report.
Sample size
Based on previously reported data for a sample of healthy females, their mean CTT was equal to 34·1 (sd 25·0) h( 25 ). Using these values, setting α = 0·05, β = 0·20, a total of sixty-two women would be sufficient to detect a difference of 9 h (i.e. 25 % change from baseline) between the placebo and active probiotic CTT.
Data collection
Data were collected, coded and stored at Georgetown University. Approximately 20 % of the data were double entered and verified for accuracy. The integrity of data collection and entry was monitored by a third party representative from Cape Cod Clinical Research, Inc.
Blinding and allocation concealment
Numerous steps were employed to ensure allocation concealment and blinding. Both placebo and active yogurts were identical in taste, consistency, appearance and nutritional value and arrived in identical packaging that was differentiated by a colour-coded label. Once the bin numbers were sent to the statistician, Cargill, Inc. had neither communication nor further knowledge of which yogurts the participants were receiving. Although the statistician was aware of the three bin numbers that corresponded to group ‘A’ and the three bin numbers that corresponded to group ‘B’, he was blinded to which was the active or control. All research personnel and the statistician were blinded throughout the study, including the initial analysis of all data.
Statistics
Initially, basic statistics (means, medians, interquartile ranges, variances, and frequency distribution) were used to describe the baseline characteristics. Either parametric or non-parametric statistical tests were used to test differences in health characteristics at baseline, primary and secondary outcomes, and the dietary measures at baseline. The normality of data was tested using the Shapiro–Wilk test.
The primary objective of the present study was to compare the CTT between an active probiotic period and a control (placebo) period on an individual basis. Therefore, the unit of analysis for the primary objective was the paired difference between the CTT for the control period minus the CTT for the active period. After verification of normality, the paired t-test was used to compare the mean CTT for the control period v. the mean CTT for the active period. Similarly, either the paired t-test or the Wilcoxon signed-rank test was used to compare secondary variables and dietary characteristics. CI for the differences were calculated using 95 % bootstrap techniques. One participant who became pregnant during the study was not permitted to have a second CTT; for her period 2, we imputed the general mean CTT.
Additional analysis consisted of constructing a general linear mixed model for testing the order of assignment (either active in period 1 and then control in period 2 or vice versa), treatment group (active or control) and the interaction between treatment and order. The assumptions for this model were examined using standard methods.
Data were coded and verified using SPSS (version 18; SPSS, Inc.). Statistical tests and models were implemented using JMP (version 9; SAS Institute, Inc.) and SAS (version 9.2; SAS Institute, Inc.) software. All tests were two-sided and P< 0·05 was considered statistically significant. Analyses were performed using the intention-to-treat principle.
Results
Recruitment, enrolment and participant flow
During the 6 months of enrolment from 30 September 2010 until 10 March 2011, 335 messages were left on the recruitment line or study website (Fig. 1). Eligibility could not be assessed for 167 women due to unsuccessful contact. Of the 168 women screened for eligibility, fifty-five did not meet the inclusion criteria. The remaining 113 participants were eligible and sixty-eight were enrolled. Of these eligible participants, forty-five either decided not to participate or were not needed to achieve the a priori sample size. Later, eight participants had protocol deviations: four participants either missed or completed their X-ray late; one participant became pregnant; one participant took the Sitz marker capsules incorrectly; one participant had clinically diagnosed diarrhoea; one participant received the wrong bin number. All sixty-eight participants enrolled were included in the intention-to-treat analysis (Fig. 1).
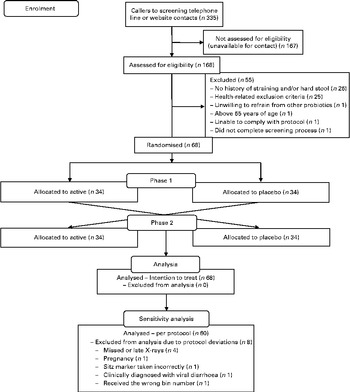
Fig. 1 Flow diagram of study participation in the cross-over trial.
Baseline demographics
All three cohorts were statistically similar with respect to demographics and health characteristics at baseline (data not shown). Baseline demographics and health are reported for the overall group (n 68; Table 1). The average age was 29 years old and the average BMI was 23 kg/m2. The majority of women had a bowel movement at least once every 3 d. There were no statistical differences among the baseline demographics and health of the active and placebo periods by initial treatment group.
Table 1 Baseline demographics and health (Mean values and standard deviations; number of participants)

* This refers to the maximum n per category; according to Institutional Review Board regulations, participants were not required to answer all baseline questions.
† On a scale of 1–10, how would you rate your overall health: 1 = very unhealthy to 10 = extremely healthy.
Compliance
The number of self-reported yogurts consumed in the 2-week periods did not differ between the groups. The group that started with the active yogurt consumed an average of 6·9 Bf-6 yogurts per week and after cross-over, they averaged 6·9 control yogurts per week. The group that started with the control averaged 6·8 four-ounce (113 g) servings of control yogurts per week and after cross-over, they averaged 6·7 four-ounce (113 g) servings of Bf-6 yogurts per week (data not shown). Overall, during the active phase, 91 % of the participants on Bf-6 yogurt tested positive for B. animalis ssp. lactis in their stools, while 94 % of the participants on control yogurt tested negative (i.e. 6 % tested positive) for B. animalis ssp. lactis (Table 2). Blinding worked appropriately as, when surveyed at the end of the study as to which order the yogurts were consumed in, 52 % of the participants who started with active yogurt correctly guessed their order, while 51 % of the participants who started on control yogurt accurately guessed their order.
Table 2 Colonic transit time and secondary outcomes by treatment group (Mean values and standard deviations; percentages and median values)
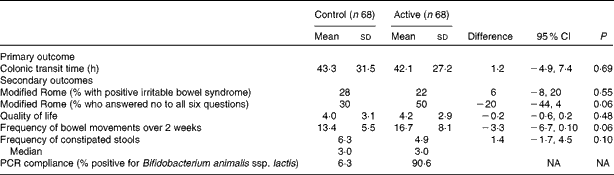
NA, not available.
Primary outcome
The distributions for CTT were statistically similar for the active and control periods, as were the medians; the average CTT was 42·1 h for the active period compared with 43·3 h for the control period (mean difference 1·2 h, 95 % CI − 4·9, 7·4; Table 2). Each treatment group had the same number of outliers.
Secondary outcomes
We examined all the other a priori determined secondary outcomes, and there were no differences between the groups (Table 2). All five nutrition components (energy, protein, carbohydrates, fat and fibre) were examined at four different times during the study and were found to be similar. Additionally, we specifically examined whether the participant diets changed during the two intervention periods, and found that there were no statistical differences (data not shown). The participant diets remained stable throughout the consumption periods.
Sensitivity analysis
The per-protocol analysis of sixty participants was analysed via imputation techniques, and the results of the primary outcome did not change (data not shown). Baseline modified Rome criteria were examined to determine the relationship to CTT success, and no relationship was found between modified irritable bowel syndrome and the CTT; the CTT was 40·3 h for those without modified irritable bowel syndrome and 47·2 h for those with modified irritable bowel syndrome (P= 0·36).
The general linear mixed model procedure( Reference Grizzle 18 , Reference Rosner 26 ), with order, treatment and interaction being terms in the model, showed that the interaction was significant (P< 0·01). This implies that the mean CTT for the active and control periods in period 2 are biased. The standard protocol then suggests examining the results of only period 1 as a traditional randomised controlled trial( Reference Rosner 26 – 28 ). Using this method, the results from period 1 showed that the mean CTT was 35·2 h for the active period v. 52·9 h for the control period (P= 0·015).
Adverse events
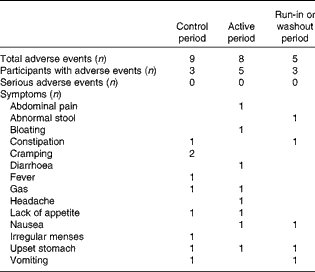
Few adverse events were reported during the entire trial; eleven participants accounted for twenty-two adverse events and no serious adverse events were reported. Both the control and active yogurts were consumed over 900 person days, with nine adverse events reported in the control period and eight adverse events reported in the active period, all of which were self-limited (Table 3).
Table 3 Adverse events by period and type
Discussion
The aim of the present study was to examine the role of Bf-6-supplemented yogurt on CTT in women with a history of straining during bowel movements or hard or lumpy stools. It was hypothesised that the Bf-6 would have an impact on the gastrointestinal tract and result in lower CTT than the standard yogurt. However, the primary results showed no clinical or statistically significant differences in the mean CTT among the active and control yogurts as the CTT were nearly identical. There were also no significant differences in the secondary outcomes.
There are a few potential reasons as to why no differences were found. According to the Food and Drug Administration regulations, one is not permitted to enrol participants in a disease state. Thus, as demonstrated in Table 1, a healthy cohort (baseline overall health 8·2; on a scale of 1–10: 1 = very unhealthy) with only limited active constipation was enrolled; only 10 % of women had bowel movements less than twice per week. Waller et al. ( Reference Waller, Gopal and Leyer 29 ) found significant differences in whole gut transit in their B. lactis probiotic intervention, but their participants were required to have one to three hard bowel movements per week. Another study by Meance et al. ( Reference Meance, Cayuela and Raimondi 16 ) on a different B. lactis probiotic found improved transit times, but subjects were randomised by baseline transit times that were considerably slower than normal standards. Another possibility is that a type II error occurred, in that Bf-6 may have an impact on CTT but only in individuals with slow CTT at baseline. No baseline CTT values were recorded, as the use of two additional X-rays was believed to be unwarranted. However, subgroup analysis showed that when examining the participants who began with placebo in period 1 and with CTT greater than 40 h (n 18), the mean difference in CTT was significant at 13·7 h. It is also possible that other more clinically relevant gastrointestinal markers or quality of life indicators are influenced by Bf-6 and need further research to elucidate.
A strength of the present study is the cross-over design, where each participant received both active and control yogurts during different periods. The net result is increased power with fewer subjects and smaller standard errors for estimation. The criticism of this design occurs when there is a significant treatment × period interaction (such as what was obtained in the present study), when the results in period 2 are influenced by the results from period 1. While there is controversy in the literature as to how to address the interaction( Reference Fleiss 17 , Reference Senn 19 ), one solution is to analyse the results in period 1 as a randomised controlled trial. For completeness, we performed this approach and the results showed a significant difference in CTT between the active period at 35·2 h (n 34) and the control period at 52·9 h (n 34) (P= 0·015) in period 1. However, since the CTT were nearly identical in the cross-over trial, we believe it is most probably a type I error. Other strengths of the present study include high rates of adherence, few protocol deviations, few adverse events and nearly 100 % complete data.
There were several limitations to the study that deserve to be mentioned. As discussed previously, it is possible that the inclusion criteria included women who were too healthy and if women were actively constipated or were required at baseline to have irritable bowel syndrome, differences may have been observed. We used a precise primary outcome of CTT because regulatory bodies in the USA and Europe accept this as an objective outcome, but other more clinically oriented outcomes may have been more appropriate measures. The literature supports our intervention period of 2 weeks, but this time frame may have been too brief to have an impact on CTT. Similarly, the washout period of 6-weeks may not have been long enough. Again, the literature supports much shorter washout periods and we were conservative with this long period. Additionally, while the CTT was the primary outcome and it appears that randomisation worked, we were ethically unable to obtain baseline CTT. We had multiple discussions among research personnel and Institutional Review Board representatives and did not believe that two additional baseline X-rays were justified. If a traditional parallel trial was conducted where baseline X-rays were performed, the sample size would have increased participants from sixty-eight to over 300 participants. Finally, not enough research has yet been conducted to definitively ascertain, but it is possible that Bf-6 has limited the ability to have an impact on CTT in healthy women.
In conclusion, the results of the paired analysis clearly showed no differences between the active and control periods. We were able to detect evidence for B. animalis ssp. lactis in the stool of patients by PCR, suggesting that intact cells made it through the digestive tract and that, therefore, Bf-6 has the potential to exhibit clinical significance. As this is one of the first studies of Bf-6, there is justification for further study of this strain under different settings and outcomes.
We thank the women who participated in the present study; the Data Safety Monitoring Board: Elizabeth Carter, Sean Karp, Felice Roggen and Alan Simon; radiologist Sandra Polin; Gwen Falony, Douwina Bosscher and Anne Frank from Cargill, Inc. for critical review of the manuscript and the following Cargill staff who formulated the test product and helped in transport to Georgetown University: Robert Loesel, Jennifer Kenney, Brian Surrat, Emelinda Gruta, Sarah Alexandroni, Alita Baker, Monica Gerds, Mark Blado, Jim Foy, Dave Williams, Jon Booker and Judy Psenko.
Acknowledgements
The present study was an investigator-initiated protocol funded by Cargill, Inc. The sponsor authors contributed to the study concept and design. The non-sponsor authors developed the initial protocol, acquired the data, supervised the double data entry, analysed the data and supplied the Data and Safety Monitoring Board reports. All non-sponsor authors were responsible for the completeness and accuracy of the results. The principal investigator and non-sponsor authors had full legal ability to publish findings. The authors have had full control of all primary data and agree to allow any data review.
The authors' contributions are as follows: D. M., F. D., C. P., K. P. and L. K. contributed to the study concept and design; D. M., A. H. and J. S. acquired the data; D. M., F. D., A. H., C. P., H. S., J. S., T. T. and R. R. analysed and interpreted the data; D. M., F. D., A. H. and T. T. drafted the manuscript; C. P., H. S., J. S. and R. R. critically reviewed the manuscript for important intellectual content; D. M., F. D., A. H. and T. T. performed the statistical analysis; D. M. and T. T. obtained funding; D. M., A. H. and J. S. supervised the study; D. M. had primary responsibility for the final content. All authors read and approved the final manuscript.
Daniel Merenstein served as a paid consultant to General Mills and Nestlé Nutrition. Kayla Polzin and Lore Kolberg were employees of Cargill, Inc. during this trial. None of the other authors has any conflicts of interest to report.





