Nerve growth factor (NGF) was the first identified and is the best-characterised member of the neurotrophic family, which consists of polypeptide growth factors. NGF influences the proliferation, differentiation, survival and death of cholinergic neurons in the central nervous system(Reference Fiore, Chaldakov and Aloe1, Reference Chao, Rajagopal and Lee2). As cholinergic neurons projecting to the hippocampus are believed to be important for memory processes(Reference Choi, Yang and Kang3), NGF is considered a therapeutic target for dementia, including Alzheimer's disease(Reference Chao, Rajagopal and Lee2). However, NGF is a high-molecular-weight polypeptide that cannot penetrate the blood–brain barrier; thus, its use requires neurosurgery(Reference Friden, Walus and Watson4). Therefore, small molecules that can readily cross the blood–brain barrier to activate receptors or potentiate the actions of NGF are required(Reference Uwabe, Iwakawa and Matsumoto5). For example, partial improvements of cognitive function in Alzheimer's disease patients have been reported with intraventricular injection of NGF protein and intravenous infusion of low-molecular-weight neuropeptides and NGF agonists to increase NGF levels(Reference Kim and Oh6).
Mori Fructus (mulberry) is the fruit of Morus alba L., of the Moraceae family. It is easily grown, cultivated worldwide and is commonly eaten as a dessert, juice, wine and vinegar. Nutritionally, Mori Fructus contains fats (1·10 %), fatty acids such as linoleic acid, palmitic acid and oleic acid, vitamin C (22·4 mg/100 g), minerals, phenolics (181 mg gallic acid equivalents/100 g) and flavonoids (29·0 mg quercetin equivalents/100 g)(Reference Ercisli and Orhan7). In traditional Oriental medicine, it has been used as an anti-ageing agent thought to act via generation of body fluids(Reference Chen, Chen and Crampton8). Mori Fructus has been shown to have various phytoconstituents with multi-bioactive functions. For example, anthocyanins, a subset of the flavonoids, are present in small berries, including the mulberry, and have antioxidant, anti-inflammatory and anti-cancer activities(Reference Peng, Liu and Chuang9). Moreover, they can cross the blood–brain barrier and modulate several cellular processes in the brain(Reference Youdim, Spencer and Schroeter10, Reference Vauzour, Vafeiadou and Rodriguez-Mateos11). Thus, there is a great deal of interest in the potential of flavonoids with regard to neuronal function and prevention of age-related neurodegeneration(Reference Vauzour, Vafeiadou and Rodriguez-Mateos11), by protecting neurons, enhancing neuronal function or stimulating neurotrophins(Reference Casadesus, Shukitt-Hale and Stellwagen12).
Previous studies showed that Mori Fructus has various biological effects, such as antioxidant, anti-inflammatory, anti-cancer, anti-obesity and hypolipidaemic activities, due to its various phytochemical constituents(Reference Peng, Liu and Chuang9, Reference Jeong, Jang and Kim13–Reference Kim, Ju and Shim15). In addition, neuro-associated actions of Mori Fructus have been reported to include an anti-stress effect(Reference Hwang and Kim16). Furthermore, it exhibited a neuroprotective effect in a cerebral ischaemia mouse model(Reference Kang, Hur and Kim17), a senescence-accelerated cognitive deficiency mouse model(Reference Shih, Chan and Liao18) and a Parkinson's disease model(Reference Kim, Ju and Shim15). Moreover, a constituent of Mori Fructus, cyanidin-3-O-β-glucopyranoside, reverses ethanol-induced inhibition of neurite outgrowth by inactivating glycogen synthase kinase 3β(Reference Chen, Bower and Xu19).
Although various neuro-associated actions of Mori Fructus extract (ME) have been reported, the effect of ME on memory has not yet been investigated. In the present study, we evaluated the memory-enhancing effect of ME, with a focus on NGF regulation, and investigated various NGF signalling events that regulate synaptic plasticity, acetylcholine (ACh) synthesisation, neuronal survival and cognitive performance.
Materials and methods
Materials
Rat monoclonal anti-5-bromo-2-deoxyuridine (BrdU), rabbit monoclonal anti-phosphorylated extracellular signal-regulated kinase (pERK), rabbit monoclonal anti-phosphorylated cyclic AMP response element-binding protein (pCREB), rabbit monoclonal anti-ERK, rabbit monoclonal anti-CREB and goat polyclonal anti-doublecortin (DCX) were purchased from Santa Cruz Biotechnology, Inc. Rabbit polyclonal anti-post-synaptic density-95 (PSD95) was purchased from Abcam. Biotinylated rabbit anti-rat antibody, horse anti-goat antibody, goat anti-rabbit antibody and avidin–biotin complex were purchased from Vector Laboratory. 3-(4,5-Dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide, chlorogenic acid, caffeic acid, ferulic acid, cinnamic acid, glucose, dimethyl sulphoxide, paraformaldehyde, 3,3-diaminobenzidine, NaCl, sucrose, ethanol, PBS, HCl, formamide, 20 × standard saline–citrate buffer, nickel chloride hexahydrate (NiCl2), phosphatase inhibitor cocktail, BrdU and mouse monoclonal anti-synaptophysin (SYN) were purchased from Sigma-Aldrich. Tetramethylethylenediamine, protein assay reagent, Tween 20, ammonium persulphate, acrylamide, enhanced chemiluminescence Western blotting detection reagent and skimmed milk were purchased from Bio-Rad Laboratory. Anti-rabbit and mouse-horseradish peroxidase secondary antibodies were purchased from Assay Designs, Inc. ChemiKine™ NGF sandwich ELISA kit and goat polyclonal anti-choline acetyltransferase (ChAT) were purchased from Chemicon International, Inc.
Preparation of extract and quantification of phenolic acids
The extract of dried mulberry fruit (ME) was the same as that used in a previous study(Reference Kim, Ju and Shim15) in which chemical profiling and standardisation of ME had been performed. Briefly, dried mulberry fruit was cultivated in Yeongcheon-si (Gyeongsangbuk-do, South Korea), purchased from Jung Do Herbal Drug Company and was extracted with 70 % ethanol for 24 h at room temperature. Then, the extract was filtered, evaporated on a rotary vacuum evaporator and finally lyophilised. The powder (yield, 20·53 %) was kept at 4°C. ME was standardised based on the concentration of cyanidin-3-O-β-glucopyranoside, and as a result, the content of cyanidin-3-O-β-glucopyranoside in ME was 0·43 (sd0·02) mg/g.
In addition, we quantified phenolic acids in ME based on the contents of chlorogenic acid, caffeic acid, ferulic acid and cinnamic acid using reverse-phase HPLC (PerkinElmer 200 HPLC system; PerkinElmer, Inc.) equipped with a photodiode array detector. Separation was carried out using a Shiseido Capcellpak C18 column (250 × 4·6 mm, 5 μm; Shiseido Co., Ltd) at 25°C. The injection volume was 10 μl. The mobile phases (A: 0·1 % acetic acid in methanol and B: 0·1 % acetic acid in water) were 10–10 % A for 0–10 min; 10–25 % A for 10–15 min; 25–35 % A for 15–25 min; 35–50 % A for 25–35 min; 50–95 % A for 35–45 min; and 95–10 % A for 45–60 min at a flow rate of 1 ml/min. The detector wavelength was set at 280 nm. Four concentrations of chlorogenic acid, caffeic acid, ferulic acid and cinnamic acid were prepared at 1, 2·5, 5 and 10 μg/ml, where 10 μl was injected as external standard. References to the calibration curve obtained with those four external standards and ME were analysed in triplicates. Chlorogenic acid, caffeic acid, ferulic acid and cinnamic acid were found in ME at mean levels of 2·43 (sd0·12), 1·59 (sd0·12), 0·21 (sd0·06) and 0·04 (sd0·00) mg/g, respectively. Before each experiment, the extract was dissolved in an appropriate vehicle and was vortex mixed for 2 min at room temperature.
Animals and treatment
Animal treatment and maintenance were carried out in accordance with the Principle of Laboratory Animal Care (National Institutes of Health publication no. 85-23, revised 1985) and the Animal Care and Use Guidelines of Kyung Hee University, Seoul, South Korea. Male ICR mice (outbred, albino, 6 weeks, 25–28 g) were purchased from Daehan Biolink Company Limited. Animals were housed five per cage, had free access to water and food and maintained under a constant temperature (23 ± 1°C), humidity (60 ± 10 %) and a 12 h light–12 h dark cycle. The mice were randomly divided into four groups (n 10 in each group): (1) a vehicle-treated group (control group); (2) a 20 mg/kg per d ME-treated group; (3) a 100 mg/kg per d ME-treated group; and (4) a 500 mg/kg per d ME-treated group. ME was dissolved in normal saline with 10 % dimethyl sulphoxide and administered orally once per d for 7 d. The orally administered doses of ME were derived from the previous report and chosen from the lower doses of it(Reference Yang, Yang and Zheng14). The control group was administered with an equal volume of saline with 10 % dimethyl sulphoxide. Independently of drug treatment, BrdU, a thymidine analogue, which incorporates into the DNA of dividing cells during the S phase of the cell cycle, was used to label the dividing cells(Reference Struikmans, Rutgers and Jansen20). Mice were intraperitoneally injected with BrdU (50 mg/kg) three times at 3 h intervals on the day before they were killed for the preparation of brain tissues, as described elsewhere(Reference Kim, Kim and Park21), with minor modification.
The step-through passive avoidance test
On day 7 of ME treatment, learning and memory were evaluated using a two-compartment step-through passive avoidance apparatus, according to the method described previously(Reference Choi, Yang and Kang3). A box was divided into bright (21 × 21 × 21 cm) and dark (21 × 21 × 21 cm) compartments by a guillotine door. The bright compartment contained a 50 W electric lamp, while the floor of the dark compartment consisted of 2 mm stainless steel rods spaced 1 cm apart. Final administrations were performed 1 h before the acquisition trial. Each mouse was placed in the bright compartment, and the door separating the two compartments was opened 10 s later. Then, after the mouse fully entered the dark chamber, the guillotine door closed and an electrical foot shock (0·1 mA/10 g) was delivered through the grid floor for 3 s. At 24 h after the acquisition trial, each mouse was placed in the bright chamber for a retention trial. Latency was defined as the time it took for a mouse to enter the dark chamber after the door opened. Latency time was recorded for up to 600 s.
Object recognition test
The experiments were carried out on day 7 of ME treatment. Prior to the test, mice were habituated to the test box, a black open field box (45 × 45 × 50 cm), for 5 min without objects. After a habituation period, mice were placed into the test box with two identical objects and allowed to explore for 3 min. The objects used in the present study were wooden blocks of the same size but different shape. The time spent by the animal exploring each object was measured (defined as the training session). At 24 h after the training session, mice were allowed to explore the objects for 3 min, in which one familiar object used in the previous training session was placed with a novel object. The time that the animals spent exploring the novel and the familiar objects were recorded (defined as the test session). The animals were regarded to be exploring when they were facing, sniffing or biting the object. The test box and objects were cleaned with 70 % ethanol between sessions. Results were expressed as percentage of novel object recognition time:
Brain tissue preparation
At 24 h after the BrdU injection, mice were immediately anaesthetised using a mixture of Zoletil 50 and Rompun solution (3:1 ratio, 1 ml/kg, intraperitoneally), perfused transcardially with 0·05 m-PBS and then fixed with cold 4 % paraformaldehyde in 0·1 m-phosphate buffer. Brains were removed and post-fixed in 0·1 m-phosphate buffer containing 4 % paraformaldehyde overnight at 4°C and then immersed in a solution containing 30 % sucrose in 0·05 m-PBS for cryoprotection. Serial 30 μm-thick coronal sections were cut on a freezing microtome (Leica) and stored in cryoprotectant (25 % ethylene glycol, 25 % glycerol and 0·05 m-phosphate buffer) at 4°C until use for immunohistochemistry. For NGF ELISA assay and Western blotting, mice were decapitated and their brains were rapidly excised. Hippocampus tissues were stored at − 70°C until performing NGF ELISA kit assay and Western blotting.
Nerve growth factor ELISA assay
Total NGF levels were detected using the NGF sandwich ELISA kit, according to the instruction manual. Briefly, hippocampus tissues were homogenised in ice-cold homogenisation buffer and centrifuged at 14 000 g for 30 min at 4°C. The resulting supernatants were used for the assay. Standards and tissue samples were incubated with a sheep anti-mouse NGF polyclonal antibody-pre-coated ninety-six-well immunoplate overnight at 4°C. The plate was washed at least four times and incubated with anti-mouse NGF monoclonal antibody (1:100 dilution) for 2 h at room temperature. After washing, the plate was incubated with donkey anti-mouse IgG polyclonal antibody (1:1000 dilution) for 2 h at room temperature. The plate was incubated with 3,3',5,5'-tetramethylbenzidine/ELISA substrate for 10 min and a stop solution was added to each well. The plate was read at 450 nm by using a spectrophotometer (Versamax microplate reader; Molecular Device), and concentrations of NGF were determined in the sample solution using a NGF standard calibration curve. Protein from the samples was normalised by using the Bio-Rad assay for total protein determination.
Immunohistochemistry
For immunohistochemical detection of BrdU, brain sections were pretreated with 50 % formamide in 2 × standard saline–citrate buffer at 65°C for 2 h before being incubated at 37°C for 30 min in 2 m-HCl. The sections were then rinsed for 10 min at 25°C in 0·1 m-boric acid (pH 8·5). Brain sections were briefly rinsed in PBS buffer and treated with 1 % H2O2 for 15 min. The sections were incubated with rat anti-BrdU antibody (1:500 dilution) overnight at 4°C in the presence of 0·3 % Triton X-100 and 3 % normal horse serum. After rinsing in PBS buffer, the sections were then incubated with biotinylated anti-rat IgG (1:200 dilution) for 90 min and then with avidin–biotin complex (1:100 dilution) for 1 h at room temperature. Peroxidase activity was visualised by incubating sections with nickel-3,3-diaminobenzidine in 0·05 m-Tris-buffered saline (pH 7·6). For the immunohistochemical study of DCX, brain sections were incubated overnight at room temperature with goat anti-DCX antibody (1:500 dilution). Then, the sections were incubated with secondary antibody following the same procedures as for BrdU. Peroxidase activity was visualised by incubating sections with 3,3-diaminobenzidine in Tris-buffered saline. After several rinses with PBS, sections were mounted on gelatin-coated slices, dehydrated and coverslipped with a histomount medium. The number of BrdU- and DCX-immunoreactive cells and the neurite length in the dentate gyrus (DG) of hippocampus were estimated by measuring at 200 × magnification using an AnalySIS LS Research (Soft Imaging System Limited). The numbers of cells or the lengths of dendrites were measured in six sections per animal by a person blind to the treatment group, and the average per section was computed. The images were photographed at 200 × magnification using an optical light microscope (Olympus Microscope System BX51; Olympus) equipped with a 20 × objective lens. Data are presented as percentages of control group values.
Western blotting
The brain tissues were lysed with a triple-detergent lysis buffer to detect pERK, pCREB, SYN, PSD95 and ChAT according to the manufacturer's instructions. The lysates were separated by 12 or 10 % SDS-PAGE and then transferred to a membrane. Membranes were incubated with 5 % skimmed milk in Tris-buffered saline and Tween 20 for 1 h and then with the primary antibodies (1:5000 dilution of pERK, ERK, pCREB and CREB; 1:500 dilution of SYN and ChAT; 1:2000 dilution of PSD95 and β-actin) overnight at 4°C, followed by incubation with horseradish peroxidase-conjugated secondary antibodies for 1 h. Immunoreactive bands were detected using an enhanced chemiluminescence detection kit, and a LAS-4000 mini system (Fujifilm Corporation) was used for visualisation. The intensities of the bands were normalised to the non-phospho-form band or β-actin band using Multi Gauge software (Fujifilm Corporation).
Statistical analysis
All quantitative data were analysed. The results are expressed as means and standard deviations for phenolic contents or standard errors of the mean for others. Statistical significance was determined by one-way ANOVA followed by Tukey's post hoc test using GraphPad Prism 4.0 software (GraphPad Software Inc.). P values less than 0·05 were deemed to be statistically significant.
Results
Effects of Mori Fructus extract on up-regulation of nerve growth factor in the mouse hippocampus
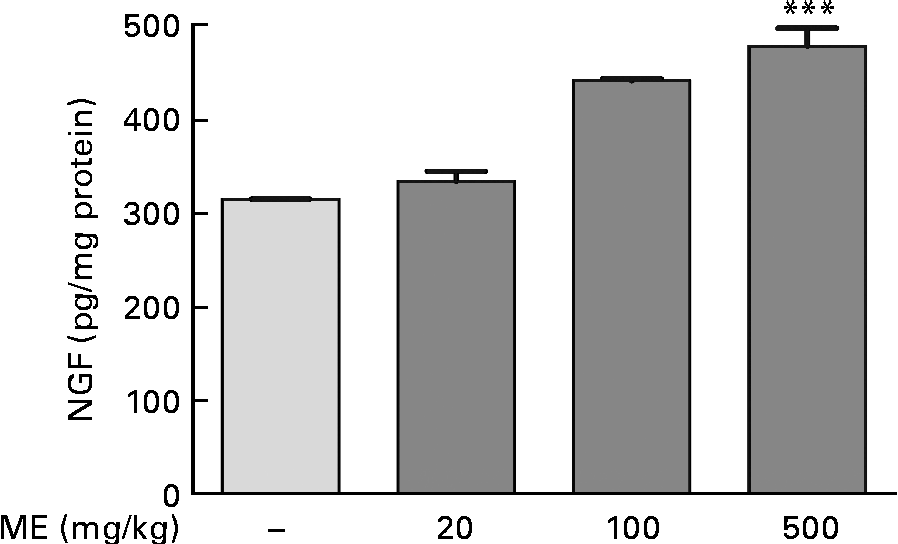
To investigate the effects of ME on NGF production in the mouse hippocampus, we performed the NGF ELISA kit assay. In our preliminary study, 100 μg/ml ME treatment for 24 h in C6 glioma cells showed NGF increase up to 122 % compared with the control group (100 %) and no significant cell toxicity was observed (data not shown). Thus, the NGF-inducing effect of ME was confirmed in the mouse hippocampus after 7 d of ME treatment. NGF concentration was 333 (sem11·1), 441 (sem2·04) and 478 (sem19·2) pg/mg protein in the mouse hippocampus after ME treatment of 20, 100 and 500 mg/kg per d, respectively, compared with the control group (314 (sem0·29) pg/mg protein). The maximal effect was shown at 500 mg/kg per d of ME treatment (Fig. 1). Therefore, these results suggest that ME acts as an NGF inducer.

Fig. 1 Effect of Mori Fructus extract (ME) on nerve growth factor (NGF) production in the mouse hippocampus. Mice were administrated with ME at 20, 100 and 500 mg/kg per d for 7 d. The NGF level in the mouse hippocampus was measured using the NGF sandwich enzyme-linked immunosorbent assay. Values are means, with their standard errors represented by vertical bars. *** Mean value was significantly different from that of the control group (P< 0·001).
Effects of Mori Fructus extract on phosphorylation of extracellular signal-regulated kinase and cyclic AMP response element-binding protein in the mouse hippocampus
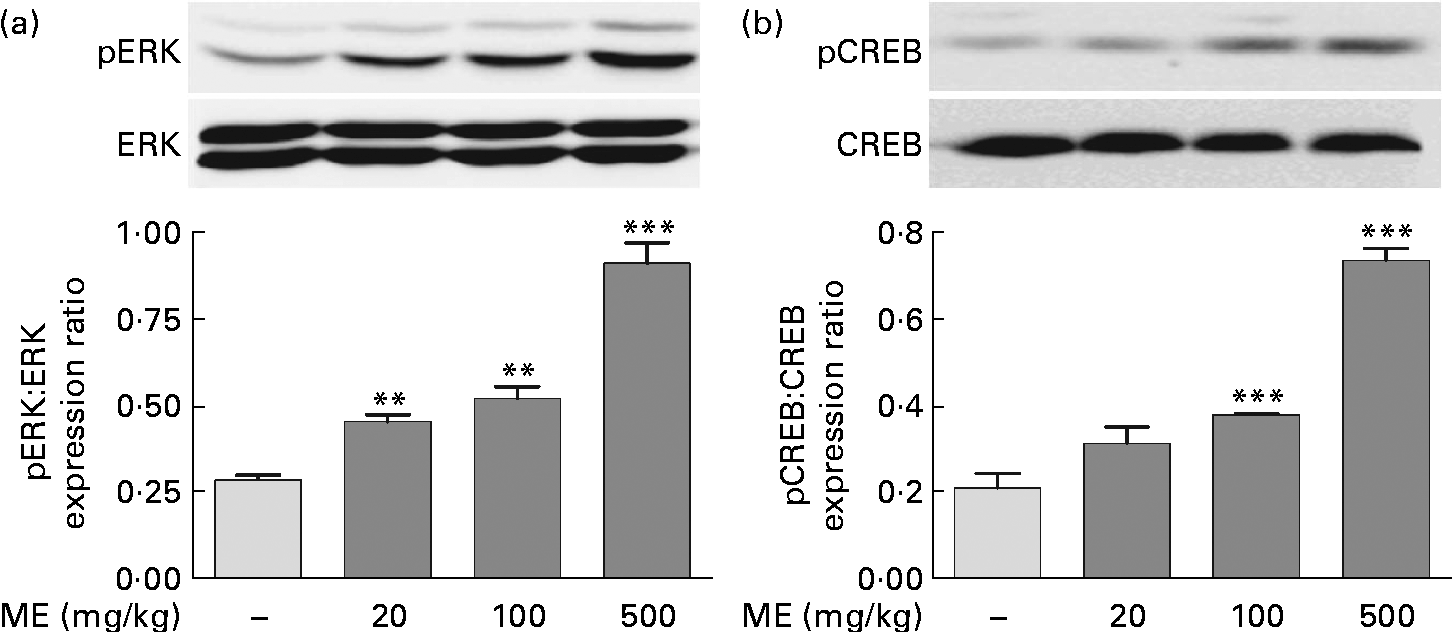
To evaluate the effects of ME on pERK and pCREB elevations, which were stimulated by NGF in the adult hippocampus(Reference Monshipouri, Jiang and Lazarovici22), Western blotting was performed using the mouse hippocampus. pERK/ERK expression level was elevated to 159 (sem7·45), 182 (sem11·9) and 321 (sem19·9) % in the ME treatment groups at 20, 100 and 500 mg/kg per d, respectively, compared with the control group (Fig. 2(a)). CREB, a transcription factor, is activated downstream of ERK and is a key signalling molecule involved in learning and memory(Reference Bourtchuladze, Frenguelli and Blendy23). pCREB/CREB expression level was elevated to 150 (sem17·8), 182 (sem0·47) and 353 (sem13·5) % at 20, 100 and 500 mg/kg per d of ME treatment, respectively, compared with the control group (Fig. 2(b)). Thus, we found that inducing effect of ME on NGF production resulted in phosphorylation of ERK and CREB.

Fig. 2 Effect of Mori Fructus extract (ME) on phosphorylation of extracellular signal-regulated kinase (ERK) and cyclic AMP response element-binding protein (CREB) in the mouse hippocampus. The phosphorylated ERK (pERK), ERK, phosphorylated CREB (pCREB) and CREB proteins in the hippocampal tissue were expressed using Western blotting after the administration of ME (20, 100 and 500 mg/kg per d, per os) for 7 d. The graphs display densitometric analyses of the expression ratios of (a) pERK:ERK and (b) pCREB:CREB. Values are means, with their standard errors represented by vertical bars. Mean value was significantly different from that of the control group: ** P< 0·01, *** P< 0·001.
Effects of Mori Fructus extract on synaptic formation and acetylcholine synthesisation in the mouse hippocampus
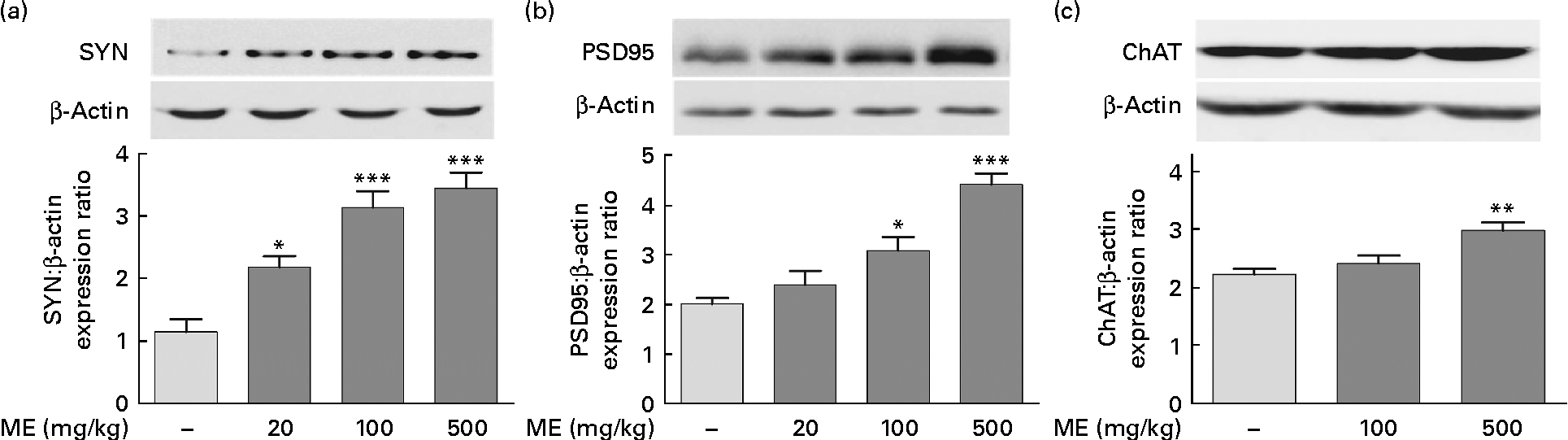
To investigate the effects of ME on the pre- and post-synaptic density in the hippocampus, SYN and PSD95 expression levels were analysed, respectively, in brain tissue. CREB phosphorylation plays key roles in the synaptic strength, resulting in learning and memory(Reference Deisseroth, Bito and Tsien24). ME treatment at 20, 100 and 500 mg/kg per d increased SYN levels in the hippocampus to 191 (sem 15·5), 275 (sem 23·5) and 302 (sem 22·1) %, respectively, compared with the control group (Fig. 3(a)). In addition, ME treatment at 20, 100 and 500 mg/kg per d increased PSD95 level in the hippocampus to 118 (sem14·3), 153 (sem13·7) and 219 (sem10·5) %, respectively, compared with the control group (Fig. 3(b)). Then, to examine the effect of ME on cholinergic function in the hippocampus, ChAT, an ACh-synthesising enzyme, expression levels were analysed, because hippocampal ACh release plays a key role during memory processing(Reference Choi, Moon and Kim25). ME treatment at 100 and 500 mg/kg per d increased ChAT level in the hippocampus to 108 (sem6·31) and 134 (sem6·22) %, respectively, compared with the control group (Fig. 3(c)). Through activation of NGF downstream signalling, ME could enhance synaptic formation and ACh synthesisation.

Fig. 3 Effect of Mori Fructus extract (ME) on pre- and post-synaptic formation and acetylcholine synthesisation in the mouse hippocampus. The synaptophysin (SYN), post-synaptic density-95 (PSD95) and choline acetyltransferase (ChAT) proteins in the hippocampal tissue were expressed using Western blotting after the administration of ME (20, 100 and 500 mg/kg per d, per os) for 7 d. The graphs display densitometric analyses of the expression ratios of (a) SYN:β-actin, (b) PSD95:β-actin and (c) ChAT:β-actin. Values are means, with their standard errors represented by vertical bars. Mean value was significantly different from that of the control group: * P< 0·05, ** P< 0·01, *** P< 0·001.
Effects of Mori Fructus extract on cell differentiation, neurite outgrowth and cell proliferation in the mouse hippocampus
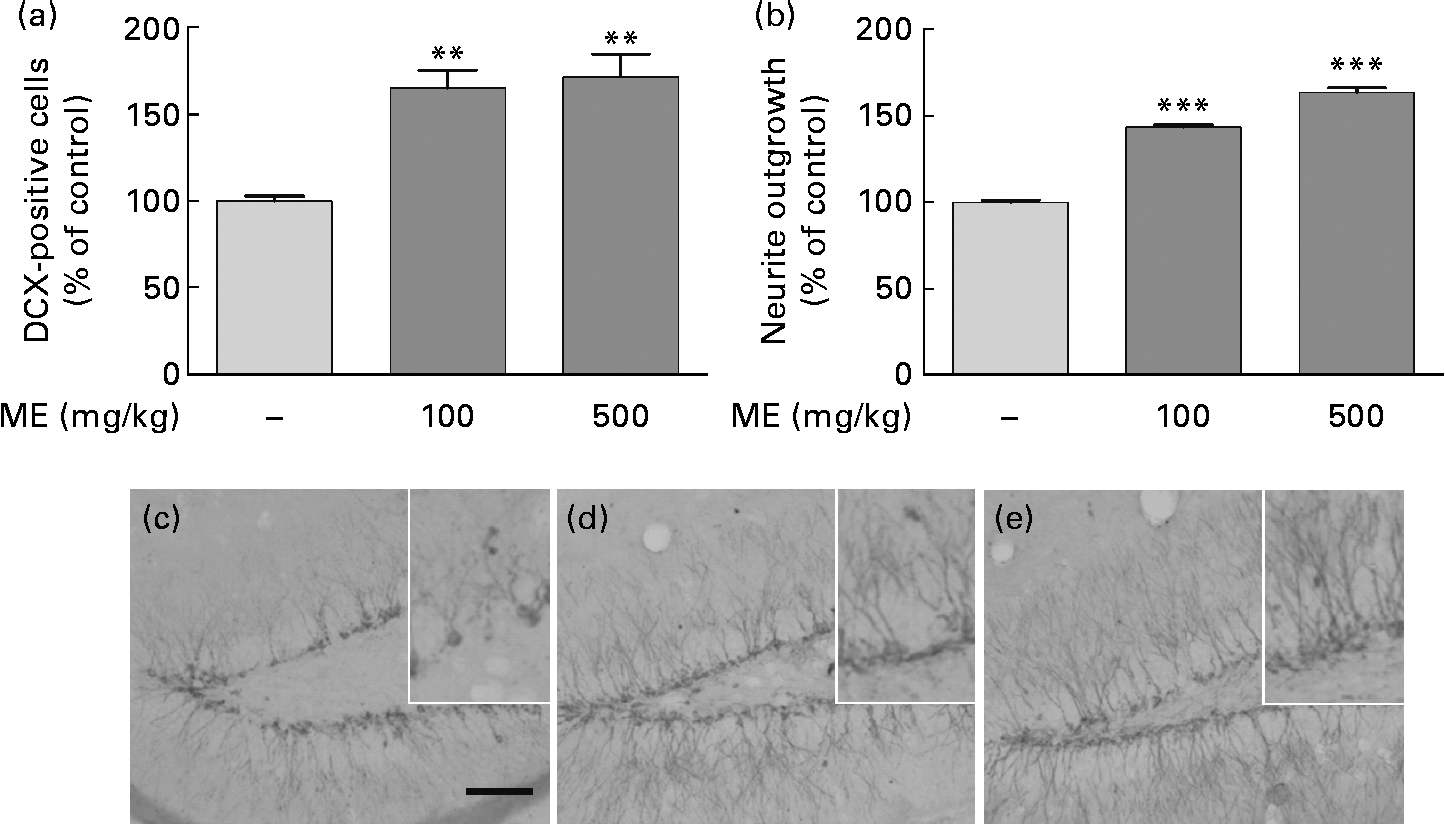
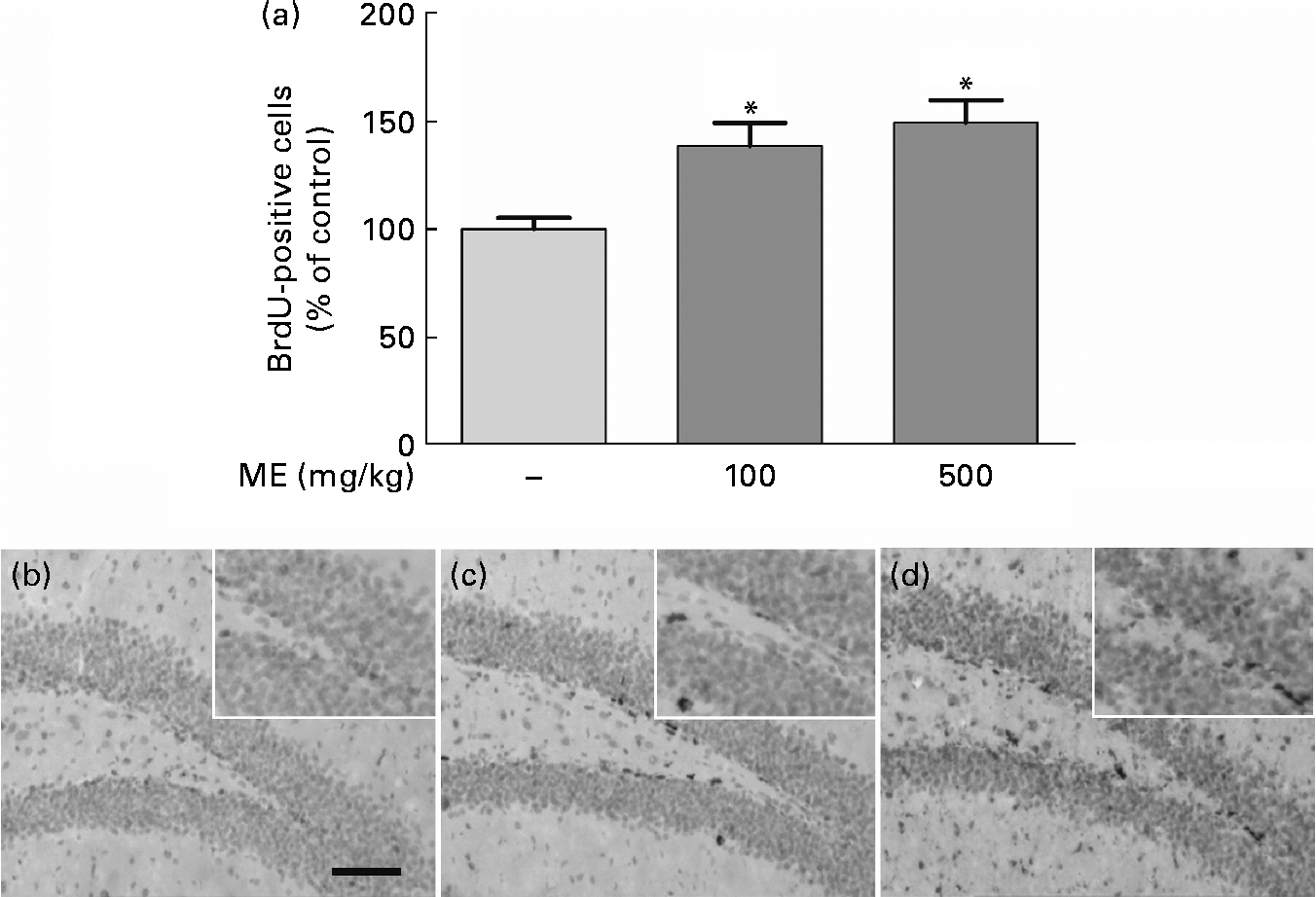
To evaluate the effects of ME on neuronal cell differentiation and neurite outgrowth, we performed DCX and BrdU immunohistochemistry in the mouse hippocampus. pERK and pCREB, the activated forms mediated by NGF, play an important role in cell differentiation, neurite outgrowth, cell proliferation and cell survival(Reference Obara, Yamauchi and Takehara26, Reference Schenning, Goedhart and Gadella27). As shown in Fig. 4(a), ME treatment at 100 and 500 mg/kg per d increased DCX-positive cells in the DG of the hippocampus to 165 (sem10·4) and 172 (sem13·5 ) %, respectively, compared with the control group. ME also promoted neurite outgrowth of DCX-positive neurons in the DG to 144 (sem1·35) and 164 (sem2·57 ) % at 100 and 500 mg/kg per d, respectively, compared with the control group (Fig. 4(b)). Then, we analysed the BrdU-positive cells in the DG of the hippocampus to investigate whether the effect of ME resulted in neuronal cell proliferation. As shown in Fig. 5, ME treatment at 100 and 500 mg/kg per d increased BrdU-positive cells in the DG to 139 (sem10·8) and 149 (sem10·5) %, respectively, compared with the control group. Therefore, ME treatment enhanced neuronal cell survival by differentiation, neurite growth and proliferation of cells in the hippocampus.

Fig. 4 Effects of Mori Fructus extract (ME) on neuronal cell differentiation and neurite outgrowth using doublecortin (DCX) immunostaining in the mouse hippocampus. Mice were treated with ME at 100 and 500 mg/kg per d for 7 d. The DCX-immunoreactivities were quantified by counting the number of (a) DCX-positive cells and by measuring the (b) neurite outgrowth in the dentate gyrus (DG). (c)–(e) Representative photographs for the DG of each group. (c) Control group; (d) 100 mg/kg per d ME-treated group; (e) 500 mg/kg per d ME-treated group. Scale bar = 50 μm. Values are means, with their standard errors represented by vertical bars. Mean value was significantly different from that of the control group: ** P< 0·01, *** P< 0·001.

Fig. 5 Effects of Mori Fructus extract (ME) on neuronal cell proliferation using 5-bromo-2-deoxyuridine (BrdU) immunostaining in the mouse hippocampus. Mice were treated with ME at 100 and 500 mg/kg per d for 7 d. (a) The BrdU-incorporated cells were quantified by counting the number of positive cells in the DG. (b)–(d) Representative photographs were shown for the dentate gyrus of each group. (b) Control group; (c) 100 mg/kg per d ME-treated group; (d) 500 mg/kg per d ME-treated group. Scale bar = 50 μm. Values are means, with their standard errors represented by vertical bars. * Mean values were significantly different from the control group (P< 0·05).
Effect of Mori Fructus extract on learning and memory enhancement in mice
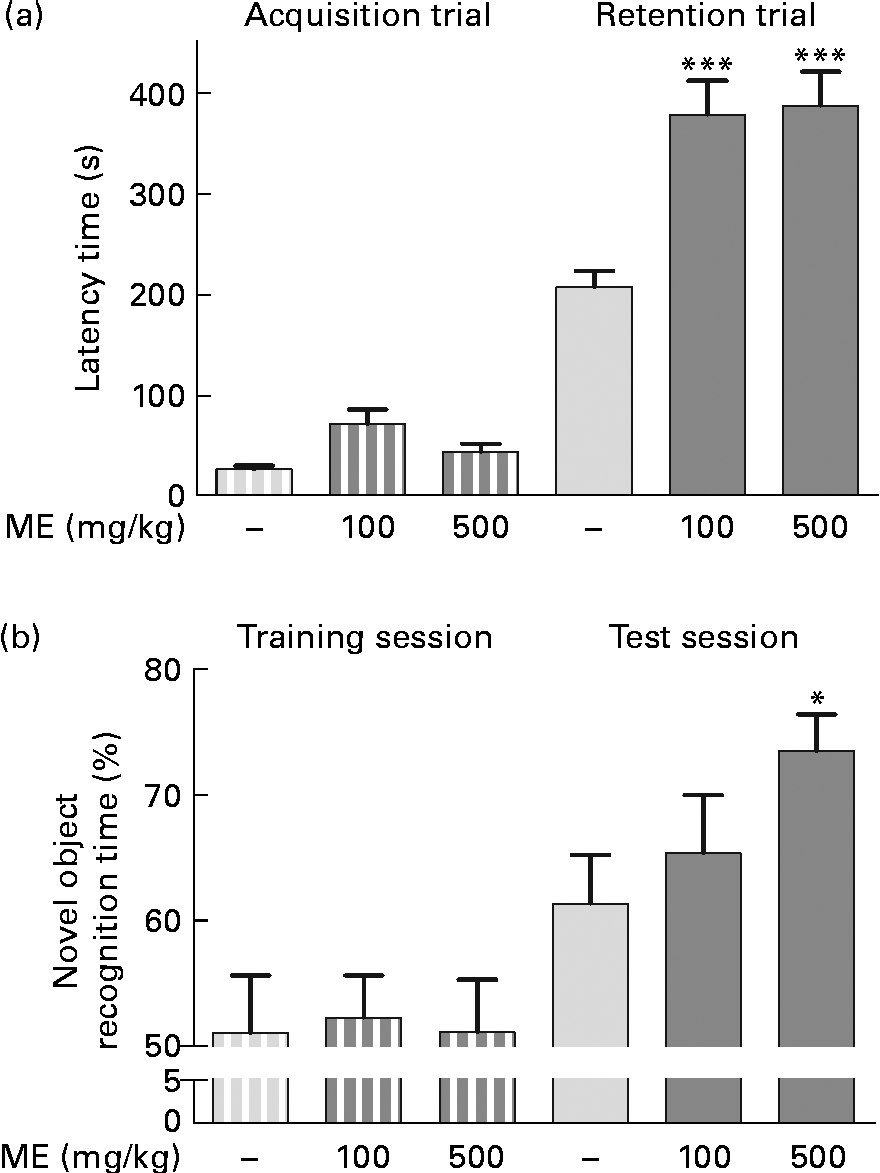
To evaluate the effects of ME on learning and memory enhancement, a passive avoidance test and an object recognition test were performed, and the retention latency period and novel object recognition time of the mice were analysed as behavioural differences, respectively. The mean retention time of the 100 and 500 mg/kg per d ME-treated groups were 378 (sem33·8) and 387 (sem33·4) s, respectively, which were significantly longer than that of the control group (207 (sem15·8 ) s), whereas the latency time of all groups for the acquisition trial was similar (Fig. 6(a)). In the object recognition test, rodents typically prefer novel or moved objects by exploring then for more time compared with familiar objects(Reference Sutcliffe, Marshall and Neill28). Mice fed 100 and 500 mg/kg ME spent more time on novel objects than the control during the test session by 65·3 (sem4·64) and 73·4 (sem2·92) %, respectively, whereas they spent a similar time on two familiar objects (Fig. 6(b)). The result suggests that ME improves learning and memory in mice.

Fig. 6 Effects of Mori Fructus extract (ME) on cognitive behaviour-related memory, as determined by a passive avoidance test and an object recognition test. Mice were treated with ME at 100 and 500 mg/kg per d for 7 d and (a) a passive avoidance test and (b) an object recognition test were conducted on day 7. Bars indicate the latency time to enter the dark compartment during the acquisition trial or training session (![]() ) and 24 h later during the retention trial or test session (
) and 24 h later during the retention trial or test session (![]() ). Values are means, with their standard errors represented by vertical bars. Mean value was significantly different from that of the control group: * P< 0·05, *** P< 0·001.
). Values are means, with their standard errors represented by vertical bars. Mean value was significantly different from that of the control group: * P< 0·05, *** P< 0·001.
Discussion
In the present study, the NGF-inducing effect of ME and its downstream pathway, phosphorylation of ERK and CREB, were investigated in a mouse model. More specifically, ME promoted NGF-mediated functions such as hippocampal synapse formation, cell differentiation, neurite outgrowth and cell proliferation, resulting in memory enhancement.
First, to determine whether ME acts as an NGF inducer, we measured NGF content in the mouse hippocampus. ME induced NGF production in a dose-dependent manner after treatment at 100 and 500 mg/kg per d for 7 d (Fig. 1). Therefore, we measured pERK and pCREB expression levels, both of which are related to NGF induction. NGF is mainly mediated by a high-affinity tyrosine kinase A receptor, which acts as a docking site for various active downstream signalling and adaptive proteins(Reference Rankin, Guy and Mearow29). The distinguishing feature of the NGF signalling pathway is activation of the mitogen-activated protein kinase pathway, which induces continuous ERK activity. In addition, ERK activation induces the nuclear translocation and nuclear activation of ribosomal S6 kinase 2 (RSK2), which in turn phosphorylates CREB to stimulate cyclic AMP response element-dependent transcription(Reference Nagase, Yamakuni and Matsuzaki30). The activation of transcription factors regulates NGF-inducible gene regulation, resulting in NGF-induced neurite formation, memory formation and neurogenesis(Reference Deisseroth, Bito and Tsien24, Reference Watson, Heerssen and Bhattacharyya31). In the present study, ME treatment activated ERK and CREB by phosphorylation, together with induction of NGF in the mouse hippocampus (Fig. 2). These results indicate that ME possesses NGF-inducing activities and phosphorylates ERK and CREB in mice.
Next, to investigate whether ME affects synapse formation and ACh synthesisation, we measured the expression of SYN, PSD95 and ChAT as pre- and post-synaptic markers and ACh-synthesising enzyme, respectively, in the mouse hippocampus. CREB is known to influence memory by modulating new synapses and the restructuring of existing synapses. SYN is the most abundant integral synaptic vesicle protein(Reference Choi, Yang and Kang3), and PSD95 is a neuronal PSD95/discs-large/zonula occludens-1 (PDZ) protein that associates with receptors and cytoskeletal elements at synapses(Reference El-Husseini, Schnell and Chetkovich32). ChAT is an enzyme that results in the formation of ACh(Reference Hellweg, Fischer and Hock33). Therefore, these are frequently used to quantify synapses. In the present study, ME increased the expression levels of SYN, PSD95 and ChAT, suggesting enhancement of synapse formation and ACh synthesisation mediated by elevated NGF levels (Fig. 3). Several synaptic proteins have been investigated with regard to their potential importance when lost or deficient in dementia, and synapse formation is related to memory formation and enhancement(Reference Pizzo and Thal34). The above results suggest that ME exhibits a memory-enhancing effect mediated by synapse formation through the NGF–ERK–CREB pathway.
Finally, to determine whether ME influenced cell survival and function, we quantified newly generated cells and neurite outgrowth in the DG of the hippocampus by DCX and BrdU staining. NGF increases the number of newly generated cells and promotes cell survival and memory enhancement(Reference Kim, Kim and Park21, Reference Kempermann, Gast and Kronenberg35). DCX is a microtubule-associated protein that is specifically expressed in virtually all migrating neuronal precursors in the central nervous system, and is correlated with cellular proliferation and dendritic growth of newly generated neurons in the DG(Reference Couillard-Despres, Winkler and Uyanik36, Reference Rao and Shetty37). In the present study, ME increased both the number of DCX-positive cells and neurite outgrowth in the DG of the hippocampus (Fig. 4). Furthermore, because BrdU is a synthetic nucleoside that is commonly used to detect proliferating cells, BrdU-incorporating cells were quantified to investigate cell proliferation. ME treatment increased the number of BrdU-positive cells in the DG of the hippocampus (Fig. 5). The production of newly generated cells in the DG of the hippocampus has been suggested to enhance memory(Reference Kempermann, Gast and Kronenberg35). Thus, the effects of ME on learning and memory enhancement were investigated, and we found that ME significantly prolonged the retention time and novel object recognition time, indicating enhancement of learning and memory (Fig. 6). Taken together, these findings showed that subchronic administration of ME markedly increased the level of NGF and the expression of pERK and pCREB, suggesting that the NGF–ERK–CREB signalling pathway plays an important role in cell synapse formation, differentiation, neurite outgrowth and memory enhancement.
NGF is a possible target for therapeutics intended to improve learning, memory and general cognitive ability, and has been successfully applied in neurodegenerative diseases such as Alzheimer's disease. Therefore, there have been many attempts to find NGF inducers from natural sources, because direct application of NGF is problematic. Moreover, phytochemical-rich fruits have been reported to reverse age-related memory impairment via an NGF-related mechanism. For example, strawberry and blueberry extracts exerted positive effects on memory and learning via up-regulation of NGF-related genes, such as ERK and CREB, in the hippocampus(Reference Vauzour, Vafeiadou and Rodriguez-Mateos11, Reference Maher, Akaishi and Abe38, Reference Williams, El Mohsen and Vauzour39). Such neuronal signalling modulation has been linked to their flavonoid phytoconstituents(Reference Vauzour, Vafeiadou and Rodriguez-Mateos11). Flavonoids, such as hesperetin, epicatechin, epigallocatechin and fisetin, have also been shown to activate ERK and CREB signalling(Reference Maher, Akaishi and Abe38, Reference Reznichenko, Amit and Youdim40–Reference Rainey-Smith, Schroetke and Bahia42), which is regulated by NGF expression(Reference Lu, Wu and Hu43). As is the case for other phytochemical-rich plants, Mori Fructus and its phytoconstituents appeared to modulate NGF and NGF-inducible genes, resulting in improvement of memory. However, cognitive function could be dependent on increased cerebral blood flow, known to be caused also by certain polyphenols such as resveratrol(Reference Kennedy, Wightman and Reay44) and other complicated conditions such as stress and ageing(Reference McEwen45). Thus, further investigation is necessary to fully understand not only toxicity, pharmacokinetics and metabolism of ME, but also functional phytoconstituents.
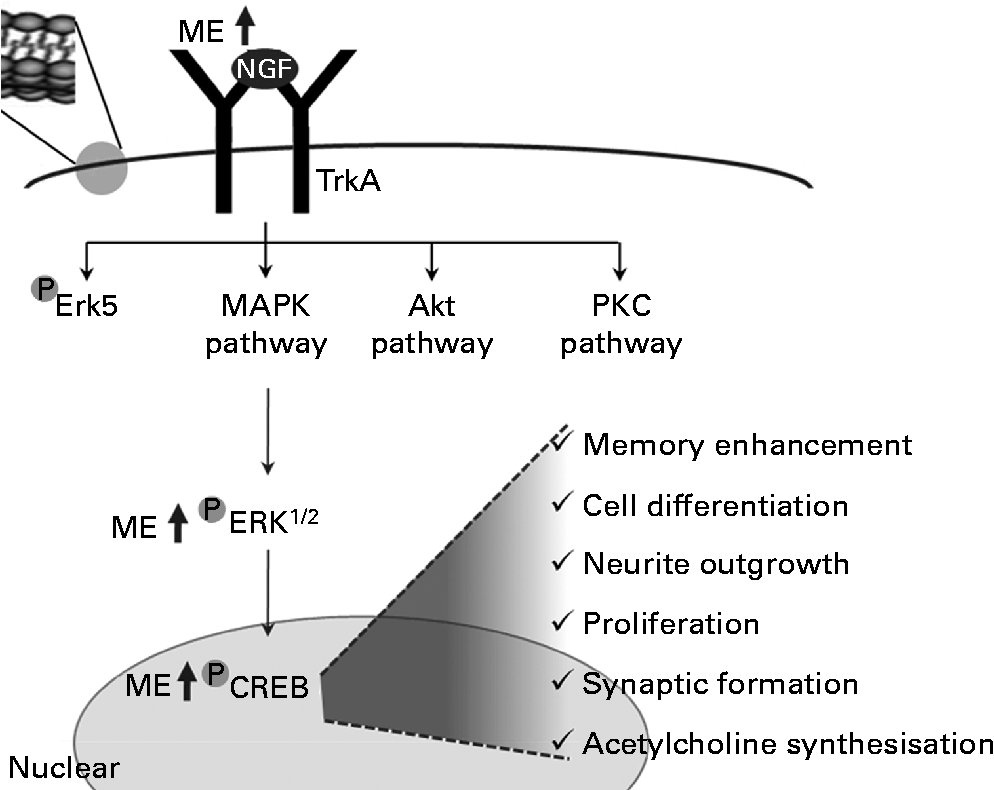
In summary, ME induced NGF release and increased the levels of pERK and pCREB. Through this regulation, it enhanced the functioning of neurons and increased synapse formation, ACh synthesisation, neuronal cell differentiation, neurite outgrowth, cell proliferation and cognition (Fig. 7).

Fig. 7 A tentative signalling diagram of Mori Fructus extract (ME) on memory enhancement by inducing nerve growth factor (NGF). MAPK, mitogen-activated protein kinase; PKC, protein kinase C; ERK, extracellular signal-regulated kinase; CREB, cyclic AMP response element-binding protein.
Acknowledgements
M. S. O. and H. G. K. were involved in the designing, writing and editing of the paper. All authors reviewed the final manuscript. The present work was supported by a post-doctoral fellowship grant from the Kyung Hee University in 2011 (KHU-20110695). The authors declare no conflicts of interest.