INTRODUCTION
Among organotin compounds (OTs), tributyltin (TBT) and triphenyltin (TPT) have been used worldwide as biocides in marine antifouling paints, to prevent the attachment and growth of marine organisms such as barnacles, mussels and algae on ships’ hulls (Snoeij et al., Reference Snoeij, Penniks and Seinen1987; Blunden & Evans, Reference Blunden, Evans and Hutzinger1989; Bosselmann, Reference Bosselmann and de Mora1996). These chemicals are responsible for many deleterious effects on non-targeted aquatic life, including fish (Fent & Meier, Reference Fent and Meier1994; Grzyb et al., Reference Grzyb, Rychlowski, Biegniewska and Skorkowski2003). In 1990, the International Maritime Organization (IMO) issued a series of recommendations on the use of TBT antifoulings, including a ban on its use on vessels less than 25 m length overall. In Japan, in the same year, bis(tributyltin)oxide (TBTO) was designated a Class 1 Specified Chemical Substance under the Law Concerning the Examination and Regulation of the Manufacture of Chemical Substances, and 7 triphenyltin (TPT) species and 13 further tributyltin (TBT) species were designated as Class 2 Specified Chemical Substances under the same law. Japanese legislation firstly restricted applications of TBT usage in shipyards and subsequently banned all application in 1991, including its use on vessels less than 25 m length overall.
In spite of these regulations, OT compounds have been detected at elevated concentrations in water, sediment, and biota from harbours, marinas and estuaries, particularly where boat activity is high and water movement is restricted (e.g. Harino et al., Reference Harino, Fukushima, Yamamoto, Kawai and Miyazaki1998; Tselentis et al., Reference Tselentis, Maroulakou, Lascourreges, Szpunar, Smith and Donard1999). Because of similar observations of persistence worldwide, IMO adopted the International Convention on the Control of Harmful Antifouling Systems (AFS Convention) in October 2001, which prohibited the use of OTs as active ingredients in antifouling systems for ships. This convention was adopted by a majority of signatories leading to a ban on the use of TBT across much of the global fleet in 2008. However, high concentrations of TBT and TPT are still detected in some aquatic ecosystems (Ohji et al., Reference Ohji, Arai, Midorikawa, Harino, Masuda and Miyazaki2007a; Harino et al., Reference Harino, Ohji, Wattayakorn, Adulyanukosol, Arai and Miyazaki2008a), especially in sediment near shipyards, due to slow degradation rates (Harino et al., Reference Harino, Yamamoto, Eguchi, Kawai, Kurokawa, Arai, Ohji, Okamura and Miyazaki2007; Langston et al., Reference Langston, Pope, Davey, Langston, O'Hara, Gibbs and Pascoe2015).
Numerous studies have been carried out on the toxic effects of OTs in marine organisms, from the molecular to the population level (Bryan et al., Reference Bryan, Gibbs, Hummerstone and Burt1986; Jha et al., Reference Jha, Hagger, Hill and Depledge2000; Ohji et al., Reference Ohji, Arai and Miyazaki2002a, Reference Ohji, Takeuchi, Takahashi, Tanabe and Miyazakib, Reference Ohji, Arai and Miyazaki2003a, Reference Ohji, Arai and Miyazakib). However, most of these are single generation studies and the significance of transgenerational factors, for example, the transfer of TBT and TPT from parent to offspring, is poorly understood (Gauthier et al., Reference Gauthier, Pelletier, Brochu, Moore, Metcalfe and Béland1998; Tanabe et al., Reference Tanabe, Prudente, Mizuno, Hasegawa and Miyazaki1998; Focardi et al., Reference Focardi, Corsolini, Aurigi, Pecetti and Sanchez-Hernandez2000; Harino et al., Reference Harino, Ohji, Brownell, Arai and Miyazaki2008b).
The surfperches (Embiotocidae) have the most evolved viviparity among the teleost fish. Ditrema temmincki, which belongs to this family, mainly inhabit seagrass beds and rocky reefs in temperate regions in Japan and the Korean Peninsula (Matsuura, Reference Matsuura, Masuda, Amaoka, Araga, Ueno and Yoshiro1984; Nakabo, Reference Nakabo, Masuda, Amaoka, Araga, Ueno and Yoshiro1984). Their mating season lasts for 3 months, from early September to early December (Nakazono et al., Reference Nakazono, Takeda and Tsukahara1981). After the eggs ripen during November and December, parturition occurs during May and June (Nakazono et al., Reference Nakazono, Takeda and Tsukahara1981; Tamura et al., Reference Tamura, Honma and Kitamura1981). The offspring generally spend several months in the parental female (Tamura et al., Reference Tamura, Honma and Kitamura1981). During this long gestation (6 months), nutrients are provided by the parental female to the young (Webb & Brett, Reference Webb and Brett1972; Wourms et al., Reference Wourms, Grove, Lombardi, Hoar and Randall1988; Nakamura et al., Reference Nakamura, Tazumi, Muro, Yasuhara and Watanabe2004) and transfer of non-essential materials, including pollutants, can also take place. This includes any TBT and TPT to which the viviparous surfperch may have been exposed during their life history. The surfperch D. temmincki therefore represents a valid and simple model to evaluate the potential for transfer of TBT and TPT between parent and juvenile viviparous fish.
The aim of the present study was to examine the difference in the accumulation pattern of butyltin compounds (BTs) including TBT and its derivatives, dibutyltin (DBT) and monobutyltin (MBT), and phenyltin compounds (PTs) including TPT and its derivatives, diphenyltin (DPT) and monophenyltin (MPT), between parental females and offspring in D. temmincki Bleeker collected from Japanese coastal waters. It also aimed to provide some clues as to the mechanism and significance of transgenerational transfer of OTs in viviparous fish.
MATERIALS AND METHODS
Sample collection
Five mature female D. temmincki were collected by set net at Otsuchi Bay located in Iwate Prefecture on the Pacific side of north-eastern Honshu Island, Japan, on 23 July 2003. The offspring were taken from these five parental females (P1 to P5) by dissecting the ovaries and weights and length determined (Table 1). The offspring were considered to be almost at the same developmental stage, just before oviposition. The number of offspring in each parental female ranged from 17 to 23 individuals. Three replicate samples of five individual offspring from each parent were analysed for OT body burdens (O1–O5) (Table 1). To test whether analysis of BTs in a single offspring was feasible, the OT concentrations in three individual offspring [O2 (1 ind.)] taken from one parental female (P2) were determined (Table 1). The OT concentrations in each parental female were also analysed. All biological samples were stored in a freezer at −80°C until chemical analysis. Seawater samples were also collected at a depth of 0.5 m at the time of fish collection, using 1 l polycarbonate bottles. The seawater samples were acidified with 1 ml of 1 M HCl immediately and stored at 4°C, in the dark, until chemical analysis.
Table 1. Total length and body weight of the parental females and offspring of Ditrema temmincki.
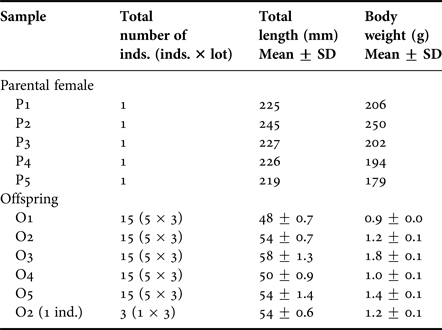
P1–5: parental female.
O1–5: offspring (three replicate samples of five individual offspring from each parent).
O2 (1 ind.): three individual offspring taken from one parental female (P2).
Chemical analysis of organotins in specimens
The method used to determine the concentrations of OTs in the biological samples was based on that of Ohji et al. (Reference Ohji, Harino and Arai2006b) with some modifications. One gram (for analysis of one individual offspring) or five grams of homogenated fish (for pooled samples) was spiked in a centrifuge tube with 100 µl acetone containing 1 µg ml−1 each of OT standards tributyltin monochloride (TBTCl)-deuterium (d)27, dibutyltin dichloride (DBTCl)-d18, monobutyltin trichloride (MBTCl)-d9, triphenyltin monochloride (TPTCl)-d15, diphenyltin dichloride (DPTCl)-d10, and monophenyltin trichloride (MPTCl)-d5 (Hayashi Pure Chemical Industries Ltd, Osaka, Japan). The mixture was extracted with 10 ml of 1 M HCl-methanol/ethyl acetate (1/1) by shaking for 10 min. After centrifugation for 10 min, the residue was extracted with a further aliquot of solvent and centrifuged again in the same way. The combined supernatants and 30 ml of saturated NaCl solution were extracted twice in a separating funnel using 15 ml of ethyl acetate/hexane (3/2). Fifty millilitres of hexane were mixed with the combined organic layers and the mixture allowed to stand for 30 min. After removal of the aqueous layer, the organic layer was dried with anhydrous Na2SO4, concentrated up to trace level by a rotary evaporator, and then further concentrated by means of a nitrogen purge. The analytes were diluted with 3 ml of ethanol, 3 ml of acetic acid-sodium acetate buffer (pH 5.0) and 10 ml of distilled water, and ethylated using 1 ml of 5% NaBEt4. After standing overnight, the lipids were saponified with 5 ml of 1 M KOH–ethanol solution by shaking for 1 h. Forty millilitres of distilled water and 10 ml of hexane were added to the solution and ethylated OTs extracted in the organic layer by shaking for 10 min. The aqueous layer containing residual OTs was extracted again by shaking for 10 min with 10 ml of hexane. The combined organic layers were dried with anhydrous Na2SO4, concentrated to 1 ml by a rotary evaporator and nitrogen gas, and cleaned using a Florisil Sep-Pak column (Waters Associates Inc.). The analytes were eluted with 5% diethyl ether/hexane, and TeBT-d36 and TePT-d20 were added as an internal standard. The final solution was concentrated to 0.2 ml.
The analytical method of OTs in seawater was based on that described by Ohji et al. (Reference Ohji, Arai, Midorikawa, Harino, Masuda and Miyazaki2007a) using a Hewlett-Packard 6890 series gas chromatograph equipped with a mass spectrometer (5973) for selected ion monitoring. Following splitless injection (1 µl) sample separation was carried out by capillary column coated with 5% phenyl methyl silicone (30 m length × 0.25 mm i.d., 0.25 µm film thickness, J&W Scientific Inc.). The column temperature was held at 60°C for the first 2 min, then increased to 130°C at 20°C min−1, 210°C at 10°C min−1, 260°C at 5°C min−1 and 300°C at 10°C min−1. Finally, the column temperature was maintained at 300°C for 2 min. The interface temperature, ion source temperature and ion energy were 280°C , 230°C and 70 eV, respectively. The concentrations of OTs in biological samples and seawater samples are expressed as Sn4+ in the present study.
Quality control of the analytical procedure was confirmed by spiking fish tissues with 1 µg of BTs and PTs. The recoveries of BTs and PTs were 86–101% and 72–90%, respectively, and their relative standard deviations (RSD) were in the range of 2.2–4.5% and 3.9–6.7%, respectively. Additional quality control tests for BTs in the biological samples were carried out using certified reference material [Institute for Reference Materials and Measurements (IRMM) CRM 477]. Measured values of BTs were within confidence intervals of the certification values. The detection limits of each BT, based on a signal-to-noise ratio of 3, were 0.1 ng Sn g−1 wet wt for biological samples and 1.0 ng Sn l−1 for seawater.
In the present study, the ratios of each OTs burden in offspring to each OTs concentration in parental female, i.e. the ratio of bioaccumulation, were calculated.
Statistics
A statistical comparison of the OT concentrations between mother and offspring, and between samples of one and five individual offspring, were carried out using the Mann–Whitney U-test (Sokal & Rohlf, Reference Sokal and Rohlf1995).
RESULTS
Butyltins
The values of the total BTs (∑BTs = TBT + DBT + MBT) in the parental females (N = 5) and the offspring (N = 5, grouped; N = 15 per parent) were 5.3 ± 1.1 (Mean ± SD) and 41 ± 7.2 ng Sn g−1 wet wt, respectively (Figure 1, Table 2). The values of ∑BTs in the offspring were significantly higher (7.1–9.7 times) than those in the parental females (Mann–Whitney U-test, P < 0.005). Among BTs, TBT values were highest, with concentrations in parental females (N = 5) and offspring (N = 5, grouped; N = 15 per parent) at 2.8 ± 1.0 and 33.7 ± 5.7 ng Sn g−1 wet wt, respectively (Figure 1, Table 2). The levels were significantly higher (9.5–17 times) in juveniles compared with maternal levels as well as ∑BTs (Mann–Whitney U-test, P < 0.005). The ∑BT and TBT concentration measured in single individuals [O2 (1 ind.)], 44 ± 2.0 and 31 ± 0.8 ng Sn g−1 wet wt, respectively (N = 3) (Table 2), was not significantly different to that determined in groups of five individuals, suggesting that BTs analysed in single individuals were representative of the wider population. The TBT, DBT and MBT values of seawater samples were below the detection limit.

Fig. 1. Ditrema temmincki. Butyltin (A) and phenyltin (B) concentration in parental females and offspring. P1–5; parental female, O1–5; offspring (three replicate samples of five individual offspring from each parent), O2 (1 ind.); three individual offspring taken from one parental female (P2).
Table 2. Butyltin and phenyltin concentrations in Detrema temmincki (ng Sn g−1 wet wt) and seawater (ng Sn l−1), and ratios of bioaccumulation (offspring/parental female).

∑BTs: TBT + DBT + MBT.
∑PTs: TPT + DPT + MPT.
P1–5: parental female.
O1–5: offspring (three replicate samples of five individual offspring from each parent).
O2 (1 ind.): three individual offspring taken from one parental female (P2).
Ratio of bioaccumulation: the ratio the ratios of each OTs burden in offspring to each OTs concentration in parental female.
The proportion of TBT, DBT and MBT in parental females was 51 ± 9.3%, 21 ± 2.2% and 28 ± 7.2% of the ∑BTs, respectively (Figure 2), compared with 82 ± 1.6%, 13 ± 0.6% and 5.3 ± 1.6%, respectively, in offspring (N = 5, grouped; N = 15 per parent). The proportion of TBT in offspring was thus higher than that of the parental females. The proportion of TBT, DBT and MBT in offspring analysed singly (72 ± 2.6%, 14 ± 1.2% and 14 ± 1.7%) was comparable to the larger sample size (N = 5, grouped; N = 15 per parent). This suggests again that BTs analysed in a single fish are representative of a larger sample size.

Fig. 2. Ditrema temmincki. Butyltin (A) and phenyltin (B) composition in parental females and offspring. P1–5; parental female, O1–5; offspring (three replicate samples of five individual offspring from each parent), O2 (1 ind.); three individual offspring taken from one parental female (P2).
Phenyltins
The values of the total PTs (∑PTs = TPT + DPT + MPT) were similar in the parental females (N = 5) and the offspring (N = 5, grouped; N = 15 per parent) – 1.1 ± 0.3 (Mean ± SD) and 1.3 ± 0.5 ng Sn g−1 wet wt, respectively (Figure 1, Table 2). Among PTs, the TPT values were highest and concentrations were similar in the parental females (N = 5) and the offspring (N = 5, grouped; N = 15 per parent) at 0.8 ± 0.3 and 1.0 ± 0.5 ng Sn g−1 wet wt, respectively (Figure 1, Table 2). No significant differences were observed in the values of ∑PTs and TPT between generations. The ∑PT and TPT concentrations in individuals analysed singly were 1.9 ± 0.2 and 1.0 ± 0.0 ng Sn g−1 wet wt, respectively (N = 3) (Table 2) comparable with ∑PT and TPT concentrations analysed in groups of five. This result confirms that PTs as well as BTs could be analysed usefully in one individual only, conserving biological resources. The ratio of ∑PT concentrations in offspring/parental females was 0.5–1.8, and was similar to the bioaccumulation ratio of TPT concentrations 0.4–1.9 (Table 2). The TPT, DPT and MPT values of seawater samples were below the detection limit.
In the parental females, the proportion of the ∑PTs present as TPT, DPT and MPT was 72 ± 18%, 14 ± 3.6% and 14 ± 19%, respectively, not significantly different to proportions in offspring at 73 ± 12%, 17 ± 3.6% and 9.8 ± 9.0%, respectively (Figure 2). The proportions of PTs were comparable whether using single offspring or five individuals. This result suggested that PTs as well as BTs could be analysed satisfactorily using only one individual.
DISCUSSION
The present study demonstrates OTs transfer from parental females to the young in the viviparous surfperch D. temmincki, although there are differences in the accumulation profile and transgenerational transfer ratio between TBT and TPT. Notably, the concentrations and proportions of TBT in the offspring were significantly higher than those in the parental females. This result suggests that TBT is most readily transferred from the parent to the offspring. TPT concentrations were significantly lower than TBT and no difference was observed between the concentration of TPT (the dominant phenyltin species) in parental females and offspring. It would seem that there are large differences in the rate of transfer of TBT and TPT to the offspring. For example, if the rate of transfer of TBT from parent to offspring was much faster than its degradation rate in the offspring, the net result would be an apparent bioconcentration in the offspring. If the rate of transfer of TPT was much slower than its biodegradation, then the concentration would lower in the offspring.
It was reported that the TBT-binding proteins (TBT-bps: TBT-bp1 and TBT-bp2) isolated from the blood of Japanese flounder Paralichthys olivaceus are members of the fish lipocalins (Shimasaki et al., Reference Shimasaki, Ohshima, Yokota, Kitano, Nakao, Kawabata, Imada and Honjo2002; Oba et al., Reference Oba, Shimasaki, Ohshima, Satone, Kitano, Nakao, Kawabata and Honjo2007; Satone et al., Reference Satone, Akahoshi, Nakamura, Lee, Honda, Shimasaki, Kawabata, Kusakabe and Ohshima2013) and that the TBT-binding ability of TBT-bp1 may have a detoxification function (Satone et al., Reference Satone, Akahoshi, Nakamura, Lee, Honda, Shimasaki, Kawabata, Kusakabe and Ohshima2013). Exposure of P. olivaceus to TBT-d27 also results in an increase in the protein concentration of TBT-bp2 in the serum (Nassef et al., Reference Nassef, Tawaratsumita, Oba, Satone, Nakayama, Shimasaki, Honjo and Ohshima2011). It is feasible that TBT-bps increase in similar fashion in response to exposure to TBT in parental females of D. temmincki.
The differences of accumulation pattern between TBT and TPT in the present study may be due to a faster rate of transfer of TBT from parent to offspring, compared with TPT. It is therefore considered that the offspring might be at higher risk from TBT (but not from TPT) than the parental females during their early growth stage in ovary in D. temmincki. The fertilization of eggs occurs in winter in this viviparous surfperch, and 6 months elapse from copulation to the birth of the young (Abe, Reference Abe1969). During the long period of gestation in this type of fish, the maternal nutrients for growth and respiration are transferred to embryonic epithelial absorptive sites, which are not in intimate association with the maternal tissue. The hindgut, fin, and branchial placenta are suspected to be placenta-analogue organs that absorb maternal nutrients from the ovarian fluid in the offspring of the surfperch (Wourms et al., Reference Wourms, Grove, Lombardi, Hoar and Randall1988; Nakamura et al., Reference Nakamura, Tazumi, Muro, Yasuhara and Watanabe2004). Since the young stays in the parental female for 6 months, OTs (and potentially other contaminants) may transfer to, and accumulate in the offspring along with essential nutrients.
The present study found differences between the BT and PT compositions in the parental female. Of the BTs, TBT and its metabolites, the sum of DBT and MBT, were found in approximately equal percentages in parental females. In contrast, TPT in parental females was largely predominant. A similar condition was found in our previous study on oviparous species such as the sea-run masu salmon O. masou (Ohji et al., Reference Ohji, Arai and Miyazaki2006a, Reference Ohji, Arai and Miyazaki2007b), Japanese eel Anguilla japonica (Ohji et al., Reference Ohji, Harino and Arai2006b, Reference Ohji, Harino and Arai2009) and brown trout (Ohji et al., Reference Ohji, Harino and Arai2010). Among BTs it was reported that high DBT and MBT levels were detected in the liver tissue of the pike while, of the PTs, high TPT concentrations were predominant (Stäb et al., Reference Stäb, Traas, Stroomberg, van Kesteren, Leonards, van Hattum, Brinkman and Cofino1996). Different capacity to degrade TBT and TPT was demonstrated in vitro in the European eel Anguilla anguilla and the rainbow trout Oncorhynchus mykiss (Fent & Bucheli, Reference Fent and Bucheli1994): liver microsomes were affected by both TBT and TPT, but the latter inhibited ethoxyresorufin O-deethylase (EROD) activity more strongly than TBT and led to the inactivation of P-450 enzymes. The results obtained in the present study might be related to the preferential dealkylation of TBT in the liver and rapid excretion via the bile relative to TPT. Although the TPT concentration was significantly lower than TBT, persistence of TPT in D. temmincki could still pose a risk for the viviparous surfperch alongside TBT.
In the present study, higher levels and proportion of TBT in offspring than those in parental female were found in D. temminki. Fish is known to have higher metabolic capacity to degrade TBT than lower trophic organisms, such as small crustaceans, in the marine ecosystem (Hall & Bushong, Reference Hall, Bushong, Champ and Seligman1996; Ohji et al., Reference Ohji, Arai and Miyazaki2002a, Reference Ohji, Arai, Midorikawa, Harino, Masuda and Miyazaki2007a). It is also known that the metabolic capacity to degrade toxic chemicals in young organisms is lower than those in adults (Padrós et al., Reference Padrós, Villalta, Gisbert and Estévez2011). Organisms have some detoxification pathways in which some enzymes are mediated, such as cytochrome P450 monooxygenase, epoxide hydrolase and other conjugating enzymes in relation to the organs such as liver (e.g. Shailaja & D'Silva, Reference Shailaja and D'Silva2003). These pathways are known to develop well in most adult organisms (Shailaja & D'Silva, Reference Shailaja and D'Silva2003). The organisms begin to eliminate or detoxify toxicants as these organs become functional, followed by a decrease in the sensitivity of adult individuals compared with the individuals in early life stages (Hall & Bushong, Reference Hall, Bushong, Champ and Seligman1996; Padrós et al., Reference Padrós, Villalta, Gisbert and Estévez2011). Therefore, low metabolic capacity to degrade TBT may cause an elevation of TBT levels in the offspring of D. temminki in the present study. It is also considered that since the offspring have higher sensitivity for TBT than parental females, high levels of TBT in offspring might induce an adverse effect in this species.
It was found that OTs were detected in both parental female and offspring in D. temminki, although the levels in the surrounding seawater were under the detection limit, and that the concentrations of TBT in the offspring were significantly higher than those in the parental females in the present study. These results suggest that the bioavailability of TBT via maternal nutrients transfer can be considered to be of major importance when comparedwith uptake via the water. Several studies regarding the transfer from mother to offspring of other chemicals have been conducted, i.e. trace metals and methyl mercury in the harp seal Phoca groenlandica (Wagemann et al., Reference Wagemann, Stewart, Lockhart and Stewart1988), and organochlorines in the bottlenose dolphin Tursiops truncatus (Law et al., Reference Law, Allchin and Morris1995). McManus et al. (Reference McManus, Wyman, Peterson and Wurster1983) found that amongst invertebrates polychlorinated biphenyls (PCBs) in the parental female copepod Acartia tonsa were released with the eggs, which are rich in lipids and provide a sink for hydrophobic compounds such as PCBs and polycyclic aromatic hydrocarbons (PAHs). Di Pinto et al. (Reference Di Pinto, Coull and Chandler1993) also suggested that depuration of PCB via egg production ultimately leads to significantly higher mortality among naupli.
Although the mechanism of the transfer of OTs from mother to offspring is thought to be different from the above chemicals and organisms, TBT and TPT are clearly capable of transfer to eggs, with potential for deleterious effects in the eggs and/or the young in viviparous fishes such as D. temmincki. TBT and TPT have adverse effects, such as physiological abnormality (10 ng l−1) in fish, even at ambient water levels (e.g. Hall & Bushong, Reference Hall, Bushong, Champ and Seligman1996), and thus these substances may have an influence on D. temmincki. Furthermore, it has been reported that TBT and TPT exposure detrimentally affects reproduction in the African catfish, herring and European minnow (Fent & Meier, Reference Fent and Meier1992, Reference Fent and Meier1994; Rurangwa et al., Reference Rurangwa, Biegniewska, Slominska, Skorkowski and Ollevier2002; Grzyb et al., Reference Grzyb, Rychlowski, Biegniewska and Skorkowski2003). Thus, TBT and TPT exposure might also affect survival, growth and reproduction during the early growth stages of the offspring while they are in the parental female of the D. temmincki.
Differences in transfer ratio of TBT and TPT between parental female to offspring were observed in the viviparous surfperch D. temmincki in the present study. The results suggested that the offspring might be exposed to a higher risk from TBT exposure (but not from TPT) than the parental females during their early growth stage in the ovary of the viviparous surfperch. The present study highlights the potential for inherited effects of organotins in viviparous fish, due to their ability to transfer and bioaccumulate in the offspring. Species such as D. temmincki thus represent a valid model to study the transfer, bioaccumulation, breakdown and elimination – and hence transgenerational risks – of both historical and emerging pollutants.
ACKNOWLEDGEMENTS
We are grateful to the staff of the International Coastal Research Center, Atmosphere and Ocean Research Institute, The University of Tokyo, for their support of the sample collection.
FINANCIAL SUPPORT
The present study was partially supported by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology, Japan (Nos. 19681005, 22651008 and 26701008).





