According to the lipoprotein-oxidative theory, oxidised LDL play an important role in initiating the atherogenic process, partly explaining the link between CVD and the lipid profile(Reference Chisolm and Steinberg1). The LDL particle consists of an apolar core of cholesteryl esters and TAG, surrounded by a monolayer of phospholipids, non-esterified cholesterol, and one molecule of apo B-100. Cholesteryl esters are the predominant lipid class in LDL, are rich in PUFA and thus easily attacked by free radicals. α-Tocopherol, the most abundant antioxidant in LDL provides protection to lipid components in LDL.
It has been described that NO also plays a determinant role in the prevention of lipid oxidation. NO readily crosses cell membranes, concentrates in lipophilic milieu where it reacts to terminate propagation reactions catalysed by lipid alkoxyl and peroxyl radical species(Reference Rubbo, Radi, Trujillo, Telleri, Kalyanaraman, Barnes, Kirk and Freeman2) and spares α-tocopherol(Reference Botti, Batthyany, Trostchansky, Radi, Freeman and Rubbo3, Reference O'Donnell, Chumley, Hogg, Bloodsworth, Darley-Usmar and Freeman4). NO can also diffuse into the lipidic core of the lipoproteins, inhibiting lipid peroxidation processes by these chain-breaking antioxidant properties(Reference Denicola, Batthyany, Lissi, Freeman, Rubbo and Radi5, Reference Trostchansky, Batthyany, Botti, Radi, Denicola and Rubbo6). Another antioxidant factor is paraoxonase-1 (PON-1), which is associated with HDL that degrades lipid hydroperoxides protecting LDL from oxidation(Reference Aviram, Hardak, Vaya, Mahmood, Milo, Hoffman, Billicke, Draganov and Rosenblat7).
Consumption of fish oil or EPA (20 : 5n-3) or DHA (22 : 6n-3) is associated with protection against CVD(Reference Calder8, Reference Connor and Connor9). One of the mechanisms, which have been demonstrated both in human(Reference Tagawa, Shimokawa, Tagawa, Kuroiwa-Matsumoto, Hirooka and Takeshita10) and in rat arteries(Reference López, Caballero, Sánchez, Puig-Parellada and Mitjavila11), involves increases in endothelium-dependent vascular relaxation through up-regulation of the endothelial NO synthase–cGMP pathway(Reference López, Orta, Casós, Sáiz, Puig-Parellada, Farriol and Mitjavila12). Long-chain n-3 PUFA are readily oxidised in vitro in homogeneous systems. However, compared with the intake of n-6 fatty acids, the intake of n-3 fatty acids prevents the so-called free radical diseases. This suggests that lipid peroxidation in vivo may not correspond with that in vitro (Reference Okuyama, Kobayashi and Watanabe13). For instance, several studies have observed that increasing the dietary intakes of EPA and DHA does not increase the oxidative susceptibility of LDL lipoproteins(Reference Higdon, Du, Lee, Wu and Wander14–Reference Wander, Du, Ketchum and Rowe16). Since molecular order and dynamics within membranes are known to be dependent on acyl chain unsaturation it was generally anticipated that lipid fluidity within lipoproteins will increase when enriched with long-chain n-3 PUFA, which in turn will increase O2 diffusibility(Reference Fischkoff and Vanderkooi17). As the physico-chemical properties of O2 and NO are very similar it was likely that the diffusibility of NO might also be improved.
In the present study we aim to provide new insights into the beneficial effect of fish or fish oil on the prevention of atherosclerosis through the antioxidant activity of NO, to contribute to understanding the mechanism of action of long-chain n-3 PUFA. We evaluated the effects of an increase in NO generation at the vascular wall on its α-tocopherol content and the diffusion of NO into native lipoproteins. Additionally, we examined by electrophoretic mobility and, through the conjugated dienes (CD), thiobarbituric acid-reactive substance (TBARS) generation and antioxidant levels whether native VLDL+LDL rich in long-chain n-3 PUFA were already oxidised. The results were contrasted with the susceptibility to oxidation of VLDL+LDL ex vivo. Furthermore, the contribution of PON-1, an antioxidant enzyme with an indirect action on LDL, was also evaluated.
Materials and methods
Animals and diets
After weaning, two groups of male Sprague–Dawley rats (Harlan Interfauna Ibérica, Barcelona, Spain) were fed semi-purified diets containing lipids at 50 g/kg for 8 weeks, as previously described(Reference López, Caballero, Sánchez, Puig-Parellada and Mitjavila11, Reference López, Orta, Casós, Sáiz, Puig-Parellada, Farriol and Mitjavila12). At the end of the feeding period, rats were fasted for 18 h before exsanguinations by withdrawal of blood from the heart, using heparin as anticoagulant, under sodium urethane (1·5 g/kg intraperitoneally) anaesthesia.
The lipids were either maize oil (CO), rich in linoleic acid (18 : 2n-6), or fish oil as menhaden oil (MO) rich in EPA and DHA. The oils were analysed for tocopherols after extraction with hexane. A sample was mixed with ethanol in the presence of 10 μm-butylated hydroxytoluene and 5 μm-α-tocopheryl acetate (internal standard). After filtration a sample was injected into an HPLC (Merck-Hitachi, Darmstadt, Germany) through a column (LiChrospher 100 RP 18, 250 mm × 4·6 mm, 5 μm; Amersham) in methanol–water (95:5) as mobile phase subjected to a flux of 1·5 ml/min. UV absorption at 290 nm was recorded. The internal standard recovery was 96–98 %. The CO contained 250 mg RRR-α-tocopherol/kg and 775 mg RRR-γ-tocopherol/kg, while MO contained 50 mg RRR-α-tocopherol/kg, thereby supplying 16·4 and 2·5 mg α-tocopherol equivalents/kg diet, respectively. The difference in α-tocopherol equivalents in oils was eliminated by adjusting diets to 67 mg RRR-α-tocopherol equivalents/kg by supplementing the CO diet with 75·6 mg/kg and the MO diet with 96·3 mg/kg of all-rac-α-tocopheryl acetate. All dietary components were from Sigma (St Louis, MO, USA) with the exception of mineral (35 AIN-76) and vitamin (10 AIN-76A, vitamin E omitted) mixes, which were obtained from ICN Pharmaceuticals (Costa Mesa, CA, USA). The fatty acid content of CO and MO was evaluated after a direct transmethylation(Reference Lepage and Roy18) and the fatty acid methyl esters were analysed with an HP5890 series II gas chromatograph fitted with a flame ionisation detector. Samples were injected through the split injection port (split ratio, 30:1) onto a SP 2330 capillary column (30 m × 0·25 mm, 0·20 μm film thickness; Supelco, Bellefonte, PA, USA)(Reference Ruiz-Sanz, Navarro, Martinez, Martin, Lacort, Matorras and Ruiz-Larrea19). Individual fatty acids were identified by comparing relative retention times with commercial standards (Nu Chek; Elysian, MN, USA). Heptadecanoic acid was used as an internal standard. The double-bond index (∑ each fatty acid % (mol/mol) × double-bond number) and the theoretical peroxidisability index (∑ each fatty acid % (mol/mol) × (double-bond number − 1)) were also evaluated. Results are shown in Table 1.
Table 1 Fatty acid content in oils
(Mean values)
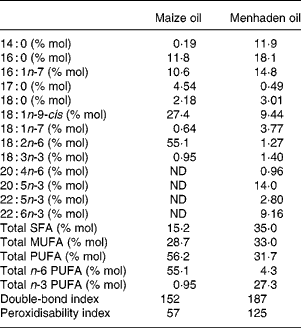
ND, not detected.
Plasma was stored at − 80°C in the presence of sucrose (60 mg/ml) to prevent changes in the oxidisability indices of lipoproteins during storage. The thoracic and abdominal aorta were dissected out and cut into segments(Reference López, Caballero, Sánchez, Puig-Parellada and Mitjavila11). The procedures and animal care were in compliance with European Union guidelines.
Isolation of VLDL+LDL and evaluation of their fatty acid composition
It is noteworthy that in rat samples the LDL concentration is the smallest fraction of plasma lipids. This is the opposite of what happens in humans(Reference Ha and Barter20), and this is why we have used VLDL+LDL.
VLDL+LDL were isolated following the technique of Esterbauer et al. (Reference Esterbauer, Gebicki, Puhl and Jürgens21) with modifications. Plasma (3 ml) density was adjusted to 1·063 g/ml by adding solid NaBr and then overlaid with 2 ml of PBS (pH 7·5) containing 0·01 % EDTA adjusted to 1·063 g/ml. Samples were centrifuged at 100 000 g at 4°C for 18 h, and the top layer (about 1 ml) was recovered. Excess NaBr and EDTA in VLDL+LDL were removed using a PD10 column (Amersham Pharmacia Biotech, UK) and VLDL+LDL were concentrated (Biomax membrane 10 000 Da; Millipore, Bedford, MA, USA). Proteins were quantified by the Bradford method (Bio-Rad, Hercules, CA, USA). The VLDL+LDL samples were immediately used for electrophoretic studies and oxidation kinetics, and the remaining solution was frozen in the presence of 0·2 mm-butylated hydroxytoluene for α-tocopherol analysis.
The evaluation of fatty acid composition of VLDL+LDL was conducted as described for dietary oils.
NO production by the aorta and NO diffusion to VLDL+LDL
NO production(Reference Vanin22) was studied immediately after extracting the aortae. Two segments of the thoracic aorta (5–10 mg) from the same rat were stripped, pre-incubated at 37°C for 20 min in 1 ml PBS, and then exposed to the spin-trapping agents, 5 mm-diethyldithiocarbamic acid and 50 μm-FeSO4.7H2O, for 30 min. An additional segment was endothelium denuded or pre-incubated for 30 min in the presence of 1 mm-N G-nitro-l-arginine, a NOS inhibitor, to assess the specificity of the assay. Aortic strips were then weighed, frozen in liquid N2, and stored at − 80°C for electron spin resonance analysis(Reference López, Caballero, Sánchez, Puig-Parellada and Mitjavila11). The resulting signal corresponded to the difference in intensity between a maximum at 3440 gauss and a minimum at 3470 gauss.
The importance of an increase in NO production by aorta is a function of its ability to diffuse to the lipidic core of VLDL+LDL. The diffusion study was conducted according to the method of Denicola et al. (Reference Denicola, Batthyany, Lissi, Freeman, Rubbo and Radi5) after incubating VLDL+LDL with a 0·35 mm-fluorescent probe 1-(pyrenyl)-methyl-3-(9-octadecenoyloxy)-22,23-bisnor-5-cholenate (PMCho). Excess PMCho was eliminated with a PD10 column (Amersham) and the eluate was degassed under Ar. The NO concentration of an aqueous solution had already been determined by oxyhaemoglobin. Lipoprotein–PMCho preparation (10–30 μg/ml) was injected into fluorimeter cuvettes in degassed 0·1 m-PBS. Subsequently, the NO solution was injected gradually, and the fluorescence (excitation wavelength, 347 nm; emission wavelength, 396 nm) was monitored. The results were normalised to a blank and the apparent second-order quenching constants between the excited state probe and NO were calculated from the slope of Stern–Volmer plots.
Oxidative state in vivo
It was important to elucidate whether native VLDL+LDL were already oxidised and to contrast these results with those of stimulated oxidation of VLDL+LDL ex vivo.
The electrophoretic mobility of lipoproteins was visualised in plasma, pre-stained with Sudan black subjected to PAGE in a non-denaturing discontinuous gradient from 2 % (at the area of application) to 3 % (in the running gel) (Lipofilm kit; Sebia; Issy-les-Moulineaux, France) at 100 V.
The electrophoretic mobility of apo B-100 in VLDL+LDL was also evaluated to detect whether the polypeptide was intact. VLDL+LDL were precipitated with a mixture of acetone and ethanol (1:1, v/v) and then delipidated with diethyl ether at − 20°C. The delipidated lipoproteins were mixed with a buffer containing 2-amino-2-(hydroxymethyl)propane-1,3-diol HCl (15·76 g/l), glycerol (60 g/l) and SDS (60 g/l). SDS-PAGE was performed in a linear gradient (4–15 %); each well was loaded with 30 μl of sample (2 μg of protein/μl) pre-heated to 80°C for 15 min. In some wells, purified apo B-100 (Sigma) was also loaded. Electrophoresis was conducted at 120 V for 2 h. Gels were fixed in 10 % acetic acid and 25 % isopropanol for 10 min, and then stained with Coomassie blue for 1 h. Completely oxidised samples (exposed to 62·5 μm-CuCl2 for 24 h) were also loaded as a positive control.
The α-tocopherol from 100 μg native VLDL+LDL protein was extracted with 5 ml water–methanol–hexane (1:1:3, by vol.) in the presence of 10 μm-butylated hydroxytoluene and 5 μm-α-tocopheryl acetate (internal standard). The hexane phase was evaporated under N2, dissolved in methanol and injected into an HPLC (Merck-Hitachi, Darmstadt, Germany) through a column (LiChrospher 100 RP 18, 250 mm × 4·6 mm, 5 μm; Amersham) subjected to a 100 % methanol flux of 1·5 ml/min. UV absorption at 290 nm was recorded. The internal standard recovery was 95–98 %.
Total antioxidant capacity in VLDL+LDL was evaluated in 100 μg VLDL+LDL protein using a kit (Randox Laboratories; Crumlin, Co. Antrim, UK).
TBARS analysis, as evaluation of malondialdehyde, was performed in 10 mg native VLDL+LDL and in 50 μl plasma at 540 nm and subtracting background absorbance at 620 nm. We used tetraethoxypropane as standard(Reference Yagi23). Butylated hydroxytoluene and EDTA were added to avoid oxidation during the process.
PON-1 activity was measured in 20 μl of plasma after incubation in 100 mm-2-amino-2-(hydroxymethyl)propane-1,3-diol HCl buffer (pH 8·0) in the presence of 2 mm-CaCl2 and 2 mm-paraoxon (Sigma). Paraoxon degradation to p-nitrophenol was monitored by the absorbance increase at 412 nm (extinction coefficient 18 290 l/mol per cm) for 5 min(Reference Beltowski, Wójcicka and Marciniak24). One unit (U) of PON-1 corresponds to 1 nmol paraoxon degradation/min.
Aortic α-tocopherol levels were also measured. A segment of abdominal aorta (20 mg) was extracted as described for β-carotene(Reference Shapiro, Mott and Machlin25). The tissue was homogenised in 1·5 ml KCl (11·5 g/l) and 0·5 ml ascorbic acid (250 mg/ml); then 2 ml ethanol and 50 μl retinol (0·1 mm) (internal standard) were added and the mixture saponified with 1 ml of 10 m-KOH. The internal standard recovery was 94–98 %. α-Tocopherol was extracted in hexane and evaluated by HPLC at 290 nm in the same conditions as for VLDL+LDL.
Stimulated oxidation of VLDL+LDL ex vivo
Cu2+ and 2,2′-azobis (2-amidinopropane) dihydrochloride (AAPH) mimic in different ways the oxidation in the vascular compartment. Cu2+ interacts with an LDL low-affinity binding site and generates Cu+, which produces chain-propagating radicals(Reference Esterbauer, Gebicki, Puhl and Jürgens21). AAPH generates alkoxyl and peroxyl radicals in the aqueous phase, thus triggering lipid peroxidation independently of transition metals(Reference Carbonneau, Cartron, Léger, Senglat and Descomps26).
VLDL+LDL (50 or 25 μg protein/ml for Cu2+ and AAPH, respectively) in oxygenated PBS at pH 7·4 were incubated at 30°C, either with 1·25 μm-CuCl2 or 1 mm-AAPH. The formation of CD was monitored by absorbance at 234 nm for 6–8 h(Reference Esterbauer, Gebicki, Puhl and Jürgens21). The background signal of an AAPH solution (1 mm) was subtracted from the absorbance values of lipoprotein oxidation assay. The lag time, the oxidation rate and the maximum amount of CD formed (molar extinction coefficient of 29 500 m/cm) were calculated.
Statistical analysis
Data are expressed as mean values with their standard errors. The results from MO-fed rats were compared with those obtained from CO-fed rats by the Student's t test for unpaired observations.
Results
Body weight
There were no differences in either the growth or final body weight of rats after 8 weeks of diet (328 (se 6) and 323 (se 9) g for the CO and MO diets, respectively). Food consumption was also similar in both animal groups.
NO production by the aorta and NO diffusion into VLDL+LDL
After incubating aortic segments with 1 mm-N G-nitro-l-arginine, the more prominent electron spin resonance signal was reduced by 90–95 % in all groups, indicating that the signal corresponds to NO. The MO diet induced a 130 % increase in aortic NO production ex vivo (Fig. 1 (a)). The endothelium-denuded segments gave a weak signal in all groups, indicating that the endothelium is the main source of NO.
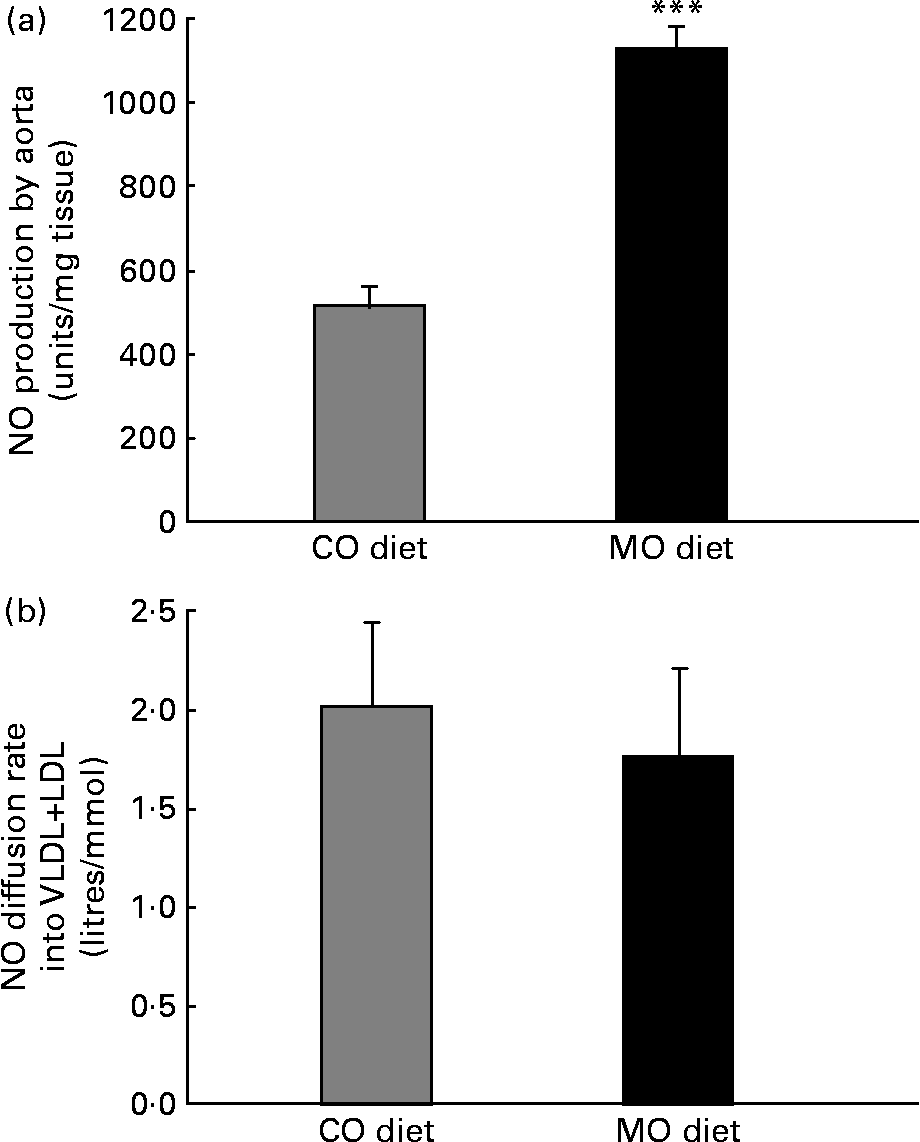
Fig. 1 Aortic NO production measured by electron spin resonance (a) and NO diffusion rate into VLDL+LDL calculated from the slope of Stern–Volmer plots (b) in rats fed a maize oil- (CO) or menhaden oil- (MO) rich diet. Values are the means of six rats per diet with their standard errors represented by vertical bars. *** Mean value was significantly different from that of the CO group (P < 0·001).
NO diffusion into the lipidic cores of VLDL+LDL was similar in both groups. The Stern–Volmer plots were 2·0 (se 0·4) and 1·7 (se 0·4) litres/mmol for CO- and MO-fed rats, respectively (Fig. 1 (b)).
Oxidative status in native VLDL+LDL in aorta and in plasma
Native VLDL and LDL from rats fed either a CO- or an MO-rich diet showed similar electrophoretic mobility in a non-denaturing gel (Table 2). Electrophoretic mobility of apo B-100 was similar in both dietary groups (Table 2). However, after oxidation by ex vivo exposure to Cu2+, apo B-100 from rats fed CO- or MO-rich diets was degraded.
Table 2 Oxidative state in native VLDL+LDL†
(Mean values with their standard errors for six rats)
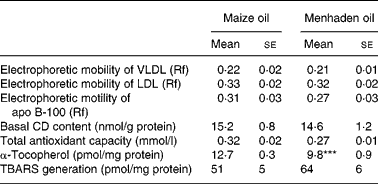
Rf, retention factor; CD, conjugated dienes; TBARS, thiobarbituric acid-reactive substances.
*** Mean value was significantly different from that of the maize oil group (P < 0·001).
† For details of diets and procedures, see Materials and methods.
The levels of CD in VLDL+LDL were similar in both dietary groups (Table 2).
The total antioxidant capacity in VLDL+LDL from both groups was not statistically different (Table 2).
There was a 23 % decrease (P < 0·001) in the α-tocopherol content of VLDL+LDL from rats fed the MO diet compared with rats fed the CO diet (Table 2). Conversely, there was a 78 % increase (P < 0·001) in the aortic tissue α-tocopherol content of rats fed the MO-rich diet (32 (se 3) pmol/mg tissue) compared with rats fed the CO-rich diet (18 (se 2) pmol/mg tissue) (Fig. 2).
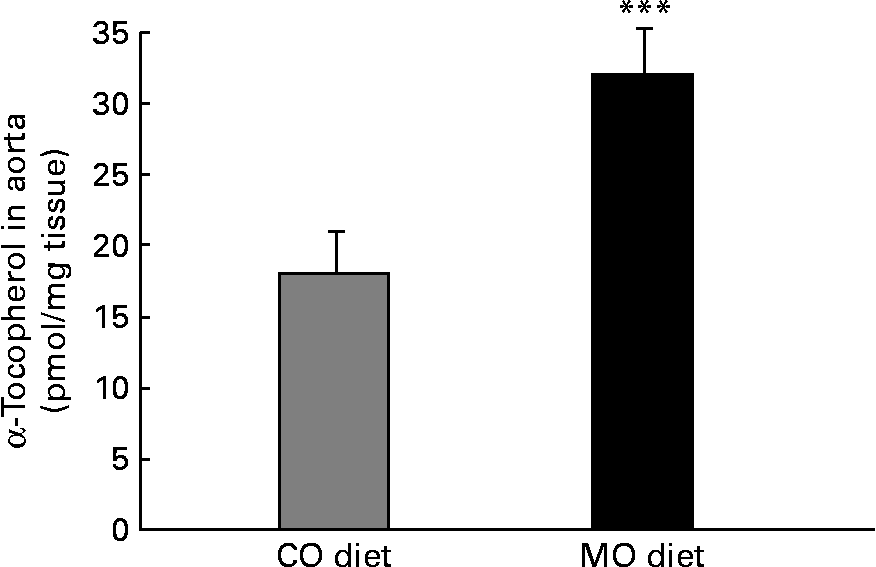
Fig. 2 Content of α-tocopherol in aorta from rats fed a maize oil- (CO) or menhaden oil- (MO) rich diet, as measured by HPLC. Values are the means of six to seven rats per diet with their standard errors represented by vertical bars. *** Mean value was significantly different from that of the CO group (P < 0·001).
We observed no significant differences in the concentration of TBARS either in native VLDL+LDL (Table 2) or in plasma (Table 3).
Table 3 Oxidative state in plasma*
(Mean values with their standard errors for six rats)
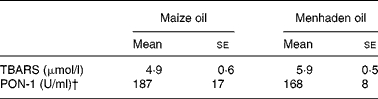
TBARS, thiobarbituric acid-reactive substances; PON-1, paraoxonase-1.
* For details of diets and procedures, see Materials and methods.
† One unit (U) of PON-1 corresponds to 1 nmol paraoxon degradation/min.
Similar PON-1 activity in plasma was detected in the two dietary groups (Table 3).
Fatty acid composition of VLDL+LDL and stimulated oxidation of VLDL+LDL ex vivo
The fatty acid composition of VLDL+LDL is shown in Table 4. The more notable differences appeared in total n-6 and n-3 PUFA content, which prevail in the lipoprotein fractions of CO- and MO-fed rats, respectively. Thus, VLDL+LDL from CO-fed rats showed higher levels of linoleic acid, γ-linolenic acid (18 : 3n-6), arachidonic acid (20 : 4n-6), and 22 : 5n-6, while lipoproteins from MO-fed rats showed higher EPA and DHA content. While the theoretical peroxidisability index in rats fed the MO-rich diet was higher than in rats fed the CO-rich diet, due to the levels of highly unsaturated fatty acids, the double-bond index was similar in both dietary groups.
Table 4 Fatty acid content in VLDL+LDL in rats fed maize oil- or menhaden oil-rich diets†
(Mean values with their standard errors for six rats)

Mean value was significantly different from that of the maize oil group, *P < 0·05, **P < 0·01, ***P < 0·001 (Student's t test).
† For details of diets and procedures, see Materials and methods.
The MO-rich diet altered the oxidation susceptibility of VLDL+LDL when exposed to 1·25 μm-CuCl2 or to 1 mm-AAPH (Fig. 3 (a)). With Cu2+ as catalyst, the lag time before the increase in CD was shortened (33 %; P < 0·01) in the lipoproteins of rats fed the MO-rich diet (89 (se 4) and 60 (se 5) min for CO and MO, respectively) (Fig. 3 (b)). The oxidation rate of fatty acids was 47 % lower (P < 0·001) in lipoproteins from rats fed the MO-rich diet (152 (se 12) μmol CD/g protein per min) compared with rats fed the CO-rich diet (289 (se 10) μmol CD/g protein per min) (Fig. 3 (c)). The maximum CD concentration was also reduced (27·0 (se 1·1) v. 19·8 (se 0·7) mmol CD/g protein for CO and MO, respectively, P < 0·001) (Fig. 3 (d)).

Fig. 3 Susceptibility of VLDL+LDL to oxidation from rats fed a maize oil (CO)- or menhaden oil (MO)-rich diet, measured in terms of conjugated diene (CD) generation. This process was continuously monitored by the change in absorbance at 234 nm. (a) Representative curve. (–♦–), Cu2+-treated VLDL+LDL from CO-fed rats; (–■–), Cu2+-treated VLDL+LDL from MO-fed rats; (–◇–), 2,2′-azobis (2-amidinopropane) dihydrochloride (AAPH)-treated VLDL+LDL from CO-fed rats; (–□–), AAPH-treated VLDL+LDL from MO-fed rats. (b) Lag time; (c) oxidation rate; (d) maximum CD level. Values are means with their standard errors represented by vertical bars (two replicates from six rats per diet). Mean value was significantly different from that of the CO group: *P < 0·05, **P < 0·01, ***P < 0·001.
When lipoproteins were exposed to the free radical generator AAPH, similar behaviour to Cu2+-induced lipoprotein oxidation was observed in the two dietary groups (Fig. 3 (a)). The lag times were 172 (se 15) and 122 (se 15) min for CO and MO, respectively (P < 0·05) (Fig. 3 (b)), which represents a reduction of 29 %. The oxidation rates of fatty acids were 89 (se 10) and 50 (se 5) μmol CD/g protein per min for CO and MO, respectively (P < 0·001), which represents a 43 % decrease (Fig. 3 (c)). The maximum CD concentration was also reduced (13·4 (se 1·1) v. 7·1 (se 0·8) mmol CD/g protein for CO and MO, respectively, P < 0·01) (Fig. 3 (d)).
Discussion
The protective effect at cardiovascular level of long-chain n-3 PUFA present in fish and fish oils has been usually attributed to anti-arrhythmic, anti-thrombotic and anti-inflammatory effects, a reduction of blood pressure and TAG levels, improvement of endothelial function, and also prevention of atherosclerosis(Reference Connor and Connor9, Reference López, Caballero, Sánchez, Puig-Parellada and Mitjavila11, Reference López, Orta, Casós, Sáiz, Puig-Parellada, Farriol and Mitjavila12, Reference Mesa, Buckley, Minihane and Yaqoob27).
The present paper gives for the first time new insights (tocopherol content in VLDL+LDL and aorta, ex vivo susceptibility of VLDL+LDL to oxidation by Cu2+ and AAPH, and PON-1) into the physiological mechanism involved in the beneficial effects of increased endothelial NO production on the vascular wall in rats postulated by our group in previous papers where the 5 % of lipids of the diet were substituted by CO or MO(Reference López, Caballero, Sánchez, Puig-Parellada and Mitjavila11, Reference López, Orta, Casós, Sáiz, Puig-Parellada, Farriol and Mitjavila12).
NO is a potent antioxidant(Reference Denicola, Batthyany, Lissi, Freeman, Rubbo and Radi5, Reference Trostchansky, Batthyany, Botti, Radi, Denicola and Rubbo6) but this activity can only be manifested when pro-oxidative reactions do not prevail, since NO may interact with ![]() to yield peroxynitrite, which promotes LDL oxidation(Reference Botti, Batthyany, Trostchansky, Radi, Freeman and Rubbo3). Whether peroxynitrite is formed in vivo and exerts any physiological or pathological activity remains a subject of debate. We previously observed, in similar conditions, no increase in
to yield peroxynitrite, which promotes LDL oxidation(Reference Botti, Batthyany, Trostchansky, Radi, Freeman and Rubbo3). Whether peroxynitrite is formed in vivo and exerts any physiological or pathological activity remains a subject of debate. We previously observed, in similar conditions, no increase in ![]() production from an MO-rich diet but induced NO-mediated vasorelaxation in tandem(Reference López, Caballero, Sánchez, Puig-Parellada and Mitjavila11). Thus, the final result may be a net gain of free NO(Reference Liochev and Fridovich28). In the present paper, we observe an endothelium-dependent NO increased production by MO that can be related to previous observations on increased NO concentrations in plasma(Reference Piolot, Blache, Boulet, Fortin, Dubreuil, Marcoux, Davignon and Lussier-Cacan29) and urine(Reference Harris, Rambjør, Windsor and Diederich30) from human subjects or in plasma from rats(Reference Erdogan, Fadillioglu, Ozgocmen, Sogut, Ozyurt, Akyol and Ardicoglu31), both indices of NO-enhanced production by fish oil-supplemented diets.
production from an MO-rich diet but induced NO-mediated vasorelaxation in tandem(Reference López, Caballero, Sánchez, Puig-Parellada and Mitjavila11). Thus, the final result may be a net gain of free NO(Reference Liochev and Fridovich28). In the present paper, we observe an endothelium-dependent NO increased production by MO that can be related to previous observations on increased NO concentrations in plasma(Reference Piolot, Blache, Boulet, Fortin, Dubreuil, Marcoux, Davignon and Lussier-Cacan29) and urine(Reference Harris, Rambjør, Windsor and Diederich30) from human subjects or in plasma from rats(Reference Erdogan, Fadillioglu, Ozgocmen, Sogut, Ozyurt, Akyol and Ardicoglu31), both indices of NO-enhanced production by fish oil-supplemented diets.
It has been described that NO concentrates in lipophilic milieu by virtue of its uncharged character, low molecular mass, and relatively high lipid/water partition coefficient(Reference Denicola, Souza, Radi and Lissi32). The NO generated at the vascular wall can act directly or through the generation of nitroalkenes by its reaction with PUFA, which eventually will release NO(Reference Schopfer, Baker and Giles33). Moreover, nitroalkenes, which reduce inflammation(Reference Cui, Schopfer and Zhang34), may also contribute to the protection against atherosclerosis. We have observed that the MO-rich diet is associated with an increase in α-tocopherol in the aortic tissue. As both the CO and MO diet were not deficient in vitamin E but contained similar tocopherol equivalents and in excess of requirements, the increased tocopherol content with MO is unlikely to be the cause of enhanced NO production through activation of endothelial NO synthase via phosphorylation of serine 1177 in the enzyme(Reference Heller, Werner-Felmayer and Werner35) but we attribute the increase in endothelial NO to the long-chain n-3 PUFA. The high levels of α-tocopherol may be explained by two contributions. First, additional RRR, RSR, RRS and RSS isomers reached the aorta from the liver because of the excess of all-rac-α-tocopherol supplemented to the MO diet (20·7 mg/kg or 27·4 %) compared with the high content of γ-tocopherol in the CO diet (38·7 mg/kg), despite the attempts to equalise tocopherol equivalents. Second, because of its antioxidant activity, NO can spare α-tocopherol from oxidation(Reference Botti, Batthyany, Trostchansky, Radi, Freeman and Rubbo3, Reference O'Donnell, Chumley, Hogg, Bloodsworth, Darley-Usmar and Freeman4).
NO is able to diffuse to the lipidic core of LDL from the vascular wall(Reference Denicola, Batthyany, Lissi, Freeman, Rubbo and Radi5) and react with radical species at near diffusion-limited rate(Reference O'Donnell, Chumley, Hogg, Bloodsworth, Darley-Usmar and Freeman4). In the present study we in fact failed to detect a difference in the ex vivo ability of chemically generated NO to reach the lipidic core of lipoproteins enriched either with n-3 PUFA or n-6 PUFA. However, the greater amount of NO generated by the MO-rich diet compared with the CO-rich diet will be equally able to diffuse into VLDL+LDL and act as antioxidant in situ. We observed that native VLDL+LDL from MO-fed rats had a higher peroxidisability index and that these lipoproteins carry much less total PUFA than those isolated from animals fed a diet enriched in CO as observed by Thomas et al. (Reference Thomas, Thornburg, Manning, Hooper and Rudel36). The absence of differences in electrophoretic mobility of either whole lipoproteins or apo B-100, together with lack of difference in CD, TBARS and total antioxidant capacity in VLDL+LDL, and in TBARS and PON-1 in plasma from the two dietary groups, even at the high dose of fish oil used in the present paper, supports the idea that these native lipoproteins are not more oxidised in vivo in spite of a lower α-tocopherol content. It should be emphasised that fish oil decreases the VLDL levels(Reference Childs, King and Knopp37) and thus the α-tocopherol plasma levels(Reference Childs, King and Knopp37, Reference Bjørneboe, Bjørneboe and Drevon38). The absence of increased oxidation in native VLDL+LDL with an increased peroxidisability index compared with rats fed a diet enriched in CO may be due to the increased NO availability, which counteracts the decrease in α-tocopherol. However, in the current in vivo study it was not possible to provide evidence of NO sparing α-tocopherol.
NO is a potent antioxidant that can diffuse in situ into lipoproteins. However, as a gas it was absent in the ex vivo assays, and thus the antioxidant effect of the increased NO production on preventing lipoprotein oxidation cannot be demonstrated in those conditions. We observed alterations in the ex vivo susceptibility to oxidation in the MO group when oxidising agents such as Cu2+ and AAPH were used. The shortening of the lag time on rats fed an MO-rich diet can be related to the lower levels of α-tocopherol. In this sense, Napolitano et al. (Reference Napolitano, Bravo, Avella, Chico, Ochoa, Botham and Rivabene39) detected a decrease in α-tocopherol in chylomicron remnant preparations from rats treated with fish oil in comparison with rats treated with CO. The reduction in oxidation rate(Reference Bonanome, Pagnan, Biffanti, Opportuno, Sorgato, Dorella, Maiorino and Ursini40) and maximum CD formation can be related either to a lower concentration of PUFA or to a more rapid decomposition of CD derived from n-3 PUFA. Reductions of lag time and oxidation rate were also shown in LDL from long-chain n-3 PUFA-fed rats(Reference Higdon, Du, Lee, Wu and Wander14, Reference Thomas, Thornburg, Manning, Hooper and Rudel36) and human subjects(Reference Wander, Du, Ketchum and Rowe16, Reference Sorensen, Marckmann, Hoy, van Duyvenvoorde and Princen41, Reference Wander, Du and Thomas42). These paradoxical results suggest the involvement of a differential distribution of fatty acids in LDL. The long chain n-3 PUFA are found in cholesteryl esters and TAG present in the core of LDL, while linoleic acid is found in surface phospholipids(Reference Napolitano, Bravo, Avella, Chico, Ochoa, Botham and Rivabene39). We can consider, in agreement of Higdon et al. (Reference Higdon, Du, Lee, Wu and Wander14), that the overall total oxidation of the VLDL+LDL particles was not increased by the MO-rich diet. However, results from different studies are contradictory. While some researches reported no difference in susceptibility of LDL particles to oxidation with fish-oil supplementation(Reference Piolot, Blache, Boulet, Fortin, Dubreuil, Marcoux, Davignon and Lussier-Cacan29, Reference Brude, Drevon and Hjermann43, Reference Higgins, Carroll, McCarthy, Corridan, Roche, Wallace, O'Brien and Morrissey44), others found a decrease(Reference Oostenburg, Mensink, Hardeman, De Vries, Brouns and Hornstra45) or even an increase(Reference Mesa, Buckley, Minihane and Yaqoob27, Reference Leigh-Firbank, Minihane, Leake, Wright, Murphy, Griffin and Williams46). Differences in study design, the supplementation dosage, the use of unreliable tests or of oxidised dietary oils may explain partly these inconsistent results.
We have to take into consideration that in the oxidation of lipoproteins ex vivo, α-tocopherol and total PUFA are involved and that NO is not present. We have thus demonstrated by different techniques that native lipoproteins were not more oxidised and that the resistance of VLDL+LDL to oxidation ex vivo does not reflect their in vivo behaviour as relevant factors such as NO are lacking.
HDL-associated PON-1 prevents LDL oxidation(Reference Aviram, Hardak, Vaya, Mahmood, Milo, Hoffman, Billicke, Draganov and Rosenblat7) and represents another mechanism by which long-chain n-3 PUFA could exert their beneficial effect at cardiovascular level. No information is currently available on the effects of fish oil on PON-1 activity in healthy humans or animals and the lack of any effect in the present study rules out an in vivo modulation of LDL oxidation by fish oil via HDL. Moreover, the similar levels of TBARS and PON-1 in plasma corroborate the absence of increased lipid peroxidation by a fish oil-rich diet.
It has been demonstrated that fish oil has a broad spectrum of beneficial effects. The increased endothelial NO production, induced in vivo, is indicative of a compensatory protective mechanism in inflammatory and vascular diseases and may be related to an increase in α-tocopherol. In the present paper, we demonstrate beneficial effects of fish oil in aortic tissue and non-deleterious effects on VLDL+LDL in spite of a higher peroxidisability index. It is likely that the fish oil-mediated NO increase and the absence of differences in the rate of diffusion into the VLDL+LDL lipidic core are additional crucial mechanisms involved in the anti-atherogenic properties of fish and fish oils.
Acknowledgements
There is no conflict of interest. The authors thank Robin Rycroft for valuable assistance in the preparation of the manuscript. The present study was supported by the Comisión Interministerial de Ciencia y Tecnología (PM 98-0182) and the Generalitat de Catalunya (2005SGR269). We would also like to thank the Universitat de Barcelona for supporting D. L.
D. L., K. C. and M. T. M. performed the assays on biological activities, M. M., A. D. and H. R. were in charge of NO diffusion studies into the lipidic core of VLDL+LDL and J. I. R.-S. evaluated the fatty acid content of oils and VLDL+LDL.








