CHD is a major cause of death in developed societiesReference Hu and Willett1. Elevated LDL-cholesterol and TAG levels are both independent risk factors for CHDReference Sprecher2–Reference Cullen4. In addition, the presence of oxidised LDL accelerates atherogenesisReference Regnstrom, Nilsson, Tornvall, Landou and Hamsten5, Reference Reaven6, which in turn increases the risk for coronary disease. Lipid-lowering drugs could effectively reduce the atherogenic lipoprotein profile in patients with hyperlipidaemia and atherosclerosisReference Wilt, Bloomfield, MacDonald, Nelson, Rutks, Ho, Larsen, McCall, Pineros and Sales7. However, dietary preventions are important to the general population, who do not yet have dyslipidaemic symptoms. In addition to conventional fruits and vegetables, some phytochemicals in traditional medicinal plants have hypolipidaemic effects, modulating gene expression in lipid and lipoprotein metabolismReference Tucker8. A number of reports support the idea. For example, Vasu et al. Reference Vasu, Hiren, Thaikoottathil and Gupta9 reported that Enicostemma littorale aqueous extract showed antioxidant effects and a cholesterol-lowering effect by reducing 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase activity in hypercholesterolaemic ratsReference Vasu, Hiren, Thaikoottathil and Gupta9 and Chu & LiuReference Chu and Liu10 showed that cranberries induced LDL receptor expression. The data suggest that medicinal plant extracts may be helpful to prevent dyslipidaemia by lowering LDL oxidation and by beneficially changing lipid gene expressions without distinctive side effects from long-term intake.
Asian plantain (Plantago asiatica) is a traditional medicine in East Asia, used to treat tumours, infectious diseases and digestive disorders, and recent studies have suggested some potential mechanisms of the health benefits of P. asiatica extracts. It was suggested that treatment with P. asiatica water extracts induced haeme oxygenase-1 expression in cultured cells without cytotoxicityReference Foresti, Hoque, Monti, Green and Motterlini11. Haeme oxygenase-1 is an inducible stress protein-degrading enzyme that opens the porphyrin ring, producing biliverdin, carbon monoxide, and the most dangerous product, free redox active Fe. Increasing evidence has indicated the critical role of haeme oxygenase-1 in cytoprotection in a variety of stressful events, such as hypoxia, bacterial lipopolysaccharides, reactive oxygen and nitrogen species and inflammatory conditions caused by oxidative stressReference Alcaraz, Fernandez and Guillen12–Reference Balla, Vercellotti, Jeney, Yachie, Varga, Eaton and Balla14. In addition, hot water extracts of P. asiatica showed significant inhibitory activity toward the proliferation of lymphoma and carcinoma cells and to herpes and adenoviral infection. P. asiatica water extracts also enhance interferon-γ secretion in human mononuclear cellsReference Chiang, Chiang, Chang and Lin15.
Compared with water extracts of plants, herbal essential oils generally contain a variety of volatile compounds that may have aromatic and medicinal properties. Some herbal essential oils are generally rich in tocopherols and phenolic compoundsReference Berrougui, Cloutier, Isabelle and Khalil16, which have strong antioxidant activity. Terpenoids found in herbal essential oils could have hypolipidaemic effects through the inhibition of hepatic cholesterol biosynthesisReference Clegg, Middleton, Bell and White17 and cholesterol nucleation in bileReference von Bergmann, Beck, Engel and Leiss18.
Similar to other essential oils, P. asiatica essential oil (PAEO) may contain a variety of volatile bioactive compounds that show antioxidant and hypolipidaemic effects. However, the composition and hypolipidaemic effects of PAEO have not been investigated. Accordingly, we analysed the composition of PAEO and tested its protective effects on LDL oxidation. We then evaluated the lipid-lowering capacity of PAEO, both in vitro and in vivo, to investigate the hypolipidaemic mechanism of PAEO by assessing the expression of key cholesterol metabolism genes.
Methods
Chemicals and reagents
Dulbecco's minimum essential medium, fetal bovine serum, liquid gentamicin reagent solution, penicillin and streptomycin, and trypsin-EDTA were purchased from Join Bio-Innovation (Seoul, Korea). Enhanced chemiluminescent Western blotting detection reagents and enhanced chemiluminescent HyperfilmTM were obtained from Amersham-Pharmacia Korea (Seoul, Korea). Anti-rabbit IgG and heavy and light (H&L) chain-specific (goat) peroxidase conjugate were purchased from Calbiochem (Darmstadt, Germany). Anti-HMG-CoA reductase was obtained from Upstate (Lake Placid, NY, USA). Anti-LDL receptor was a generous gift from Dr Allen D. Cooper (Stanford School of Medicine, Palo Alto, CA, USA). PowerScript RT was obtained from Clontech (Palo Alto, CA, USA). The oligo dT15 primer and random hexamers were obtained from Promega (Madison, WI, USA). Taq DNA polymerase was obtained from iNtRON Biotechnology (Kyungki-Do, Korea). All other reagents used were purchased from Sigma Chemicals (St Louis, MO, USA). HepG2 cells were obtained from the Korean Cell Line Bank (Seoul, Korea).
Preparation of Plantago asiatica essential oil
Leaves of P. asiatica were harvested from the Arboretum of Korea University (Seoul, Korea) in June 2005. The leaves were stored in a plastic bag at − 70 °C before analysis. For steam distillation, 20 g leaves were ground using a commercial blender and steam-distilled and extracted simultaneously with 500 ml distilled water and 30 ml diethyl ether for 2 h at atmospheric pressure. The extract was dried over anhydrous Na2SO4 and concentrated to 300 μl using a gentle stream of N2 gas. Extractions were performed in triplicate.
Analysis of Plantago asiatica essential oil by gas chromatography–mass spectrometry
GC–MS analysis was conducted using a gas chromatograph (Agilent 6890 N; Agilent Technologies, Palo Alto, CA, USA) with a mass spectrometer (Quattro GC/MS/MS; Macromass, Manchester, UK); the gas chromatograph was equipped with a capillary column (50 m length × 0·25 mm diameter × 0·2 μm film thickness, AT-1701; Alltech, PA, USA). A 1 μl sample of the extract was injected (splitless mode) into each column. Oven temperature was programmed from 40 °C, with an initial holding time of 2 min, to 120 °C at the rate of 3 °C/min, and then finally to 200 °C at the rate of 5 °C/min. The flow rate of He, the carrier gas, was 1·0 ml/min. The injector and detector temperatures were held at 280 and 240 °C, respectively. The parameters of the mass spectrometer were optimised for the best resolutions at 69, 219, 502 and 614 m/z using perfluorotributyl amine. The measurement of mass was conducted using an EI-positive ion source at 240 °C in the SCAN mode in the mass range of 33–350 m/z. Total ion chromatograms of the samples were analysed using MassLynx 4.0 (MassLynx 4.0 SCN 474; Micromass), and the compounds were positively identified with the aid of the Wiley mass spectral database (2002; John Wiley & Sons, Hoboken, NJ, USA).
Cell culture and Plantago asiatica essential oil treatments
HepG2 cells were seeded in six-well Falcon plates at 106 cells/ml in Dulbecco's minimum essential medium supplemented with 10 % fetal bovine serum, 1 % liquid gentamicin reagent solution, and 1 % penicillin and streptomycin. The cells were cultured at 37°C in a humid atmosphere containing 5 % CO2 until 60–80 % confluent and were then used for RT-PCR and Western blot assay. The culture medium was replaced on alternate days, and the cells were kept in medium free of serum and antibiotics during treatment. HepG2 cells were incubated in fresh Dulbecco's minimum essential medium with or without experimental additives. Cells were exposed to 0, 0·005, 0·01, 0·025, 0·05, 0·1 or 0·2 mg/ml concentrations of PAEO for 24 h.
Quantification of low-density lipoprotein oxidation
Fresh human LDL was isolated from serum according to a previously described methodReference Lee, Moye, Campos, Williams and Sacks19, and the protein content of the isolated LDL was determined using a Bio-Rad protein kit (Bio-Rad, Hercules, CA, USA), with bovine serum albumin (Sigma, St Louis, MO, USA) as the standard. The stock LDL fraction was dialysed against degassed PBS (pH 7.4) in the dark for 24 h. The dialysis solution was changed at least four times. The dialysed LDL was diluted to 600 mg protein/l with 0·01 m-sodium phosphate buffer (pH 7.4). For the control incubation tubes, 30 μl LDL (600 mg/l) was mixed with 5 μl 50 μm-CuSO4 solution and 15 μl 0·01 m-sodium-phosphate-buffer (pH 7.4) and incubated at 37°C for up to 18 h. For the experimental incubation tubes, 30 μl LDL (600 mg/l) was pre-incubated with essential oil in 0, 0·1, 0·2 or 0·3 mg PAEO/ml for 5 min, after which 5 μl 50 μm-CuSO4 solution was added to initiate oxidation, followed by incubation at 37°C for 6 or 12 h. The oxidation was then stopped by the addition of 2·5 μl 27 mm-EDTA and cooled to 4°C. The degree of LDL oxidation was monitored by measuring the production of thiobarbituric acid-reactive substances (TBARS). In brief, the LDL incubation tubes were immediately treated with 100 μl ice-cold 10 % TCA to precipitate protein and were incubated for 15 min on ice. The incubation mixture was then centrifuged at 2200 g for 15 min at 4°C. A 100 μl sample of supernatant fraction was placed into a new screw-topped 1·5 ml tube, and an equal volume of 0·67 % (w/v) thiobarbituric acid was added. The mixture was then heated at 95°C for 25 min and then cooled on ice. TBARS were then determined by measuring the absorbance at 532 nm. The calibration was done using a malondialdehyde standard solution prepared from 1,1,3,3-tetramethoxypropane. The value of TBARS was expressed as μmol malondialdehyde/mg LDL protein.
Animals and feeding protocol
The mice were obtained from Orient Bio (Gyeonggi-Do, Korea). The animals were divided into groups of six mice weighing 15–19 g. Mice, aged 6 weeks, were fed normal chow supplemented with water (control), 0·03 mg PAEO/mouse per d (PA1) or 0·15 mg PAEO/mouse per d (PA2) every d for 3 weeks. The mice were fasted for 16–19 h overnight and blood samples were collected in purple-topped EDTA tubes once per week. Plasma samples were obtained by blood centrifugation at 4°C and 3000 rpm for 15 min and stored at − 20°C for lipid analysis and were used within 1 week. After the treatment ended, the mice were killed and several organs were quick-frozen in liquid N2 and stored at − 80°C for total RNA and protein extraction.
Determination of total cholesterol, triacylglycerol and glucose in plasma
The plasma was separated from the blood after centrifugation. Total cholesterol, TAG and glucose levels were measured enzymically (Asan Chemical, Seoul, Korea). The protein concentration was determined using a Bio-Rad protein kit, with bovine serum albumin as the standard.
Isolation of total RNA and reverse transcriptase-polymerase chain reaction
Total RNA was extracted from mouse cells and liver using a TRI reagent kit (Sigma) according to the manufacturer's protocol and suspended in diethylpyrocarbonate-treated water. For cDNA synthesis, 2 μg total RNA was reverse transcribed using the PowerScript reverse transcriptase (Clontech, Mountain View, CA, USA) according to the manufacturer's protocol, using a combination of oligo dT15 primer and random hexamers, resulting in 20 μl cDNA. PCR was performed using 1 unit Taq DNA polymerase (iNtRON Biotechnology, Gyeonggi-Do, Korea) with 1 μl cDNA template. In vitro PCR primers were designed using published nucleotide sequences and shown in Table 1Reference Andreou and Prokipcak20, Reference Hasumi, Suzuki, Matsui, Koike, Ito and Yamanaka21.
Table 1 Polymerase chain reaction primer sequences

HMG-CoA, 3-hydroxy-3-methyl-glutaryl-CoA.
* Primers are shown 5′ → 3′.
PCR using the primers for the LDL receptor and HMG-CoA reductase was performed with an initial cycle of 5 min at 95°C, 30 s at 62°C and 30 s at 72°C, and a final extension for 5 min at 72°C. PCR using the 18S rRNA and CYP7A1 templates was similar, with the exception of the annealing temperature (18S, 30 s at 60°C; CYP7A1, 30 s at 57°C) and number of cycles (18S, ten cycles; CYP7A1, thirty-four cycles). The 18S rRNA transcripts were used as internal controls.
In vivo PCR primers were designed using published nucleotide sequences for the mouse LDL receptor from Hanaka et al. Reference Hanaka, Abe, Itakura and Matsumoto22 and the sequences for mouse HMG-CoA reductase and CYP7A1 from Han et al. Reference Han, Chung, Lee and Rhee23 and β-actin from Wood et al. Reference Wood, Hunter and Trayhurn24 (Table 1). PCR using the LDL receptor primer was performed with an initial cycle of 4 min at 94°C, followed by thirty cycles of 30 s at 94°C, 30 s at 42°C and 30 s at 72°C, and a final extension for 5 min at 72°C. PCR using the HMG-CoA reductase, CYP7A1 and β-actin primers was performed similarly, with the exception of the annealing temperature (HMG-CoA reductase, 57°C; CYP7A1, 52°C; β-actin, 50°C) and number of cycles (HMG-CoA reductase, twenty-nine cycles; CYP7A1, thirty cycles; β-actin, twenty-three cycles). The β-actin transcripts were used as internal controls.
Western blotting
The cells were lysed and the liver was homogenised in a buffer containing 10 mm-tri(hydroxymethyl)-aminomethane-HCl (pH 7.4), 0·1 m-EDTA, 10 mm-NaCl, 0·5 % Triton X-100 and protease inhibitor cocktail (1 ml/100 ml cell lysate; Sigma) at 4°C. The lysate was clarified by centrifugation at 14 000 rpm for 10 min at 4°C. The protein concentration was determined using a Bio-Rad protein kit, with bovine serum albumin (Sigma) as the standard. Equal amounts of protein were boiled in a sample buffer (5 % β-mercaptoethanol) for 5 min. Samples were separated using SDS-PAGE and were blotted onto a nitrocellulose membrane (0·45 μm, PROTRAN Nitrocellulose Transfer Membrane; Schleicher & Schuell BioScience GmbH, Dassel, Germany). Non-specific protein-binding sites were blocked by incubation in PBS (pH 7.4), 0·1 % Tween 20 and 5 % skimmed milk. To examine LDL receptor expression, the samples were incubated with an anti-LDL receptor antibody at 1/2000 or anti-HMG-CoA reductase (Upstate, Lake Placid, NY, USA). After washing several times with PBS–0·1 % Tween 20, the membrane was incubated with 1/5000 anti-rabbit IgG and heavy and light (H&L) chain-specific (goat) peroxidase conjugate secondary antibody (Calbiochem, Darmstadt, Germany). Immunoreactive bands were detected by enhanced chemiluminescent Western blotting detection reagents (Amersham-Pharmacia Korea, Seoul, Korea) and exposed to high-performance chemiluminescence film for 10 s. Protein immunoblots were scanned using a 690 Bio-Rad densitometor, using the Multi-Analyst program (Bio-Rad). The density of each band was quantified using Sigmagel (Jandel Scientific, San Rafael, CA, USA).
Statistical analyses
Each experiment was repeated at least three times. One-way ANOVA followed by Tukey tests were used to compare treatments. Student t tests were used for comparisons between two groups. Statistical significance was indicated by P < 0·05. Data are reported as the mean values and standard deviations.
Results
Plantago asiatica essential oil composition
According to the GC–MS analysis of the PAEO leaves, the primary volatile compounds were linalool (82·5 %) and fenchylalcohol (9·1 %) and minor components were bicyclogermacrene (3·7 %), menthen-2,3-diol (2·7 %) and eugenol (2·0 %) (Table 2).
Table 2 Analysis of Asian plantain (Plantago asiatica) essential oil by gas chromatography–mass spectrometry

t, Trace amount (less than 0·05 % of the total peak area).
Plantago asiatica essential oil suppressed human Cu2+-mediated low-density lipoprotein oxidation in vitro
The oxidation of LDL has been implicated in the development of atherosclerosisReference Witztum25; thus, we examined the protective effect of PAEO against Cu2+-mediated LDL oxidation. The isolated human LDL was incubated with CuSO4 for 6 or 12 h, and a TBARS assay was performed to quantify LDL oxidation. PAEO significantly inhibited the formation of TBARS at all PAEO concentrations. Similar reductions in LDL oxidation were found in both 6 and 12 h incubations. At 0·3 mg/ml, Cu2+-mediated LDL oxidation was inhibited up to 94·4 % after 6 h of incubation and 89·5 % after 12 h of inhibition (Fig. 1). These results suggest that PAEO has strong antioxidant activity and inhibits LDL oxidation, which may be beneficial for the prevention of atherogenesis.
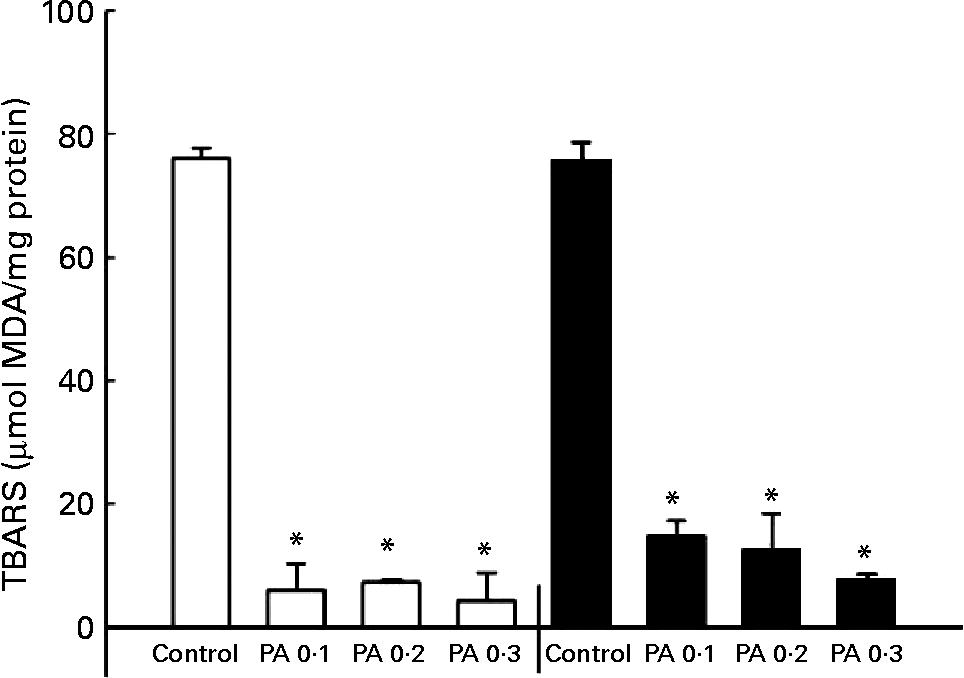
Fig. 1 Effect of Asian plantain (Plantago asiatica) essential oil (PAEO) on Cu2+-induced LDL oxidation at 6 h (□) and 12 h (■) incubation. The 30 μl isolated human LDL (600 mg/l) was pre-incubated with 0, 0·1, 0·2 or 0·3 mg PAEO/ml for 5 min (control, PA 0·1, PA 0·2 and PA 0·3, respectively). Then 5 μl 50 μm-CuSO4 solution was added, and the solution was incubated at 37°C for 6 or 12 h. Oxidation was stopped by the addition of 2·5 μl 27 mm-EDTA, and the degree of LDL oxidation was quantified using a thiobarbituric acid-reactive substances (TBARS) assay. MDA, malondialdehyde. Values are means (n 4), with standard deviations represented by vertical bars. * Mean value was significantly different from that of the control (P < 0·05).
Effects of Plantago asiatica essential oil on expression of the low-density lipoprotein receptor, 3-hydroxy-3-methyl-glutaryl co-enzyme A reductase and CYP7A1 in vitro
HepG2 cells were incubated with 0, 0·005, 0·01, 0·025, 0·05, 0·1 or 0·2 mg PAEO/ml for 12 h, after which the expressions of the LDL receptor, HMG-CoA reductase and CYP7A1 were assessed by RT-PCT (Fig. 2 (A)). The PAEO treatments did not affect gene expression at low concentrations; however, the mRNA level of LDL receptors was significantly elevated in treatments with 0·05, 0·1 and 0·2 mg PAEO/ml. In contrast, the expression of HMG-CoA reductase and CYP7A1 was reduced significantly. At 0·2 mg PAEO/ml, the induction of the LDL receptor was 63 % and the reduction in HMG-CoA reductase and CYP7A1 was 37 and 36 %, respectively (Fig. 2 (A)). Western blot analysis showed results similar to those of the RT-PCR analysis (Fig. 2 (B)). At 0·05 mg PAEO/ml, the LDL receptor was significantly elevated (13 %), whereas the HMG-CoA reductase level was reduced by 24 %. The expression of cholesterol 7-α hydroxylase was unaltered (data not shown).
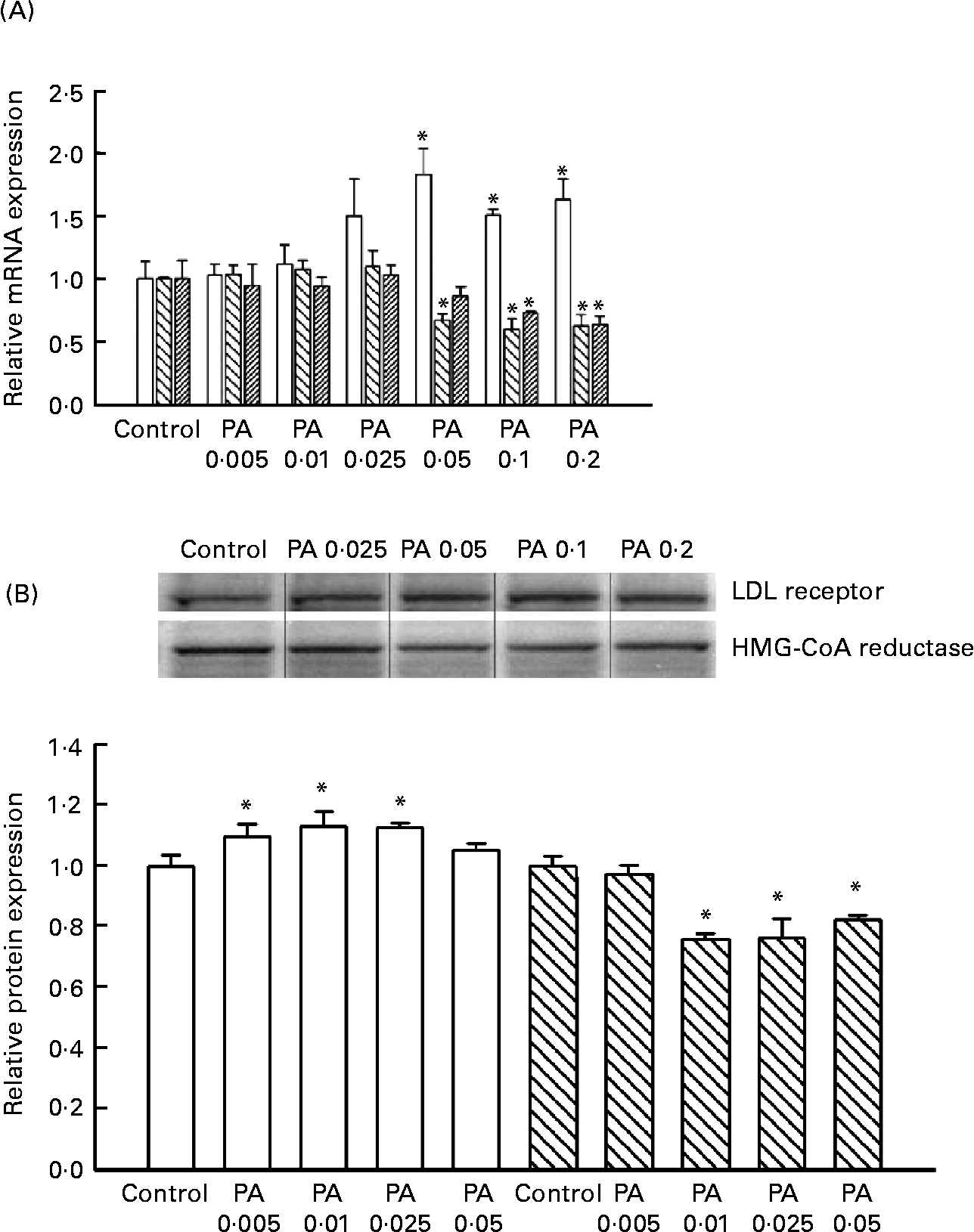
Fig. 2 Effect of Asian plantain (Plantago asiatica) essential oil (PAEO) on (A) mRNA expression and (B) protein expression of the LDL receptor (□), 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase (![]() ) and CYP7A1 (▨). HepG2 cells were incubated with 0, 5, 10, 25, 50, 100 or 200 μg PAEO/ml for 24 h (control, PA 0·005, PA 0·01, PA 0·025, PA 0·05, PA 0·1 and PA 0·2, respectively). The ‘fold-induction’ of each mRNA species was calculated as the ratio of the level of mRNA in treated cells to that of the corresponding mean value in control cells. The amount of mRNA and protein expressions in each sample was normalised to β-actin. The band density was quantified using SigmaGel (Jandal Scientific, San Rafael, CA, USA). Values are means (n 3–6), with standard deviations represented by vertical bars. * Mean value was significantly different from that of the control (P < 0·05).
) and CYP7A1 (▨). HepG2 cells were incubated with 0, 5, 10, 25, 50, 100 or 200 μg PAEO/ml for 24 h (control, PA 0·005, PA 0·01, PA 0·025, PA 0·05, PA 0·1 and PA 0·2, respectively). The ‘fold-induction’ of each mRNA species was calculated as the ratio of the level of mRNA in treated cells to that of the corresponding mean value in control cells. The amount of mRNA and protein expressions in each sample was normalised to β-actin. The band density was quantified using SigmaGel (Jandal Scientific, San Rafael, CA, USA). Values are means (n 3–6), with standard deviations represented by vertical bars. * Mean value was significantly different from that of the control (P < 0·05).
Hypolipidaemic effects of Plantago asiatica essential oil in C57BL/6J mice
After oral administration of a low level of PAEO (PA1; 0·03 mg/mouse per d) for 1 or 2 weeks, TAG levels were not changed; however, plasma cholesterol levels decreased significantly after 2 weeks ( − 17 %; Figs. 3 (A) and (B)). After 3 weeks, the plasma cholesterol and TAG levels were significantly lower than those of the control by 29 and 46 %, respectively (P < 0·05). At a high level of PAEO (0·15 mg PAEO/mouse per d; PA2), plasma TAG levels decreased significantly after 1 week; however, they did not decrease more with further treatment (Fig. 3 (B)). Plasma cholesterol levels were significantly lower than those of the control after 2 weeks and showed a similar trend at 3 weeks (Fig. 3 (A)). The reductions in total cholesterol, TAG and glucose levels were 21, 29 and 26 %, respectively, after 3 weeks of high-PAEO feeding (Fig. 3). Plasma glucose levels were also significantly lower in the PA1 group after 3 weeks (Fig. 3). The reductions were 34 and 26 %, respectively, at low and high levels of PAEO.

Fig. 3 (A) Plasma total cholesterol, (B) TAG and (C) glucose concentrations in mice fed normal chow supplemented with water (control), 0·03 mg Asian plantain (Plantago asiatica) essential oil (PAEO)/mouse per d (PA1) or 0·15 mg PAEO/mouse per d (PA2) every day for 3 weeks. Values are means (n 6), with standard deviations represented by vertical bars. Mean value was significantly different from that of the control: * P < 0·05, ** P < 0·01, *** P < 0·001.
Effects of Plantago asiatica essential oil on mRNA and protein levels of the low-density lipoprotein receptor, 3-hydroxy-3-methyl-glutaryl-CoA reductase and CYP7A1 in vivo
Because plasma cholesterol levels were reduced significantly at both low and high levels of PAEO feeding, we further analysed cholesterol-related gene expression in the mouse liver after 3 weeks of PAEO feeding. The level of hepatic LDL receptor mRNA in PAEO-treated animals was significantly higher than in control animals (P < 0·05; Fig. 4), and greater induction was observed in the PA1 than in the PA2 group. However, the LDL receptor protein levels were only marginally elevated in the PA1 animals (Fig. 5). The HMG-CoA reductase mRNA and protein levels in the liver were significantly lower after PAEO administration than in the control group (P < 0·05; Figs. 4 and 5). HMG-CoA reductase transcription was down regulated by 41 % in the PA1 and 46 % in the PA2 animals. Protein levels were also significantly reduced in the PA2 group by 11 %. The CYP7A1 mRNA level was unchanged after PAEO feeding.

Fig. 4 Effects of Asian plantain (Plantago asiatica) essential oil (PAEO) on the expression of the LDL receptor (□), 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase (![]() ) and CYP7A1 (▨). C57BL/6J mice were administered PAEO at 0·03 mg PAEO/mouse per d (PA1) or 0·15 mg PAEO/mouse per d (PA2) every day for 3 weeks. The mRNA of LDL receptor, HMG-CoA reductase and CYP7A1 was normalised to the quantity of β-actin. The ‘fold-induction’ of each mRNA species was calculated as the ratio of the level of mRNA in treated mice to that of the corresponding mean value in control mice. Values are means (n 6), with standard deviations represented by vertical bars. * Mean value was significantly different from that of the control (P < 0·05).
) and CYP7A1 (▨). C57BL/6J mice were administered PAEO at 0·03 mg PAEO/mouse per d (PA1) or 0·15 mg PAEO/mouse per d (PA2) every day for 3 weeks. The mRNA of LDL receptor, HMG-CoA reductase and CYP7A1 was normalised to the quantity of β-actin. The ‘fold-induction’ of each mRNA species was calculated as the ratio of the level of mRNA in treated mice to that of the corresponding mean value in control mice. Values are means (n 6), with standard deviations represented by vertical bars. * Mean value was significantly different from that of the control (P < 0·05).

Fig. 5 Effects of Asian plantain (Plantago asiatica) essential oil (PAEO) on the protein levels of the LDL receptor (□) and 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase (![]() ). C57BL/6J mice were administered PAEO at 0·03 mg PAEO/mouse per d (PA1) or 0·15 mg PAEO/mouse per d (PA2) every day for 3 weeks. The band density was quantified using SigmaGel (Jandal Scientific, San Rafael, CA, USA). Values are means (n 6), with standard deviations represented by vertical bars. * Mean value was significantly different from that of the control (P < 0·05).
). C57BL/6J mice were administered PAEO at 0·03 mg PAEO/mouse per d (PA1) or 0·15 mg PAEO/mouse per d (PA2) every day for 3 weeks. The band density was quantified using SigmaGel (Jandal Scientific, San Rafael, CA, USA). Values are means (n 6), with standard deviations represented by vertical bars. * Mean value was significantly different from that of the control (P < 0·05).
Discussion
Our objective was to determine the hypolipidaemic effects of PAEO both on cultured hepatocytes and in C57BL/6 mice. We examined the hypolipidaemic mechanism of PAEO by assessing the expression of key hepatic cholesterol metabolism genes and also measured the protective effects of PAEO on LDL oxidation.
PAEO treatment strongly protected against LDL oxidation initiated by Cu ions. The major compound in PAEO, as determined by GC–MS, was linalool, which can affect LDL metabolism by reducing LDL oxidation and by altering the receptor affinity, probably due to their lipophilic activityReference Naderi, Asgary, Ani, Sarraf-Zadegan and Safari26. The oxidised LDL particles have low affinity to the LDL receptor and have markedly increased affinity to macrophage scavenger receptors, such as SR-A and CD36, which can accelerate foam cell formation in the major blood vessels. Thus, prevention of LDL oxidation by linalool can contribute to increase of the affinity of LDL to the LDL receptor, which is in line with our findings. Oxidised LDL particles are preferentially taken up by macrophage receptors in blood vesselsReference Brown and Goldstein27, and the antioxidant activity of PAEO may prevent this process.
In cultured HepG2 cells, there were profound effects of PAEO on the expression of cholesterol metabolism genes. The LDL receptor was notably up regulated, whereas HMG-CoA reductase and CYP7A1 were significantly down regulated. Parallel changes in the protein levels of the LDL receptor and HMG-CoA reductase were observed in Western blot analyses. Hypolipidaemic effects of PAEO were subsequently confirmed in vivo by feeding PAEO to mice for 3 weeks. Interestingly, plasma cholesterol and TAG levels were reduced significantly after 2 or 3 weeks of feeding, especially in the high-dose treatment. The up regulation of the LDL receptor and the down regulation of HMG-CoA reductase in the liver of treated mice confirmed the in vitro results.
Cholesterol reduction could be achieved by multiple mechanisms: by reducing plasma cholesterol, decreasing hepatic synthesis, or enhancing cholesterol degradation and excretion. LDL receptor induction is a major factor in reducing plasma cholesterol levels, and the direct inhibition of HMG-CoA reductase or its expression could effectively control hepatic cholesterol biosynthesis. Furthermore, bile acid formation from cholesterol in the liver is a major pathway for cholesterol degradation, and cholesterol 7-α hydroxylase, which is encoded by CYP7A1, is a rate-limiting enzyme in the classical pathway of bile acid formationReference Cooper28.
PAEO increased LDL receptor mRNA and protein levels in vitro and LDL receptor mRNA levels in vivo, but there were no significant effects on the LDL receptor protein in vivo, even though it showed a tendency similar to its in vitro response. This is not surprising because in vitro cell-culture systems are often more sensitive than in vivo animal models for evaluating the expression of the LDL receptor. In animals, cholesterol homeostasis is maintained by cooperation with extra-hepatic organs, including muscles and the kidney, which also express the LDL receptor. Hepatic LDL receptor expression might not be a critical response to PAEO in vivo.
HMG-CoA reductase was significantly lower in the PAEO groups than in the control group, both in vitro and in vivo. The reduction of HMG-CoA reductase could lower hepatic cholesterol biosynthesis, and may be a major mechanism by which PAEO lowers plasma cholesterol level. Under normal cellular conditions, both HMG-CoA reductase and the LDL receptor are induced in cholesterol depletion of hepatocytes via the activation of sterol regulatory element-binding proteinsReference Smith, Osborne, Brown, Goldstein and Gil29, Reference Smith, Osborne, Goldstein and Brown30. These two genes were regulated in opposite directions in the present studies: the LDL receptor was up regulated and HMG-CoA reductase was down regulated and these findings are in line with previous studies of HMG-CoA reductase inhibitorsReference Clarke, Fogelman and Edwards31, Reference Molowa and Cimis32. Cholesterol degradation to bile acids in the liver is largely dependent on the expression of CYP7A1. The CYP7A1 mRNA level decreased in response to PAEO in HepG2 cells, but not in mice. In addition, the plasma TAG concentration, which is an independent risk factor for coronary diseaseReference Sprecher2, Reference Cullen4, was also significantly lower in mice fed PAEO than in control mice.
In conclusion, PAEO showed hypocholesterolaemic effects in vitro and in vivo. The suppression of HMG-CoA reductase expression may be a major mechanism leading to this effect; the up regulation of the LDL receptor, especially in vitro, may be an alternative mechanism. Reduced TAG levels and the antioxidant capacity of PAEO may provide additional heart-protective benefits if PAEO is ingested at appropriate concentrations. Further human studies are needed to determine whether dietary feeding of PAEO can reduce plasma cholesterol levels as observed in the present in vitro and in vivo studies. In addition, future research will examine whether purified compounds from PAEO, such as linalool or fenchylalcohol, have hypocholesterolaemic effects in vitro and in vivo.
Acknowledgements
We thank Dr Allen D. Cooper for providing the LDL receptor antibody. The present study was supported by a grant from the BioGreen 21 Program, Rural Development Administration, Republic of Korea (grant no. 20050401-034-749-180-02-00), a grant from the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (A050376), and also supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF-2006-F00131 (I02849)).








