CVD is an important public health problem and the leading cause of death both in the USA and worldwide( 1 , Reference Benjamin, Virani and Callaway 2 ). The most common type of CVD is atherosclerotic coronary artery disease (CAD). Because calcification is a developmental phase of atherosclerotic plaque, computed tomography (CT) scanning can be used to identify calcified coronary plaques and provides a quantification of coronary artery Ca (CAC)( Reference Grayburn 3 ). The Agatston, or CAC score, is a validated predictor of cardiovascular events( Reference Detrano, Guerci and Carr 4 – Reference Yeboah, McClelland and Polonsky 8 ), and longitudinal change in scores has been used as an indicator of progressive change in the burden of calcified atherosclerotic plaque in the coronary arteries( Reference McClelland, Bild and Burke 9 – Reference Shea, Booth and Miller 11 ).
Dietary modification has long been one of the cornerstone strategies for prevention of CVD( Reference Eckel, Jakicic and Ard 12 – 14 ). Thus far, there is no consensus on ‘an optimal diet or dietary pattern’ that reduces risk or progression of atherosclerotic CAD. Low-carbohydrate diets (LCD) have significant popular appeal as a strategy for weight loss and weight management( Reference Astrup, Meinert Larsen and Harper 15 , Reference Hu and Bazzano 16 ). However, the perception that LCD will have detrimental effects on CAD, due to their higher levels of fat and cholesterol( Reference Eckel, Jakicic and Ard 12 , Reference Hu, Stampfer and Manson 17 , Reference Hooper, Summerbell and Higgins 18 ), has resulted in some professional organisations, including the American Dietetic Association cautioning against LCD( Reference Stein 19 ). Although evidence from randomised controlled trials has shown that short-term weight loss resulting from LCD improve risk factors for atherosclerotic CAD, as weight may regain gradually over the course of dietary intervention, the long-term relationship of LCD to atherosclerotic CAD remains controversial( Reference Hu and Bazzano 16 ). Moreover, the effects of low-carbohydrate dietary patterns in free-living populations, particularly those with subclinical atherosclerosis, are unclear. We took advantage of a large number of persons with subclinical atherosclerosis at baseline and data collected during follow-up in the Multi-Ethnic Study of Atherosclerosis (MESA) to examine the relationship between low-carbohydrate dietary patterns and CAC prevalence, incidence and progression. We bore in mind that there are many ways to formulate a diet that is low in carbohydrates and studied three variants of such diets.
Methods
Study population
Objectives and design of the MESA study have been described previously( Reference Bild, Bluemke and Burke 20 ). In brief, participants were recruited from six regions in the USA (Forsyth County, NC; Northern Manhattan and the Bronx, NY; Baltimore City and Baltimore County, MD; St Paul, MN; Chicago and the village of Maywood, IL; and Los Angeles County, CA). Signed informed consent was obtained from all participants for the procedures in the MESA study, and expedited institutional review board approval was obtained for this secondary analysis.
Data from 6814 men and women aged 45–84 years who were free of clinical CVD at their baseline examination (July 2000 and August 2002) were available at the outset. After excluding 705 individuals who provided insufficient or implausible data on dietary information (consuming >25104 or <2510 kJ/d), 256 individuals who reported implausible physical activity (>24 h/d), and 239 individuals who had uncertain results of cardiac CT scans at baseline (one zero and the other non-zero), the current analysis included data from 5614 men and women.
Dietary assessments
All participants were asked to complete a 120-item FFQ at baseline, assessing their usual intake of specific foods and beverages over the past year. The FFQ utilised the Block format and was validated among Non-Hispanic Whites, African Americans and Hispanic Americans with modification to include Chinese foods( Reference Block, Woods and Potosky 21 ). For each food item, the consumption frequency (times/d, week or month) and estimated food-specific serving size (small, medium or large) were recorded. Frequency options included nine responses ranging from ‘rare or never’ to ‘≥2 times/d’ for food items( Reference Block, Woods and Potosky 21 ). Some processing errors of input of some food frequency variables were subsequently discovered in a subset of MESA participants. These errors were corrected by re-entering all available forms and imputing frequency and serving size values for those whose original records no longer existed.
Daily nutrient intakes were estimated by multiplying frequency and serving size (portion size gram weights) for each food/beverage consumed by the nutrient content of that food or beverage (Nutrition Data Systems for Research (NDSR), Nutrition Coordinating Centre, University of Minnesota, Minneapolis). NDSR estimates and direct outputs of daily nutrient intake, including total energy, carbohydrate, fat (total, saturated, monounsaturated fat and polyunsaturated fat) and protein (total, animal and vegetable protein) were measured as percentage kJ. In NDSR, animal protein includes protein primarily from dairy products, meat, fish, poultry, eggs and other animal foods; whereas vegetable protein includes protein contributed by nuts, legumes, cereal, fruit, vegetables and other plant products. In studies examining the reproducibility of a single FFQ, correlations ranged between 0·5 and 0·7 for nutrient intakes measured at intervals of 1–10 years( Reference Willett 22 ).
Assessment of coronary artery calcium
At baseline and each follow-up visit after 11–59 months (median timing 18 and 39 months for the two visits, respectively), CAC measurements were obtained in two heart passes for each participant. Scanning was performed using an electron beam CT scanner or a multi-detector CT system that utilised helical scanning with reconstruction in 5 mm thick cuts and 350 mm field of view. Two CT scans were acquired for each participant. The methodology for acquisition of the scans has been described previously along with the comparability and reproducibility of image data collected using different scanners( Reference Carr, Nelson and Wong 23 , Reference Detrano, Anderson and Nelson 24 ). These scans were read independently at a centralised reading centre (Harbor-UCLA Research and Education Institute in Torrance, California). Scans were read by a blinded, experienced reader at the MESA CT reading centre( Reference Nelson, Kronmal and Carr 25 ). Measurements from the two CAC scans were averaged to quantify the amount of Ca present, using the Agatston scoring method( Reference Agatston, Janowitz and Hildner 6 ). All scans were brightness adjusted with a standard phantom containing four bars of known Ca density.
Additional measurements
At baseline, information on demographic characteristics, lifestyle risk factors and medical conditions was obtained using interviewer and self-administered questionnaires. The highest level of education completed was utilised as a measure of socioeconomic status. Current alcohol drinking and tobacco use were also assessed at baseline. Physical activity in metabolic equivalents (MET) was evaluated using a semi-quantitative questionnaire adapted from the Cross-Cultural Activity Participation Study( Reference Ainsworth, Irwin and Addy 26 ). Leisure time physical activity was calculated as the sum of minutes per week of intentional exercise, including walking, sports, dance and conditioning (e.g. aerobics, bicycling, running, jogging, rowing, swimming, judo, karate) multiplied by the activity’s individual MET value. History of hypertension was defined as systolic blood pressure ≥140 mmHg, or diastolic blood pressure ≥90 mmHg or taking a medication for hypertension. Prevalent diabetes was defined as fasting blood glucose ≥7mmol/l or the use of glucose-lowering agents. Anthropometric measurements and laboratory data were determined as previously described through a standardised physical examination and venepuncture( Reference Bild, Bluemke and Burke 20 ). BMI was calculated as weight (kg) divided by height (m) squared.
Statistical analysis
Dietary intake of macronutrients, including carbohydrate, fat (total, saturated and unsaturated fat) and protein (total, animal and vegetable protein), was expressed as a percentage of total energy intake (% kJ). Participants were divided into deciles for each of macronutrient intake, and a point value was assigned for each stratum. For fat and protein, participants in the highest stratum received 9 points for that macronutrient, in the next stratum received 8 points, and so on down to participants in the lowest stratum, who received 0 points. For carbohydrate, the order of the strata was reversed; those with the lowest intake received 9 points and those with the highest carbohydrate intake received 0 points (Table 1). A total of three LCD scores were generated: an overall LCD score was calculated as the sum of point scores of total carbohydrate, fat and protein; an animal-based LCD score was calculated as the sum of point scores of carbohydrate, saturated fat and animal protein; and a plant-based LCD score was calculated as the sum of point scores of carbohydrate, unsaturated fat (as the sum of polyunsaturated and monounsaturated fat, excluding trans-fat) and vegetable protein( Reference Halton, Willett and Liu 27 ).
Table 1 Decile cut points for the respective macronutrient categories used to generate the low-carbohydrate diet (LCD) scores*
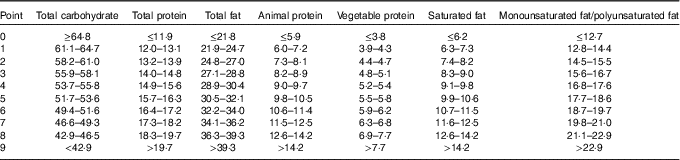
* All cells refer to the percentage of energy intake; the overall LCD score was the sum of reversed decile number for carbohydrate, plus decile numbers for total fat and total protein; the animal-based LCD score was similarly based on carbohydrate, animal protein and saturated fat; and vegetable-based LCD score was based on carbohydrate, vegetable protein and unsaturated fat.
We examined the following outcomes: whether or not CAC was present at baseline (defined as Agatston >0); the amount of CAC present at baseline for those with non-zero baseline scores; progression in CAC from baseline to follow up; and new development of CAC from a previous score of zero during follow-up. Since the frequency of CAC was close to 0·5 at baseline (the rare disease assumption was not met), we used multivariable relative risk regression models to examine the relationship between LCD scores (categorised in quintiles) and presence of CAC at baseline. We used Cox proportional hazard regression models to examine the relationship between LCD score quintiles and new development of CAC during follow-up. Follow-up time was defined as the time from baseline exam to incident CAC. For those participants with positive CAC scores at baseline, we further fitted robust regression models to minimise the influence of outliers in examining the relationship between LCD scores and amount and progression of CAC. Progression of CAC was defined as absolute change in CAC from baseline to the end of follow-up among those participants with positive scores at baseline. All models were adjusted for age, sex, race, educational level, BMI, physical activity, HDL-cholesterol, LDL-cholesterol, TAG, total energy intake, alcohol use, smoking, diabetes status and history of hypertension at baseline (models for progression of CAC were further adjusted for follow-up time). All analyses used the lowest quintile of LCD scores (indicating a high carbohydrate, low fat and protein diet) as the reference category. We used restricted cubic splines without imposing any particular structure on the observed data to examine the shape of the relationship between each LCD score and CAC( Reference Desquilbet and Mariotti 28 ). We performed a sensitivity analysis excluding participants with diabetes at baseline, and another sensitivity analysis to examine the relationship with the CAC increases (binary) combining those with zero and non-zero scores at baseline to increase the sample size. We explored potential racial/ethnic differences in associations by testing interaction terms for LCD scores by race/ethnicity in above multivariable models. We replicated all above analyses using CAC scores which were not adjusted using the standard phantom.
Results
The study sample included 5614 men and women with a mean age 62·5 (sd 10·3) years. The sample was 48 % male and 40 % non-Hispanic White. The correlation coefficients were 0·93 between the overall and animal LCD scores, 0·74 between the overall and vegetable LCD scores and 0·52 between the animal and vegetable LCD scores (all P <0·0001). Unadjusted baseline characteristics of participants are shown in Table 2 according to quintiles of the overall LCD score. Participants in higher quintiles of the overall LCD score (those who consumed less carbohydrate and more protein or fat) were slightly younger, more likely to have finished high school and to drink alcohol or smoke cigarettes. On average, they had higher BMI, higher glucose levels and were more likely to have diabetes but had lower levels of systolic blood pressure, diastolic blood pressure and were less likely to be hypertensive than their counterparts consuming a diet with higher levels of carbohydrate and lower levels of fat and/or protein. There were no differences in the levels of total, LDL, HDL-cholesterol, TAG or physical activity across quintiles of the overall LCD score.
Table 2 Baseline characteristics by quintile (Q) of the overall low-carbohydrate diet (LCD) score (Mean values and standard deviations; medians and interquartile ranges)
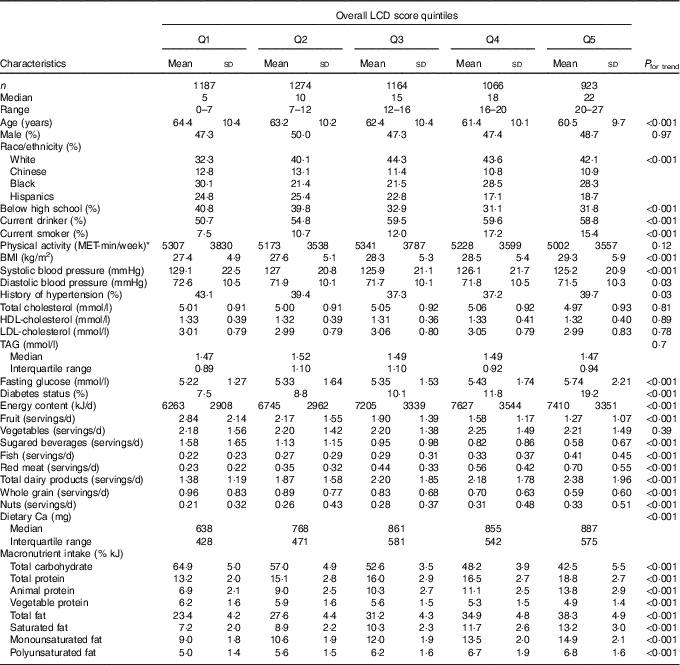
MET, metabolic equivalent.
* Leisure time physical activity was calculated as the sum of minutes per week of intentional exercise (walking, sports, dance and conditioning) multiplied by the activity’s individual MET value.
The median (25th–75th percentile) intake of carbohydrate, fat and protein expressed as a percentage of energy were 53·7 (48·1–59·5), 30·5 (25·9–35·0) and 15·6 (13·5–17·7) %, respectively. In terms of diet, those with higher overall LCD scores had higher intake of total energy, Ca, fish, red meat, dairy products and nuts, lower intakes of sugared beverages, fruit and whole grain. Similar trends were observed for animal- and plant-based LCD scores (including red meat intake), with the exception that participants with higher animal-based scores had lower intake of vegetables, higher intake of dairy products and did not have higher intake of nuts, while those with higher plant-based scores had higher intake of vegetables and nuts but lower intake of dairy products (online Supplementary Tables S1 and S2).
Of the 5614 individuals included in our analysis, 2722 (48·5 %) participants had positive CAC scores at baseline and 264 participants developed newly positive CAC during the follow-up. Among those with positive CAC at baseline, the median (25th–75th percentile) increase in CAC was 47 Agatston units (12–145 units) over the course of follow-up.
Table 3 shows the cross-sectional results of multivariable-adjusted relative risk regression models with presence or absence of CAC at baseline (defined by non-zero or zero Agatston score, respectively) as the outcome and robust regression models with amount of CAC at baseline as the outcome. No evidence for association was observed between the quintiles of overall LCD score and the presence of CAC at baseline or between the quintiles of overall LCD score and amount of CAC at baseline among those with non-zero Agatston scores (P for linear trend = 0·43 for presence v. absence of CAC and 0·59 for amount of CAC at baseline). Similarly, there were no consistent trends for either the animal-based or plant-based LCD scores. Table 4 shows the adjusted associations of LCD scores at baseline with the new development of CAC during follow-up and progression of CAC from baseline to follow-up. For either incidence of CAC or progression of CAC, no significant trends were identified across quintiles of the overall, animal-based or plant-based LCD scores. In the restricted cubic spline models, there was no significant linear relationship for any of the LCD scores and CAC, after adjustment for all of the aforementioned confounders. Results were similar among non-diabetic participants (n 4986; online Supplementary Tables S3 and S4). The sensitivity analysis for binary outcome of CAC increase showed similar results. None of the associations were modified by race/ethnicity (P interaction by race/ethnicity >0·20 for all). Analyses using unadjusted CAC scores did not alter the results.
Table 3 Low-carbohydrate diet (LCD) score and prevalence and amount of coronary artery calcium (CAC) at baseline (cross-sectional) (Ratios and 95 % confidence intervals; coefficients and standard errors)
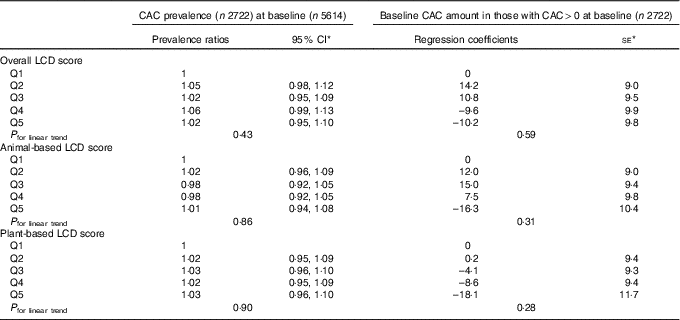
Q, quintile.
* Adjusted for age, sex, race, educational level, BMI, physical activity, HDL-cholesterol, LDL-cholesterol, TAG, total energy intake, alcohol use, smoking and tobacco use, history of hypertension and diabetes status at baseline.
Table 4 Low-carbohydrate diet (LCD) score and coronary artery calcium (CAC) incidence and change (longitudinal) (Hazard ratios and 95 % confidence intervals; coefficients and standard errors)
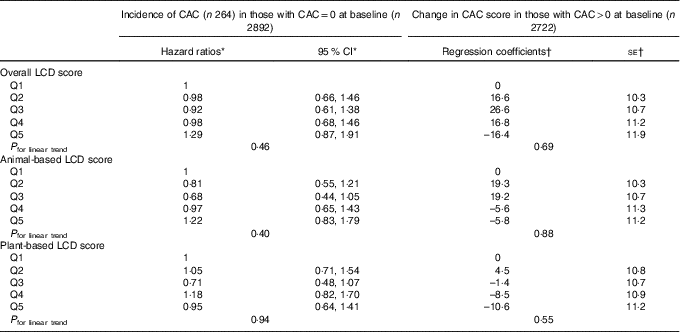
Q, quintile.
* From Cox proportional hazard regression model. Time to event was from baseline to incident CAC. The model was adjusted for age, sex, race, educational level, BMI, physical activity, HDL-cholesterol, LDL-cholesterol, TAG, total energy intake, alcohol use, smoking and tobacco use, history of hypertension and diabetes status at baseline.
† From robust regression adjusted for above covariates plus the interval between CAC exams.
Discussion
In this multi-ethnic, community-based population, we observed no evidence for association between low-carbohydrate dietary patterns and the prevalence, incidence or progression of atherosclerosis as represented by the CAC score. This study is featured by the examination of multiple low-carbohydrate dietary patterns and their relation to the presence or development of CAC in a community-based multi-ethnic cohort. Considering the public perception that LCD may increase cholesterol and in turn increase the risk of CVD, it is important to note that although our finding does not suggest benefit from a LCD in terms of CAC risk, neither does it suggest that diets low in carbohydrates and high in fat and/or protein are associated with greater levels of coronary atherosclerotic plaque or with the progression and/or new development of coronary atherosclerosis( Reference Eckel, Jakicic and Ard 12 , Reference Hu, Stampfer and Manson 17 , Reference Hooper, Summerbell and Higgins 18 ).
It has not been feasible to conduct long-term trials of LCD. However, short-term clinical trials have evaluated the effects of LCD on earlier stage cardiovascular risk markers, such as cholesterol metabolism and glucose synthesis( Reference Hu and Bazzano 16 , Reference Mansoor, Vinknes and Veierod 29 ). Dietary carbohydrate is positively related to HDL concentration and inversely related to TAG concentration( Reference Hu and Bazzano 16 ). Controlled feeding trials have demonstrated that dietary saturated fat, when substituted for carbohydrates, has neutral effects by increasing both LDL-cholesterol and HDL-cholesterol without changing the total:HDL ratio( Reference Mensink, Zock and Kester 30 ). In terms of glucose metabolism, dietary carbohydrates generally reduced markers of glycaemia. In an isoenergetic feeding study in adults without diabetes, limiting dietary carbohydrates lowered glycated albumin and fructosamine( Reference Juraschek, Miller and Selvin 31 ), both of which have been associated with higher risk of cardiovascular events( Reference Selvin, Rawlings and Lutsey 32 ). In a meta-analysis of twenty-three feeding trials, isoenergetic replacement of 5 % dietary energy from carbohydrate with 5 % dietary energy from either saturated fat or unsaturated fat did not alter fasting glucose but lowered HbA1c( Reference Imamura, Micha and Wu 33 ). The findings of those intermediate biomarkers strengthened our understanding of LCD on atherosclerosis and potentially placed emphases on what are the main priorities in the prevention of CVD. However, the results should be interpreted with caution because those biomarkers only partially predict atherosclerosis and diets in weight loss trials cannot represent usual diets in general population.
Prospective cohort studies, despite their limitations, provide the best approach to assessment of possible associations between low-carbohydrate dietary patterns and CVD outcomes in free-living, general populations. Thus far, evidence from such studies has been inconsistent with most identifying no association and a few identifying positive association( Reference Halton, Willett and Liu 27 , Reference Bueno, de Melo and de Oliveira 34 – Reference Lagiou, Sandin and Lof 36 ). For example, a prospective cohort study of young Swedish women reported that low-carbohydrate, high-protein diets were associated with higher risk of incident CHD( Reference Lagiou, Sandin and Lof 36 ). In that study, median carbohydrate intake accounted for 49 % of total daily energy intake. In contrast, data from the Nurses’ Health Study cohort did not identify an association between low-carbohydrate dietary patterns and risk of incident CHD( Reference Halton, Willett and Liu 27 ). In that cohort, median carbohydrate intake expressed as a percentage of total daily energy was 44·2 %. Some potential explanations for these differences in results may be differences in analytic methods, population characteristics and types of diets across the study populations. Neither of those studies was able to directly measure a continuous marker of subclinical disease, such as CAC, and examine its development over time. Our data suggest that diets low in carbohydrates and high in fat and/or protein are not associated with greater levels of coronary atherosclerotic plaque or with new development and/or the progression of coronary atherosclerosis.
Very little is known about whether consumption of dietary carbohydrates is associated with atherosclerotic progression among those who have had atherosclerosis already, and, other than the present study, little or no information exists specifically for low-carbohydrate dietary patterns. Mozaffarian et al. Reference Mozaffarian, Rimm and Herrington (37) reported progression when carbohydrates were replaced by saturated fat and monounsaturated fat but not when carbohydrates were replaced by total fat, polyunsaturated fat or protein among postmenopausal women with a relatively low total fat intake at baseline. In our study, we examined low-carbohydrate dietary patterns where any type of protein or fat replaced carbohydrate, among a population-based cohort of men and women from a variety of race and ethnic backgrounds. Participants generally consumed moderate to high levels of carbohydrates, and about 15·2 % of participants consumed a LCD (defined as ≤45 % of energy from carbohydrates); therefore, extrapolation to other populations should be done in a cautious manner.
Although neither animal-based nor plant-based LCD appeared to have cardiovascular effects, the two LCD might still exert in the cardiovascular system through different fat and protein sources( Reference Halton, Willett and Liu 27 , Reference Bueno, de Melo and de Oliveira 34 – Reference Lagiou, Sandin and Lof 36 ). For example, in the present study, animal-based LCD included more dairy foods but less vegetables, while plant-based LCD included more vegetables but less dairy products (differences in nut intake were minimal). Current evidence is inconclusive to support one low-carbohydrate pattern over another. In a dose–response meta-analysis, a 200 g/d higher vegetable intake was associated with 16 % lower risk of future CAD( Reference Aune, Giovannucci and Boffetta 38 ). On the other hand, data from both the MESA and the European Prospective Investigation into Cancer and Nutrition-Netherlands cohort suggested that higher saturated fat from dairy products was associated with lower risk of CHD, while saturated fat from other sources were not associated with CHD( Reference de Oliveira Otto, Mozaffarian and Kromhout 39 , Reference Praagman, Beulens and Alssema 40 ). In summary, while animal-based LCD patterns and plant-based LCD patterns had similar associations with subclinical atherosclerosis, their roles in the cardiovascular system are complicated and unlikely identical.
The plant-based low-carbohydrate dietary patterns have some commonality with the Mediterranean diets and Dietary Approaches to Stop Hypertension (DASH), that is, two diets are associated with lower CVD risk( Reference Appel, Moore and Obarzanek 41 , Reference Estruch, Ros and Salas-Salvado 42 ). Similar to the Mediterranean diet patterns, the plant-based LCD emphasise choosing unsaturated fats. On the other hand, the Mediterranean diets tend to limit refined carbohydrates but promote consuming more whole grains products, fruits, vegetables and legumes; whereas the plant-based low-carbohydrate dietary patterns limit total carbohydrates. The DASH pattern emphasises consumption of low-fat dairy products, fruits, vegetables, nuts and whole grain products, while reduces Na intake, which may in turn reduce CVD risk by lowering blood pressure. Compared to DASH, the low carbohydrate principle tends to include more meat products but also eliminate some plant foods such as fruit and whole grains which are known to be beneficial to health.
The conclusions that can be drawn from this study are subject to some limitations. Dietary intake is not perfectly measured by FFQ. However, measurement error in the assessment of dietary intake, and resulting misclassification, should be non-differentially distributed as diet was assessed before the outcome (CAC) was measured in this prospective study. Although we adjusted for a variety of important confounders, residual confounding due to imperfect measurements, unmeasured confounders or unknown confounders cannot be entirely excluded in this observational study. A relatively short period of follow-up was available to examine development and progression of CAC. Although CAC is a strong predictor of CAD( Reference Folsom, Kronmal and Detrano 5 , Reference Yeboah, McClelland and Polonsky 8 ), the association between CAC amount and CAD risk may not be linear( Reference Pletcher, Tice and Pignone 43 , Reference Tanenbaum, Kondos and Veselik 44 ). Finally, the amount of carbohydrates consumed in this sample was largely moderate to high.
A variety of strengths lend confidence to our findings. The prospective design ensures a temporal relationship between exposure and outcome. The available sample size allowed for detection of a clinically important increase in the risk of CAC development or progression. Using an α level of 0·05 and two-tailed testing, we calculated power ranging from 82 to 99 % to detect effect sizes observed in the present study for the associations between LCD scores and CAC outcomes( Reference Castelloe 45 ). Moreover, the inclusion of four race-ethnic groups, sampled from a community-based population, increases the generalisability of our findings. Finally, the MESA study had rigorous, standardised data collection procedures and extensive quality control, though the FFQ had processing errors which were corrected by re-entering the data and making some imputations( Reference Bild, Bluemke and Burke 20 ).
In summary, evidence from this prospective cohort study emphasises some of the variation inherent in formulating LCD and suggests that low-carbohydrate dietary patterns are not associated with prevalence, incidence or progression of atherosclerosis as reflected by CAC. This conclusion is made within the range of consumption found in a large free-living cohort free of CVD at baseline.
Acknowledgements
The authors thank the other investigators, the staff and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
This research was supported by the National Heart, Lung, and Blood Institute (grant number: N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169); National Center for Research Resources (grant number: UL1-TR-000040 and UL1-TR-001079). The National Heart, Lung, and Blood Institute and National Center for Research Resources are part of National Institutes of Health of the USA. The contents herein are solely the responsibility of the authors and do not necessarily represent the official view of National Institutes of Health. The National Institutes of Health had no role in the design, analysis or writing of this article.
T. H. formulated the research question, designed the study, analysed the data and wrote the article; D. R. J., L. M. S. participated in data collection; L. M. S. had primary responsibility for final content; L. A. B., A. G. B. critically reviewed the manuscript; all authors reviewed and approved the manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518003513






