Non-technical Summary
A recently discovered fossil site in southern Mississippi, approximately 27–28 million years old, represents the first of this age from the Gulf Coastal Plain outside of Florida. The fossils, which include well-preserved plant material as well as reptiles, amphibians, fishes, land mammals, and marine mammals, were found in deltaic deposits near the base of the Catahoula Formation – a geologic unit broadly distributed across the Gulf Coastal Plain. Interestingly, the land mammals are represented primarily by species previously known almost exclusively from the Great Plains, the northern Rocky Mountains (Montana), and the John Day region of Oregon, with only a few apparently endemic to the Gulf Coast. They include a possum, a shrew, a squirrel, a “mountain beaver,” a small true beaver, and several other rodents, as well as weasel- and badger-like carnivores, a small primitive dog-like carnivore, a tapir, a “hippo-like” species, a small horse, a rhinoceros, and a giant wild-boar-like omnivore. Marine mammals are represented by a small “sea-cow” and a small dolphin-like whale. The close association of the land mammals from this site in Mississippi with those from so much farther north and west provides important new information on the geographic ranges of these animals across North America at this time, because by about 23 million years ago, the mammals of the Midcontinent and those of the Coastal Plain were much different. This, in large part, likely reflects the changing climate of the interior of the continent, which was becoming more arid, relative to that along the southern and southeastern coasts, which remained subtropical coastal forests.
Introduction
Fossil mammal assemblages representative of the Arikareean North American Land Mammal Age (ca. 18–30 Ma) are known primarily from the Great Plains, where this age was typified (Wood et al., Reference Wood, Chaney, Clark, Colbert, Jepsen, Reeside and Stock1941; Tedford et al., Reference Tedford, Skinner, Fields, Rensberger, Whistler, Galusha, Taylor, Macdonald, Webb and Woodburne1987, Reference Tedford, Swinehart, Swisher, Prothero, King, Tierney, Prothero and Emry1996, Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004), but also from the John Day Formation, Oregon (e.g., Fremd et al., Reference Fremd, Bestland and Retallack1994; Hunt and Stepleton, Reference Hunt and Stepleton2004; Albright et al., Reference Albright, Woodburne, Fremd, Swisher, MacFadden and Scott2008), and the Renova Formation, Montana (Rasmussen, Reference Rasmussen1977; Calede, Reference Calede2016, Reference Calede2020). Figure 1 shows the general location of various sites in these areas, which are mentioned often throughout the text. There are also, however, a few rare and isolated sites known from the Gulf Coastal Plain. Notable among these, also shown in Figure 1, are the White Springs, Cowhouse Slough, SB-1A, Brooksville 2, and Buda local faunas of Florida, and the Toledo Bend Fauna of easternmost Texas (Patton, Reference Patton1969; Frailey, Reference Frailey1979; Morgan, Reference Morgan and Morgan1989, Reference Morgan1993, Reference Morgan1994; Albright, Reference Albright1991, Reference Albright1994, Reference Albright1996, Reference Albright, Terry, LaGarry and Hunt1998a, Reference Albrightb, Reference Albright1999; Hayes, Reference Hayes2000; MacFadden and Morgan, Reference MacFadden and Morgan2003; Morgan et al., Reference Morgan, Czaplewski and Simmons2019). Additionally germane to this discussion is the fauna from Florida’s I-75 locality. Originally considered Whitneyan in age by Patton (Reference Patton1969), then later placed near the Whitneyan/Arikareean boundary by Hulbert (Reference Hulbert2001), more recent analysis estimates its age as late Whitneyan (Morgan et al., Reference Morgan, Czaplewski and Simmons2019).

Figure 1. (1) Index map of the contiguous USA, plus Saskatchewan, Canada, showing the general location of Arikareean-aged sites across the Great Plains and western U.S. mentioned throughout the text. Saskatchewan: Kealey Springs LF; Montana: faunas from the Cabbage Patch beds (Renova Formation), including the Tavenner Ranch LF; South Dakota: Wounded Knee-Sharps and Blue Ash (late Whitneyan) faunas; Nebraska: faunas from Ridgeview, Wagner, Agate, and Pine Ridge sites; California: Kew Quarry LF; and Oregon: faunas from the John Day Formation. (2) Sites of Arikareean age in the Gulf Coast region plus Florida (numbered); star denotes Jones Branch locality.
Several taxa from these localities are apparently endemic to the Gulf Coastal Plain region and support the view that it represented a unique biogeographic province separate from the central and northern Great Plains region during the late Oligocene–Early Miocene (Quinn, Reference Quinn1955; Wilson, Reference Wilson1956; Patton, Reference Patton1969; Webb, Reference Webb1977; Frailey, Reference Frailey1979; Albright, Reference Albright, Terry, LaGarry and Hunt1998a; Hayes, Reference Hayes2000; MacFadden and Morgan, Reference MacFadden and Morgan2003). On the other hand, the presence of numerous additional taxa that were originally described from the more northern localities indicates that a faunal link between that region and the Gulf Coastal Plain was never entirely severed (Albright, Reference Albright, Terry, LaGarry and Hunt1998a). Such taxa include, at the generic level, Miohippus, Diceratherium, Moropus, Nexuotapirus, Daphoenodon, Daeodon, and Arretotherium, all of which are members of the late Arikareean Toledo Bend Fauna. The known ranges of these taxa as determined through geochronological and chronostratigraphic techniques (radioisotopic dating of volcanic horizons and magnetostratigraphy) at their Great Plains and John Day localities provide the primary means of dating Arikareean sites in the Gulf Coastal Plain (and Florida) where high-resolution temporal control is, in most cases, lacking (see discussions in Tedford and Hunter, Reference Tedford and Hunter1984; Albright, Reference Albright, Terry, LaGarry and Hunt1998a).
This report adds several more taxa to that list, but from even farther back in time—the early Arikareean (Ar1 and Ar2). These additions are from an important new locality in the Gulf Coastal Plain discovered by avocational fossil collectors (A. Weller and R. Rains of Waynesboro, Mississippi) yielding an assemblage of taxa that constitutes the Jones Branch Local Fauna (LF) (Albright et al., Reference Albright, Phillips, Starnes, Stringer and Weller2016a, Reference Albright, Starnes and Phillipsb). As with those faunas noted above, the Jones Branch LF includes new species apparently endemic to the Gulf Coastal Plain, but also shares several taxa known previously from Midcontinental Arikareean (and pre-Arikareean) localities. These include Miohippus, Diceratherium, Protapirus, and Elomeryx, all of which lend further support for at least some level of faunal continuity between these two regions during this time. Interestingly, however, are several small mammals previously known only from the Midcontinent and Pacific Northwest such as Mesoscalops, Hesperopetes, Downsimus, Apeomys, Kirkomys, and Leptochoerus, that also indicate at least some level of faunal continuity between these two regions and the Gulf Coastal Plain during this time. The occurrence of these taxa in the Gulf Coastal Plain is somewhat surprising given the difference in paleoenvironments that existed during the Oligocene between this region and the more northern localities. Thus, the endemism noted above inferring that these regions represented different biogeographic provinces by the late Oligocene–Early Miocene was not yet as strongly apparent during the early to mid-Oligocene. This will be discussed further throughout the paper.
Sedimentological and stratigraphic work at the site has determined that the fossils are derived from a distributary channel lag at the base of the Catahoula Formation, which rests unconformably on interbedded marine marl/clay beds of the subjacent, Oligocene, Paynes Hammock Formation. An abundance of teleostean otoliths dominated by sciaenids (drums and croakers; Stringer, Reference Stringer2016), together with the muddy ‘shell-hash’ nature of the matrix from which the fossils are recovered (Fig. 2), which includes the bivalve Donax sp., provides strong indicators of a tidally influenced, estuarine paleoenvironmental setting—exactly that expected given the inferred coastal location of the Jones Branch site during the mid-Oligocene (Fig. 3). The remains of terrestrial and marine-adapted mammals, in addition to remains of reptiles, amphibians, fishes, and terrestrial plants, washed into this setting. Studies on the latter groups will be addressed elsewhere (e.g., Cicimurri et al., Reference Cicimurri, Ebersole, Stringer, Starnes and Phillips2025).

Figure 2. Image showing the ‘shell-hash’ lithology of the deposit from which fossils at the Jones Branch site were recovered. Note crocodilian osteoderm among oyster shells.

Figure 3. Inferred Oligocene shoreline for the southeastern United States based on mapped surface geology (Ebersole, Reference Ebersole2016).
Originally thought to be temporally equivalent with the Toledo Bend Fauna because of the presence of rhinoceros, tapir, and anthracothere material, which are common mammalian components at Toledo Bend (Albright, Reference Albright1999), more detailed study revealed that the representatives of these groups at Jones Branch are earlier occurring species and that few, if any, taxa are shared. Like the Toledo Bend Fauna, but in contrast to faunas inferred to be of similar age in Florida, the Jones Branch LF apparently lacks camels and oreodonts. However, a single lower molar of an oreodont was recently identified by LBA in the collection of material from Toledo Bend at the South Carolina State Museum. This specimen will be noted in more detail in another report. Also, out of hundreds of specimens from Toledo Bend, only two, a ramal fragment with m2–3 and an isolated m1, represent the occurrence of a camel there. The Jones Branch LF currently represents the only assemblage of early Arikareean age yet known from the Gulf Coastal Plain outside of Florida, and greatly adds to our understanding of this region during the Oligocene.
Geologic setting, stratigraphy, and age of the Jones Branch Local Fauna
The Catahoula Formation (mid-Oligocene–Upper Miocene in Mississippi) extends from Texas to Alabama with a maximum thickness exceeding 245 meters in central Louisiana (Dockery and Thompson, Reference Dockery and Thompson2016). Exposures are common throughout its outcrop belt, except where it is masked by younger units, such as extensive terraces and the alluvium of modern stream courses. In eastern Mississippi, it unconformably overlies the Paynes Hammock and Chickasawhay formations, and, in places, the Vicksburg Group stratigraphically below the Chickasawhay, and it underlies the Hattiesburg Formation. In this area, the Catahoula Formation is composed of unweathered gray-/green-colored, fissile clays interspersed with interbedded distributary channel and thick fine-grained to coarse graveliferous sands of an emergent delta with marginal marine, brackish water, and terrestrial influences.
At the Jones Branch locality, the basal clays of the siliciclastic sequence of the Catahoula Formation are interrupted by a series of thin syndepositional channel lenses along a narrow horizon. These fissile clays preserve an excellent macroflora of lignitized broadleaf and palmetto fossils and grade upward into massive, more uniform, non-fossiliferous freshwater clays. The fossil fauna, however, appears to be derived from a single horizon consisting of a tidally influenced, estuarine, muddy ‘shell-hash’ lag within one of these distributary channels that rests directly on the subjacent glauconitic, sandy clay marl, and soft limestones of the Paynes Hammock Formation. This phosphate nodule-bearing lag contains a rich assortment of fossil mollusks, plus marine and terrestrial vertebrates, and appears to be somewhat bimodal in distribution. The larger marine vertebrate and invertebrate fauna, such as the oysters and dugong ribs, show signs of abrasion from reworking, whereas, in contrast, the terrestrial vertebrate assemblage is nearly pristine and suggests less post-depositional reworking or transportation. Vertebrate fossils include numerous rodents, carnivores, perissodactyls, artiodactyls, sirenians, reptiles, amphibians, sharks, and teleostean fish remains.
At the site, the Paynes Hammock/Catahoula Formation boundary is marked by a thin, fine-grained, sandy, indurated ledge, with well-preserved primary structures of ripple marks on its upper surface interpreted as once belonging to a sandy tidal flat. Based on the mapping of the geology of Wayne County (May et al., Reference May, Baughman, McCarty, Glenn and Hall1974), the fossil horizon lies well below the last regional occurrence of the benthic foraminifera Heterostegina, which marks the uppermost beds of the Catahoula Formation in the shallow subsurface. Important with respect to determining the age of the Jones Branch LF, therefore, is the age of the top of the Paynes Hammock Formation, which is much more amenable to dating due to its deposition in a marine environment than is the fluvial and deltaic Catahoula Formation. Additionally, many of the mammals from the site are also biochronologically diagnostic and therefore provide data that further refine its age.
In Dockery and Thompson (Reference Dockery and Thompson2016, fig. 343; reproduced from Dockery, Reference Dockery1996, fig. 7), the Paynes Hammock Formation and the Chickasawhay Limestone are shown to be correlative with calcareous nannoplankton zone NP24 and with the Ciperoella ciperoensis (previously Globigerina; see Olsson et al., Reference Olsson, Hemleben, Coxall and Wade2018) and Globorotalia [=Paragloborotalia] opima planktonic foraminiferal zones, respectively (also see Siesser, Reference Siesser1983). These are the zones to which Poag (Reference Poag1966, p. 399; 1972) correlated the two formations, which he considered “a single biostratigraphic unit” based on “the apparent absence of a distinct faunal change” between them (i.e., C. ciperoensis was found in both units). More recent work since these earlier publications (e.g., Poag, Reference Poag1966, Reference Poag1972; Dockery, Reference Dockery1996) has refined the boundaries and bounding ages of Oligocene calcareous nannofossil and planktonic foraminiferal zones (Berggren and Pearson, Reference Berggren and Pearson2005; Wade et al., Reference Wade, Pearson, Berggren and Pälike2011; Agnini et al., Reference Agnini, Fornaciari, Raffi, Catanzariti, Pälike, Backman and Rio2014; Olsson et al., Reference Olsson, Hemleben, Coxall and Wade2018; and especially Coccioni et al., Reference Coccioni, Montanari, Bice, Brinkhuis and Deino2018). For example, as seen in Figure 4, the Ciperoella ciperoensis Partial-Range Zone (PRZ), equivalent to Oligocene Planktonic Foraminifera Zone O6, correlates with the lower part of NP25 (Chattian), the latter of which has a basal age of 26.8 Ma (from Gradstein et al., Reference Gradstein, Ogg, Schmitz and Ogg2012, tables A3.1, A3.4; Agnini et al., Reference Agnini, Fornaciari, Raffi, Catanzariti, Pälike, Backman and Rio2014; Coccioni et al., Reference Coccioni, Montanari, Bice, Brinkhuis and Deino2018; Speijer et al., Reference Speijer, Pälike, Hollis, Hooker, Ogg, Gradstein, Ogg, Schmitz and Ogg2020). It is important to note, however, that C. ciperoensis ranges from about mid-Rupelian to early Aquitanian (Zone O3 to within Zone M1a, Olsson et al., Reference Olsson, Hemleben, Coxall and Wade2018). Similarly, the Paragloborotalia opima Highest-Occurrence Zone (HOZ), equivalent to Zone O5 with a basal age of 27.4 Ma, correlates with the upper part of NP24 (lower Chattian), although the total range of this taxon is from early Rupelian to early Chattian (about 32.3 to 26.9 Ma; Coccioni et al., Reference Coccioni, Montanari, Bice, Brinkhuis and Deino2018).

Figure 4. Biochronologic range of selected mammalian taxa recovered from the Jones Branch site. Data mainly from Tedford et al. (Reference Tedford, Swinehart, Swisher, Prothero, King, Tierney, Prothero and Emry1996, Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004) and various chapters in Janis et al. (Reference Janis, Scott and Jacobs1998, Reference Janis, Gunnell and Uhen2008). The Jones Branch horizon (star) is within a distributary channel lag of the Catahoula Formation that rests immediately above the Paynes Hammock Formation. The basic stratigraphy from Dockery and Thompson (Reference Dockery and Thompson2016, fig. 343) is correlated to NALMAs, planktonic foraminifer zones, calcareous nannoplankton zones, and to the GPTS based on the most recent interpretations of those zones as follows: GPTS base from GTS2020 (Speijer et al., Reference Speijer, Pälike, Hollis, Hooker, Ogg, Gradstein, Ogg, Schmitz and Ogg2020); planktonic foraminifer zones (but not boundary dates) from Wade et al. (Reference Wade, Pearson, Berggren and Pälike2011); boundary dates for planktonic foraminifer and calcareous nannoplankton zones from Agnini et al. (Reference Agnini, Fornaciari, Raffi, Catanzariti, Pälike, Backman and Rio2014), Coccioni et al. (Reference Coccioni, Montanari, Bice, Brinkhuis and Deino2018), and GTS2020; boundary dates for the Arikareean from Albright et al. (Reference Albright, Woodburne, Fremd, Swisher, MacFadden and Scott2008); base of Chickasawhay and Bucatunna formations from magnetostratigraphy of Miller et al. (Reference Miller, Thompson and Kent1993). Ar1 = early early Arikareean; Ar2 = late early Arikareean; Ar3 = early late Arikareean; Ar4 = late late Arikareean; Wt, Whitneyan; Or, Orellan. Dotted line represents the absence of Sinclairella beyond Ar1 with the exception of its presence in Florida’s Ar3 Buda LF.
Poag (Reference Poag1972, text-fig. 3) noted the presence of the following biochronologically important foraminifera in the Paynes Hammock Formation: Ciperoella ciperoensis, Ciperoella angulisuturalis, and Chiloguembelina cubensis. The first two were also found in the underlying Chickasawhay Limestone. As noted above, the range of C. ciperoensis spans most of the Oligocene into the lowest Miocene, but the latter two taxa constrain the age of the Paynes Hammock/Chickasawhay formations to between 29 Ma and 27.41 Ma (i.e., the LO of C. angulisuturalis and HCO of Chiloguembelina cubensis, respectively = Oligocene Planktonic Foraminifera Zone O4 of Wade et al., Reference Wade, Pearson, Berggren and Pälike2011; also see GTS2012, Appendix 3; Coccioni et al., Reference Coccioni, Montanari, Bice, Brinkhuis and Deino2018; Olsson et al., Reference Olsson, Hemleben, Coxall and Wade2018). Although C. cubensis was not recorded from Poag’s section PH2 (Reference Poag1966, text-fig. 7), he did note its occurrence in the lowest level of section S19 and in a lower and upper level of section CX; C. angulisuturalis was noted from only the lower part of section S19. With a 91% faunal resemblance across sections PH2, S19, and CX, Poag (Reference Poag1966) surmised that the differences may have been due to deposition of each of these sections in somewhat different environments.
Coccioni et al. (Reference Coccioni, Montanari, Bice, Brinkhuis and Deino2018) provided two solutions for the Rupelian/Chattian boundary, which they placed at the HCO of Chiloguembelina cubensis and within the lower part of magnetochron C9n in the Monte Cagnaro, Italy, location chosen for the base Chattian GSSP, those dates being 27.82 Ma and 27.41 Ma. In the most recent time scale, GTS2020, 27.82 Ma falls below chron C9n, within C9r; hence the use herein of 27.41 Ma, which falls within C9n (Fig. 4). Although these revisions preclude earlier correlations of the Paynes Hammock/Chickasawhay formations to the Ciperoella ciperoensis PRZ and Paragloborotalia opima HOZ planktonic foraminiferal zones, the new correlations are still consistent with the calcareous nannoplankton zone NP24 correlation of Siesser (Reference Siesser1983) and Dockery (Reference Dockery1996), the planktonic foraminifera zone P21 correlation of Poag (Reference Poag1972), and the P20–P21a correlation of Miller et al. (Reference Miller, Thompson and Kent1993). These data, including the placement of the HCO of Chiloguembelina cubensis at 27.41 Ma, provide a revised correlation of the Paynes Hammock/Chickasawhay formations with the Ciperoella angulisuturalis/Chiloguembelina cubensis Concurrent Range Zone of late Rupelian age (Wade et al., Reference Wade, Pearson, Berggren and Pälike2011), in turn inferring that the top of the Paynes Hammock Formation is essentially coincident with the Rupelian/Chattian boundary.
Magnetostratigraphic analysis of the Paynes Hammock and Chickasawhay formations further supports this correlation. Miller et al. (Reference Miller, Thompson and Kent1993) determined that the Paynes Hammock Formation possibly spanned chrons C10r through C10n and that the underlying Chickasawhay Limestone ranged from uppermost C11r at its base (30 Ma, Vandenberghe et al., Reference Vandenberghe, Hilgen, Speijer, Gradstein, Ogg, Schmitz and Ogg2012, table 28.3; 29.97 Ma, Speijer et al., Reference Speijer, Pälike, Hollis, Hooker, Ogg, Gradstein, Ogg, Schmitz and Ogg2020, table 28.1) through C10r. These correlations also indicate a late Rupelian age for the Paynes Hammock and Chickasawhay formations (Fig. 4). Additionally, Miller et al. (Reference Miller, Thompson and Kent1993, table 1) reported several 87Sr/86Sr isotope values that further support this age assignment. Values that range from 0.707927 to 0.708075 for the Chickasawhay Limestone (plus a value of 0.707788 by Denison et al., Reference Denison, Koepnick, Fletcher, Dahl and Baker1993) fall within the Rupelian section of the 87Sr/86Sr curve of MacArthur et al. (Reference MacArthur, Howarth, Shields, Zhou, Gradstein, Ogg, Schmitz and Ogg2020, fig. 7.2, LOESS 6 Look-up table). Dockery and Thompson (Reference Dockery and Thompson2016) also noted that Denison et al. (Reference Denison, Koepnick, Fletcher, Dahl and Baker1993) reported 87Sr/86Sr isotope values for the Paynes Hammock Formation of 0.707865 derived from samples of Ostrea blandpiedi Howe, Reference Howe1937, also indicative of the Rupelian (McArthur et al., Reference MacArthur, Howarth and Bailey2001, Reference MacArthur, Howarth, Shields, Zhou, Gradstein, Ogg, Schmitz and Ogg2020). These values are consistent with Coccioni et al.’s (Reference Coccioni, Montanari, Bice, Brinkhuis and Deino2018) reported value of 0.708105 ± 0.00001 for the base of the Chattian (0.708040 in MacArthur et al., Reference MacArthur, Howarth, Shields, Zhou, Gradstein, Ogg, Schmitz and Ogg2020, LOESS 6 Look-up table).
To the extent that the top of the Paynes Hammock Formation must be very near the Rupelian/Chattian boundary (27.41 Ma, solution 2 of Coccioni et al., Reference Coccioni, Montanari, Bice, Brinkhuis and Deino2018), the stratigraphic level of the Jones Branch site near the base of the overlying Catahoula Formation suggests a very earliest Chattian age, especially in consideration of the global regression noted to have occurred at that boundary (Van Simaeys et al., Reference Van Simaeys, De Man, Vandenberghe, Brinkhuis and Steurbaut2004), which may be responsible for the unconformity between the Paynes Hammock and Catahoula formations. Albright et al.’s (Reference Albright, Woodburne, Fremd, Swisher, MacFadden and Scott2008, p. 230) biostratigraphic work in the John Day Formation, Oregon, resulted in an estimated age of “about 28 Ma” for the Ar1–Ar2 boundary, as did the more recent work by Calede (Reference Calede2020) in the Cabbage Patch beds in the northern Rocky Mountains, Montana (ca. 28.1 Ma). This, together with all the data noted above, supports an estimated age for the Jones Branch LF very near the Rupelian/Chattian boundary and within the early Arikareean. The mammals described and discussed in detail below will bear substantially on, and help refine, the age of the Jones Branch LF site.
Materials and methods
Fossils were recovered from a site near the town of Waynesboro, Mississippi, within a small tributary of Jones Branch, which in turn is a tributary of the Chickasawhay River. The fossil-bearing horizon extends for approximately 10–15 meters along the creek bottom, and specimens were recovered primarily by shoveling matrix into screens that were simultaneously washed in the creek and sorted. Small specimens, such as rodent teeth, shark teeth, snake vertebrae, and otoliths, were recovered by sieving matrix through fine-mesh screens (see Cicimurri et al., Reference Cicimurri, Ebersole, Stringer, Starnes and Phillips2025, for further details). Most of the specimens reported herein are curated at the Mississippi Museum of Natural Science, although a few are in the collections of the South Carolina State Museum. Detailed information regarding the location of the site can be found at those institutions.
Geochronology/chronostratigraphy
The version of the global Geomagnetic Polarity Time Scale (GPTS) used for the temporal framework in this contribution is the Paleogene portion of both GTS2012 (Vandenberghe et al., Reference Vandenberghe, Hilgen, Speijer, Gradstein, Ogg, Schmitz and Ogg2012) and GTS2020 (Speijer et al., Reference Speijer, Pälike, Hollis, Hooker, Ogg, Gradstein, Ogg, Schmitz and Ogg2020), as well as the work of Coccioni et al. (Reference Coccioni, Montanari, Bice, Brinkhuis and Deino2018). The terms “Fauna” and “Local Fauna” (LF) follow Tedford (Reference Tedford1970) and definitions in Woodburne (Reference Woodburne1987, Reference Woodburne2004). The Ar1 (early early), Ar2 (late early), Ar3 (early late), and Ar4 (late late) divisions of the Arikareean North American Land Mammal Age (NALMA) follow Tedford et al. (Reference Tedford, Skinner, Fields, Rensberger, Whistler, Galusha, Taylor, Macdonald, Webb and Woodburne1987, Reference Tedford, Swinehart, Swisher, Prothero, King, Tierney, Prothero and Emry1996, Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004), with revised temporal boundaries of these divisions following Albright et al. (Reference Albright, Woodburne, Fremd, Swisher, MacFadden and Scott2008). Bioevent terminology follows definitions in Woodburne (Reference Woodburne2004), Wade et al. (Reference Wade, Pearson, Berggren and Pälike2011), and Bergen et al. (Reference Bergen, Truax, Kaenel, Blair, Browning, Lundquist, Boesiger, Bolivar and Clark2019): first appearance datum (FAD); last appearance datum (LAD); highest common stratigraphic occurrence (HCO); highest stratigraphic occurrence (HO); highest occurrence zone (HOZ); lowest stratigraphic occurrence (LO); Concurrent Range Zone (CRZ); partial range zone (PRZ); mega-annum (million years), a radioisotopically calibrated numerical age (Ma); millions of years, elapsed time or duration (Myr); thousands of years, elapsed time or duration (Kyr).
Anatomical abbreviations
AP and TR, antero-posterior (length) and transverse (width) measurements, respectively; P or M, upper premolars and molars, respectively; p or m, lower premolars and molars, respectively. Measurements were taken with a Kobalt electronic caliper with digital display (to the nearest 0.01 mm) or using a reticule in the eyepiece of a microscope.
Repositories and institutional abbreviations
Amherst College Museum (ACM), Amherst, Massachusetts; American Museum of Natural History (AMNH), New York; Carnegie Museum of Natural History (CM), Pittsburgh, Pennsylvania; College of Charleston Natural History Museum (CCNHM), also known as the Mace Brown Museum of Natural History, South Carolina; Frick: American Mammals Collection (F:AM) at the AMNH; Florida Museum of Natural History (FLMNH), University of Florida, Gainesville; Field Museum of Natural History (FM), Chicago, Illinois; John Day Fossil Beds National Monument (JODA), Kimberly, Oregon; Natural History Museum of Los Angeles County (LACM), California; Mississippi Museum of Natural Science (MMNS), Jackson; South Carolina State Museum (SCSM), Columbia (note: only “SC” is used as the prefix in catalogue numbers for specimens at the SCSM); University of Florida/Florida Geological Survey (UF/FGS), Gainesville; University of Nebraska State Museum (UNSM), Lincoln; United States Geological Survey (USGS); Yale Peabody Museum, Princeton University Collection (YPM PU), New Haven, Connecticut.
Systematic paleontology
Mammalia Linnaeus, Reference Linnaeus1758
Metatheria Huxley, Reference Huxley1880
Herpetotheriidae Trouessart, Reference Trouessart1879
Herpetotherium Cope, Reference Cope1873
Type species
Herpetotherium fugax (Cope, Reference Cope1873).
Herpetotherium sp.

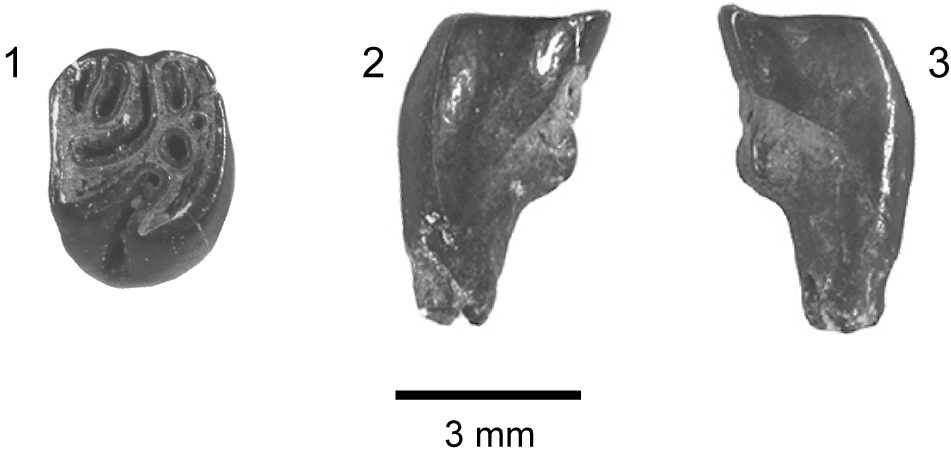
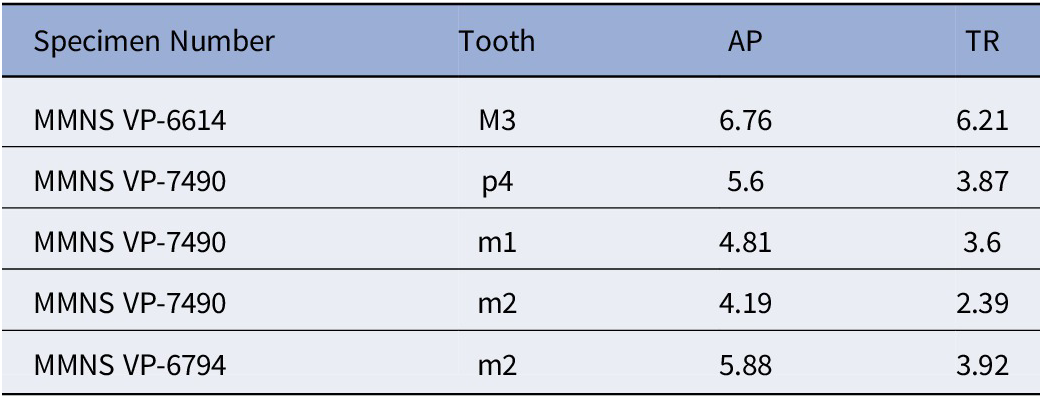
Figure 5. Marsupials, apatemyids, talpoids, lagomorphs, sciurids, aplodontiids, and Eutypomys from the Jones Branch LF, lower Catahoula Formation, Mississippi: (1) Herpetotherium sp., MMNS VP-11645, right m2; (2) ?Sinclairella sp., MMNS VP-6629, left ?dP4; (3) Mesoscalops irwini n. sp., MMNS VP-8239, left m2, (holotype); (4–6) Oligolagus welleri n. gen. n. sp., MMNS VP-6604, left ramal fragment with p4–m1, (holotype); (4) labial view, (5) lingual view, (6) occlusal view; (7) ?Hesperopetes sp., MMNS VP-7715, left m2; (8) Downsimus rainsi n. sp., MMNS VP-7334, right m2, (holotype); (9–18) Eutypomys sp., (9) MMNS VP-7481, left maxilla fragment with M1, (10) MMNS VP-8632, left dP4, (11) MMNS VP-8326, right P3, (12) MMNS VP-8705, right P3, (13) MMNS VP-6943, right P4, (14) MMNS VP-7782, left M1 or M2, (15) MMNS VP-6584, left p4, (16) MMNS VP-7482, left m1, (17) MMNS VP-6945, right m2, (18) SC2013.28.1, left m3.
Referred specimen
MMNS VP-11645, right m2.
Description
MMNS VP-11645, an m2, measures 1.5 mm AP × 1.0 mm TR. The trigonid is higher and slightly narrower transversely than the talonid. The paraconid is broken and missing, but an anterior cingulid is apparent and it extends to the labial surface of the protoconid. The metaconid is worn. The protocristid extends nearly transversely across the crown. The twinned entoconid and hypoconulid are separated by a distinct sulcus, and the somewhat anteroposteriorly compressed entoconid is connected to the posterior surface of the metaconid via an entocristid. A posterior cingulid extends “from the hypoconulid along the posterior wall of the talonid to the base of the crown at the posterobuccal corner of the hypoconid” just as described for lower molars of Herpetotherium by Korth (Reference Korth1994, p. 380). There is no lingual cingulid.
Remarks
Herpetotherium is an extremely long-ranging genus spanning over 30 million years and at least eight NALMAs, from the Uintan through the Barstovian (Korth and Cavin, Reference Korth and Cavin2016). Based on measurements of m2 length, Korth (Reference Korth1994, fig. 2) and Korth and Cavin (Reference Korth and Cavin2016, fig. 5) showed that (1) the Chadronian-aged species, H. marsupium (Troxell, Reference Troxell1923a) and H. valens (Lambe, Reference Lambe1908), were the largest; (2) the late Chadronian specimens of the type species, H. fugax, averaged the smallest; and (3) size somewhat stabilized with a slightly larger m2 in the Orellan through early Arikareean H. fugax (the Arikareean occurrence of the latter was reported by Hayes [Reference Hayes2005, Reference Hayes2007] from Nebraska and Florida) and in another Arikareean species, H. youngi (McGrew, Reference McGrew1937). The later Arikareean Herpetotherium merriami (Stock and Furlong, Reference Stock and Furlong1922) was distinguished from H. youngi and H. fugax by its larger size. The tiny tooth from Jones Branch is similar in size to the small late Chadronian specimens of H. fugax.
Identification of the Jones Branch species is somewhat equivocal because differentiation depends largely on the arrangement, size, and presence/absence of the stylar cusps on the upper molars (e.g., Korth and Cavin, Reference Korth and Cavin2016)—elements not yet recovered from Jones Branch. The Jones Branch tooth is only slightly smaller than m2s from the mid-Arikareean age Brooksville 2 LF (1.56–1.81 mm, n = 3), which, based on the variable stylar cusp morphologies, Hayes (Reference Hayes2005) referred to H. fugax. Herpetotherium sp. cf. H. merriami from Florida’s I-75 LF is much larger than the species from Jones Branch based on measurements in Hayes (Reference Hayes2005). Until upper molars are recovered from Jones Branch, which may shed light on which species is represented, referral beyond Herpetotherium sp. is deferred.
Placentalia Owen, Reference Owen1837
Apatotheria Scott and Jepsen, Reference Scott and Jepsen1936
Apatemyidae Matthew, Reference Matthew1909
Sinclairella Jepsen, Reference Jepsen1934
Referred specimen
MMNS VP-6629, left ?dP4.
Description
MMNS VP-6629 is a small, odd, triangularly shaped tooth, measuring 3.43 mm AP × 2.80 mm TR. Its cap-like morphology, absence of roots, and very slight degree of wear on the principal cusps suggest that it may be deciduous. It has a large, prominent parastyle occupying the anterolabial corner. Between the parastyle and paracone is a weak cingulum. The paracone and metacone are similar in size, larger than the parastyle, slightly transversely compressed, connected by a centrocrista, with a small, shelf-like labial cingulum between them; the paracone is taller than the metacone. The protocone is larger than the paracone and metacone, but not as tall. The peak of the protocone lies directly lingual to the paracone (i.e., it is in line with the paracone transversely). However, rather than cusp-like, the protocone is transversely compressed, forming more of a ridge than a cusp due to a posterolingual extension from its peak that ends at the posterolingual point of the tooth (Fig. 5.2). An anterior cingulum extends from the parastyle along this entire ridge to the posterolingual point of the tooth. Between the paracone and protocone, and anterior to the peaks of those cusps, is a prominent, distinctly isolated, paraconule. There is also a slightly smaller, but similarly prominent metaconule anterolingual to the metacone. The posterior cingulum is broad and shelf-like.
MMNS VP-6629 resembles the M1 of Sinclairella dakotensis Jepsen, Reference Jepsen1934, and S. simplicidens Czaplewski and Morgan, Reference Czaplewski and Morgan2015, in size, in the presence of a prominent parastyle, and in the connection of the paracone and metacone by a centrocrista; but it differs in several other aspects. First, the M1s of Sinclairella are more quadrangular than triangular primarily due to the presence of a hypocone, which is absent in the Jones Branch tooth (see Czaplewski and Morgan, Reference Czaplewski and Morgan2015, fig. 3B). Next, the Jones Branch tooth has a particularly prominent paraconule and metaconule; the M1 of Sinclairella has only a very weak paraconule and no metaconule. And finally, the Jones Branch tooth has a transversely compressed, ridge-like protocone, rather than the typical cusp-like morphology on the M1 of Sinclairella. The tooth’s distinctly triangular shape resembles the M1 of the Uintan-aged Apatemys uintensis (Matthew, Reference Matthew1921) based on a comparison with Matthew (Reference Matthew1921, fig. 2) and Czaplewski and Morgan (Reference Czaplewski and Morgan2015, fig. 4C, redrawn from Matthew, Reference Matthew1921, fig. 2), but it too appears (based on Matthew, Reference Matthew1921, fig. 2) to lack the prominent paraconule and metaconule present in MMNS VP-6629. The tooth in no way resembles the non-molariform, transversely compressed P4 of Sinclairella illustrated by Jepsen (Reference Jepsen1934, pl. 1, fig. 2), Clemens (Reference Clemens1964, fig. 1), and Tornow and Arbor (Reference Tornow and Arbor2017, fig. 6).
Remarks
Apatemyids constitute a unique family of small, rare, purportedly arboreal insectivorous mammals from the Paleogene of Europe and North America thought to have occupied a niche similar to that of the extant lemuroid primate Daubentonia, the aye-aye of Madagascar (e.g., Silcox et al., Reference Silcox, Bloch, Boyer and Houde2010; Czaplewski and Morgan, Reference Czaplewski and Morgan2015). Ranging from the early Tiffanian to early Arikareean in North America, the only member of the family known to have extended into the early Arikareean is Sinclairella. Sinclairella dakotensis is known from the Duchesnean through early early Arikareean of western North America, including Oregon (West, Reference West1973; Cavin and Samuels, Reference Cavin and Samuels2012; Samuels, Reference Samuels2021). A second species, S. simplicidens, was described by Czaplewski and Morgan (Reference Czaplewski and Morgan2015, p. 1) from Florida’s early late Arikareean (Ar3) Buda LF, who concluded that “this late occurrence probably represents a retreat of this subtropically adapted family into the Gulf Coastal Plain subtropical province at the end of the Paleogene and perhaps the end of the apatemyid lineage in North America.”
To the extent that deciduous teeth are unknown for Sinclairella, it still seems reasonable that MMNS VP-6629 is a dP4, given its size and general similarity to M1s of that taxon (and Apatemys uintensis). However, it is equally important to note that the presence on this tooth of such a prominent paraconule and metaconule is anomalous, thus suggesting that the specimen might represent a heretofore unknown, early Arikareean, Gulf Coastal Plain endemic apatemyid; hence the “?” in the taxonomic heading (see Kornicker, Reference Kornicker1979). Although it is possible that the Jones Branch specimen belongs to one of the two species of Sinclairella noted above, only the recovery of additional specimens amenable to such a diagnosis (upper molars of the Jones Branch species or a dP4 of the two known species) can confirm this. Regardless of its identity, the Jones Branch specimen provides only the second Oligocene record of an apatemyid beyond the Great Plains and Oregon, and east of the Mississippi River, and further supports the conclusion reached by Czaplewski and Morgan (Reference Czaplewski and Morgan2015) regarding a late Paleogene Gulf Coastal Plain refugium for this taxon. Figure 4 shows the absence of any record of Sinclairella between Ar1 levels in the Great Plains and Oregon and the later Ar3 occurrence in Florida.
Eulipotyphla Waddell et al., Reference Waddell, Cao, Hauf and Hasegawa1999
Talpoidea Fischer von Waldheim, Reference Fischer von Waldheim1817
Proscalopidae Reed, Reference Reed1961
Mesoscalops Reed, Reference Reed1960
Holotype
MMNS VP-8239, left m2.
Diagnosis
Narrow, V-shaped trigonid and talonid, with talonid slightly wider anteroposteriorly; differs from Proscalops in absence of labial cingulid, absence of accessory cupids at lingual termination of anterior and posterior cingula, absence of lingual invagination of talonid, and smaller size; resembles Mesoscalops in narrow, V-shaped trigonid and talonid, in absence of labial cingulid, absence of accessory cupids at lingual termination of anterior and posterior cingula, and absence of lingual invagination of talonid (= presence of entocristid and metacristid); differs from Mesoscalops scopelotemos K. Reed, Reference Reed1960, and M. montanensis Barnosky, Reference Barnosky1981, in much smaller size (measurements below) and distinct difference in height between trigonid and talonid (trigonid about twice the height of talonid).
Description
Dental terminology follows Barnosky (Reference Barnosky1981, fig. 6). This tiny m2 measures 1.8 mm AP × 1.44 mm TR at the trigonid. Respective measurements for 25 m2s of M. scopelotemos are 2.45–3.05 mm AP × 1.65–2.25 mm TR (K. Reed, Reference Reed1960, table 1) and for one m2 of M. montanensis is 2.8 mm AP × 1.9 mm TR (Barnosky, Reference Barnosky1981). The trigonid is strongly V-shaped and very narrow anteroposteriorly. The talonid is similarly sharply V-shaped, just slightly broader anteroposteriorly than the trigonid, and only half its height. The trigonid and talonid are entirely separate (i.e., the anterior surface of the talonid [the cristid obliqua] does not abut the posterior surface of the trigonid [the protocristid]—it extends all the way lingually, terminating in a metastylid). The paraconid and metaconid are of similar size and there is only a slight, shallow invagination between them; this may be a function of wear. The metaconid and metastylid are worn, but the metastylid is still distinguishable as a separate cusp. The entoconid is the most robust of the lingual cusps, and there is no lingual invagination between it and the metastylid (i.e., the talonid shows no lingual invagination because of the presence of an entocristid and metacristid). When viewed from the posterior aspect, both the trigonid and talonid have been worn into a transversely concave morphology. There is no labial or lingual cingulid, but the anterior and posterior cingula, although not shelf-like, extend along the entire base of the crown. They do not terminate lingually into distinct accessory cuspids. Instead, the lingual termination of both cingula appears as simple, subtle swellings—the posterior swelling (not a true entostylid) slightly more prominent than the anterior.
Etymology
Named for Mr. Kelly J. Irwin, herpetologist with the Arkansas Game and Fish Commission, who found MMNS VP-8239 while sorting screen-washed matrix from the Jones Branch site.
Remarks
The Proscalopidae are mole-like eulipotyphlans that were originally considered endemic to the North American northern Great Plains (Barnosky, Reference Barnosky1981) until Geisler (Reference Geisler2004) described four humeri of Oligoscalops from Mongolia. The Jones Branch specimen, therefore, represents the first and only Paleogene talpoid in North America beyond the northern Great Plains, northern Rocky Mountains, and Oregon. Like the true moles (Talpidae), the Proscalopidae have highly specialized adaptations for burrowing (Barnosky, Reference Barnosky1982).
According to Barnosky (Reference Barnosky1981), the Proscalopidae are represented by four genera: the Chadronian Cryptoryctes C. Reed, Reference Reed1954; the Chadronian and Orellan Oligoscalops K. Reed, Reference Reed1961; the Whitneyan and mainly Arikareean Proscalops Matthew, Reference Matthew1901; and the Hemingfordian to early Barstovian Mesoscalops K. Reed, Reference Reed1960. Gunnell et al. (Reference Gunnell, Bown, Hutchison, Bloch, Janis, Gunnell and Uhen2008), however, considered Cryptoryctes a micropternodontid soricomorph—an insectivore group entirely unrelated to Talpoidea. Based on measurements in Gunnell et al. (Reference Gunnell, Bown, Hutchison, Bloch, Janis, Gunnell and Uhen2008), the average length of m2 increases in size, respectively, for Oligoscalops (approx. 2 mm), Proscalops (2.8 mm), and Mesoscalops (approx. 3 mm). The Jones Branch tooth is smaller than any of them (1.8 mm).
Mesoscalops and Proscalops resemble the Jones Branch species in having narrow trigonids and talonids (Macdonald, Reference Macdonald1963; Barnosky, Reference Barnosky1981, Reference Barnosky1982). However, Proscalops differs from Mesoscalops and the Jones Branch species in several traits. First, Mesoscalops and the Jones Branch species lack the deep lingual invagination, particularly on the talonid, seen so prominently in species of Proscalops (e.g., Reed, Reference Reed1961, pl. 1, fig. 4; Barnosky, Reference Barnosky1982, pl. 1, fig. 5) due to the absence of an entocristid and metacristid in Proscalops. The morphology of the anterior and posterior cingula also differs. In Proscalops tertius K. Reed, Reference Reed1961, the anterior and posterior cingula are shelf-like, and both terminate lingually into prominent anterior and posterior accessory cuspids (Hutchison, Reference Hutchison1968, fig. 6). In Proscalops evelynae (Macdonald, Reference Macdonald1963) (based on specimens examined at the UNSM, e.g., UNSM 20500) the anterior cingulid starts about half-way along the anterior surface of the tooth and continues around the anterolingual corner (around the paraconid), and the posterior cingulid is shelf-like. In Mesoscalops and the Jones Branch tooth, the anterior and posterior cingula extend along the entire base of the crown, they do not wrap around onto the lingual surface, they are not shelf-like, and they do not terminate lingually into prominent accessory cuspids.
Mesoscalops irwini n. sp. is similar in size to the mainly Hemingfordian to Barstovian talpid Mystipterus Hall, Reference Hall1930. But the latter has a prominent labial cingulid, the trigonid and talonid are wider anteroposteriorly than in the Jones Branch species, and they are not entirely separate (i.e., the cristid obliqua [or entocristid] abuts the posterior surface of the trigonid [the protocristid], whereas in Mesoscalops irwini n. sp., it extends all the way lingually, terminating in a metastylid) (see Hutchison, Reference Hutchison1968, fig. 20; Rasmussen, Reference Rasmussen1977). Another talpid, Mioscalops Ostrander et al., Reference Ostrander, Mebrate and Wilson1960 (previously Scalopoides Wilson, Reference Wilson1960, but see Korth and Evander, Reference Korth and Evander2016) resembles the Jones Branch species in having a cristid obliqua that extends lingually to a metastylid, but differs in its larger size, in having a prominent labial cingulid, and anterior and posterior cingula with accessory cuspids at their lingual termination (Hutchison, Reference Hutchison1968).
Although Mesoscalops is known primarily from the Hemingfordian to Barstovian of Montana, Wyoming, and South Dakota (Barnosky, Reference Barnosky1981), the Jones Branch species much more closely resembles that taxon than the mainly Arikareean Proscalops. This older age of the Jones Branch species does not, however, preclude assignment to Mesoscalops. As Barnosky (Reference Barnosky1981, p. 330) concluded, Mesoscalops was “most closely related either to a P[roscalops] miocaenus-like form or P. tertius, both Whitneyan species.” It is hoped that additional screening of matrix from the Jones Branch site will eventually yield further remains of this talpoid, in turn providing a more detailed assessment of its affinities with the known species from the Great Plains and Rocky Mountains regions.
Lagomorpha Brandt, Reference Brandt1855
Oligolagus new genus
Type species
Oligolagus welleri new genus new species
Diagnosis
As for species.
Etymology
See below.
Holotype
MMNS VP-6604, left ramal fragment with p4–m1.
Diagnosis
Small size; non-rooted, ever-growing, high-crowned lower cheek teeth; prominent lingual and labial reentrants extend along entire length of teeth with minimal cement; no “lingual bridge” (Dawson, Reference Dawson1958) as seen in Palaeolagus haydeni Leidy, Reference Leidy1856, P. philoi Dawson, Reference Dawson1958, and P. hemirhizis Korth and Hageman, Reference Korth and Hageman1988 (note: Prothero and Whittlesey [Reference Prothero, Whittlesey, Terry, LaGarry and Hunt1998, p. 50] suggested that P. hemirhizis is more likely an “artificial construct of two different species [P. temnodon Douglass, Reference Douglass1902, and P. haydeni] …. lumped together…”); enamel of lingual surface of talonid abuts posterior surface of trigonid slightly labial to its lingual-most point (resulting in prominent lingual reentrant); trigonid and talonid joined by cement; thick enamel with thinning apparent only along lingual half of anterior surface of trigonid; no crenulations on posterior edge of trigonid or anterior edge of talonid; no posterolophid.
Differs from Oligocene ochotonids (e.g., Desmatolagus dicei Burke, Reference Burke1936, and D. gazini Burke, Reference Burke1936, but note that Fostowicz-Frelik and Meng, Reference Fostowicz-Frelik and Meng2013, considered Desmatolagus a paraphyletic stem group of lagomorphs) in absence of rooted cheek teeth (Burke, Reference Burke1936; Dawson, Reference Dawson1965; Erbajeva, Reference Erbajeva, Tomida, Li and Setoguchi1994); differs from Megalagus Walker, Reference Walker1931, in much smaller size and absence of low-crowned, rooted cheek teeth; differs from Archaeolagus Dice, Reference Dice1917, in having prominent lingual reentrant between trigonid and talonid along entire length of tooth and in absence of lingual bridge; smaller than Palaeolagus intermedius Matthew, Reference Matthew1899, and P. philoi; similar in size to P. haydeni, P. hypsodus Schlaikjer, Reference Schlaikjer1935, and P. burkei Wood, Reference Wood1940; differs from P. haydeni (abundantly represented in the Brule Formation) in the absence of the prominent, anteroposteriorly extending swelling seen low on the lingual surface of the ramus below the p3–m1 into which the posterior portion of the incisor resides and in the anteriormost point of the V-shaped masseteric fossa. In P. haydeni, it extends to a point between m1 and m2, whereas in Oligolagus welleri n. gen. n. sp. it extends slightly farther forward, to a point even with the talonid of m1. The Chadronian-aged Chadrolagus Gawne, Reference Gawne1978, Limitolagus Fostowicz-Frelik, Reference Fostowicz-Frelik2013, and the rare early Orellan-aged Litolagus Gawne, Reference Gawne1978, all have prominent lingual bridges that develop in early wear stages (Fostowicz-Frelik, Reference Fostowicz-Frelik2013).
Description
The teeth in MMNS VP-6604 are considered to be the p4 and m1 based on the opposing angles they show as they extend down into the jaw fragment (Fig. 5.4; also see Wolniewicz and Fostowicz-Frelik, Reference Wolniewicz and Fostowicz-Frelik2021, fig. 12H) and on comparisons with numerous specimens of P. haydeni from the Brule Formation. The p4 of MMNS VP-6604 measures 2.3 mm AP × 2.1 mm TR (max); m1 measures 2.4 mm AP × 2.2 mm TR (max). A break in the lower part of the lingual surface of the dentary shows that they are non-rooted and apparently ever-growing, resulting in their very high-crowned morphology. The trigonid is higher than the talonid, and the two columns are joined by cement; but there is very little cement in the prominent reentrants, both lingual and labial, that separate the trigonid from the talonid, especially for teeth this high-crowned, compared with species of Palaeolagus. The enamel on both teeth is thick with little thinning anywhere around the tooth except along the lingual half of the anterior surface of the trigonid. Although there is an enamel connection lingually between the talonid and trigonid, it does not form the lingual bridge resulting from wear noted by Dawson (Reference Dawson1958), and particularly characteristic of P. hemirhizis, P. haydeni, and P. philoi, due in large part to the paucity of cement in the lingual reentrant. In Palaeolagus, once the lingual bridge forms, all evidence of a lingual reentrant is lost. In Oligolagus welleri n. gen. n. sp., the lingual reentrant extends along the entire length of the tooth due, in part, to the nature of the lingual trigonid–talonid connection (the enamel of the lingual surface of the talonid connects to, or abuts, the posterior surface of the trigonid slightly labial to its lingual-most point) and to the minimal amount of cement in the lingual reentrant, as noted above (Fig. 5.5). There are no crenulations on the posterior edge of the trigonid or anterior edge of the talonid, and the teeth show no posterolophid.
Etymology
Named for the Oligocene age of the Jones Branch LF and for Andy Weller of Waynesboro, MS, who discovered the Jones Branch site, who alerted GEP to its fossils, and who donated numerous specimens utilized in this study, including this one.
Remarks
Dawson (Reference Dawson, Janis, Gunnell and Uhen2008) recognized the two traditional families of North American lagomorphs, the Leporidae and the Ochotonidae, and in her figure 17.4 she showed three leporid genera spanning the early Arikareean: Palaeolagus, Megalagus, and Archaeolagus. More recent analysis based on detailed study of the cranial morphology has found that Palaeolagus (specifically P. haydeni) shares a mixture of characters associated with both leporids and ochotonids, thus supporting its phylogenetic status as a stem lagomorph (e.g., Wolniewicz and Fostowicz-Frelik, Reference Wolniewicz and Fostowicz-Frelik2021). Megalagus, also considered a stem lagomorph by Fostowicz-Frelik and Meng (Reference Fostowicz-Frelik and Meng2013), is larger than Oligolagus welleri n. gen. n. sp. with lower crowned, rooted teeth. Archaeolagus differs from O. welleri in having the development of the enamel bridge that connects the trigonid and talonid lingually, and in having the typical leporid enamel pattern in which it is thicker labially than lingually, thin to absent lingually, and often absent on the anterior surface of the trigonid and posterior surface of the talonid.
Several species of Palaeolagus, a long ranging group known primarily from the Chadronian through Arikareean of the Great Plains (Dawson, Reference Dawson1958; Emry and Gawne, Reference Emry and Gawne1986; Korth and Hageman, Reference Korth and Hageman1988), have been described, including the Chadronian-aged P. temnodon and P. primus Emry and Gawne, Reference Emry and Gawne1986, the early Orellan-aged P. hemirhizis (but see note above regarding the validity of this taxon), the common late Orellan–early Whitneyan-aged P. haydeni, the late Orellan–Whitneyan-aged P. intermedius and P. burkei, and the early Arikareean-aged P. hypsodus and P. philoi. Palaeolagus hypsodus and P. philoi are known from deposits considered “approximately equivalent to Gering-Monroe Creek beds of Nebraska” according to Dawson (Reference Dawson1958, p. 29), and Bailey (Reference Bailey2004) noted both species from the Ar1-aged Ridgeview LF, Nebraska, as did Hayes (Reference Hayes2007) from the Wagner Quarry LF.
Oligolagus welleri n. gen. n. sp. is similar in size to P. hypsodus, and both lack the lingual bridge trigonid–talonid connection—a feature present in and characteristic of P. temnodon, P. haydeni, P. hemirhizis, P. intermedius, and P. philoi, in which the enamel on the lingual surfaces of the trigonid and talonid merge upon wear. Palaeolagus burkei also lacks the lingual bridge, and together with P. hypsodus comprise Dawson’s (Reference Dawson1958) “P. burkei group” in which the trigonid and talonid are united “solely by cement during most of life” and which have inflated auditory bullae relative to the other species (Dawson, Reference Dawson1958, p. 19; Dawson, Reference Dawson, Janis, Gunnell and Uhen2008; Fostowicz-Frelik, Reference Fostowicz-Frelik2013).
Oligolagus welleri n. gen. n. sp. differs from P. hypsodus in showing generally thicker enamel and thinning enamel only on the lingual portion of the anterior surface of the trigonid. Palaeolagus hypsodus and P. philoi lack enamel on the anterior surface of the trigonid, but the former also lacks enamel on the lingual surface of the trigonid and talonid (Dawson, Reference Dawson1958). Oligolagus welleri n. gen. n. sp. differs from P. philoi in slightly smaller size, in its absence of a posterolophid, and in the absence of enamel crenulations on the anterior wall of the talonid (Dawson, Reference Dawson1958).
The thick enamel on the teeth of the Jones Branch specimen, the degree of hypsodonty, the nature of the talonid–trigonid lingual connection, the prominent lingual reentrant and paucity of cement within resulting in the absence of a lingual bridge even upon wear, the absence of ventro-medial swelling along the ramus for the posterior incisor, and the anterior extension of the ridge of the masseteric fossa to the m1 preclude referral of the Jones Branch species to Palaeolagus, and support its referral to a genus other than those known from the Arikareean of the Midcontinent. Although additional material, particularly the p3 and upper cheek teeth, would help support this, the presence of a previously unknown genus in the Gulf Coastal Plain seems reasonable given the different paleoecological setting that existed there relative to that of the Midcontinent during mid-Oligocene time.
Rodentia Bowdich, Reference Bowdich1821
Sciuridae Fischer de Waldheim, Reference Fischer von Waldheim1817
Hesperopetes Emry and Korth, Reference Emry and Korth2007
Type species
Hesperopetes thoringtoni Emry and Korth, Reference Emry and Korth2007.
?Hesperopetes sp.
Referred specimens
MMNS VP-7539, left p4; MMNS VP-7715, left m2.
Description
The p4, MMNS VP-7539, measures 1.98 mm AP × 1.71 mm TR. The metaconid and hypoconid are the largest cusps, the protoconid is nearly the size of the metaconid, and the entoconid is the smallest; but all are highly worn. The wear of the metaconid and protoconid precludes a determination as to the nature of the trigonid and to metalophulids I and II (tooth morphology follows Bell, Reference Bell2004). The small but prominent mesostylid is connected to the posterior surface of the metaconid, but entirely separated from the entoconid. There is also a worn mesoconid between the protoconid and hypoconid. The crenulations of the talonid are also worn to the extent that they are nearly indistinguishable relative to those of the better preserved m2, MMNS VP-7715.
The m2, which measures 2.04 mm AP × 2.19 mm TR, is parallelogram-shaped, with four robust cuspids and two long, prominent roots (Fig. 5.7). The anterolingually located metaconid is the largest, highest, and most prominent cusp and slightly anterior to the protoconid. Extending labially from the metaconid, and forming the anterior margin of the tooth, is a lophid, likely metalophulid I; but there is no metalophulid II. In other described species of Hesperopetes (and Sciurion), there is typically a small, oval, trigonid basin enclosed between the metalophulid I and II (see below). This anterior lophid does not connect to the protoconid; instead, it terminates labially as a small, subtle cuspid that might be considered an anteroconid. This cuspid-like structure is appressed to, but distinctly separate from, the anterior surface of the protoconid. The protoconid and posterolabially located hypoconid are similar in size, and between them is a small, but distinct mesoconid, which is more closely appressed to the hypoconid than to the protoconid. A prominent, arcuate posterolophid connects the hypoconid to the posterolingually located entoconid. Between the entoconid and the metaconid is a small mesostylid more distinctly separated from the entoconid than from the metaconid. The talonid basin is crenulated.
Remarks
Hesperopetes Emry and Korth, Reference Emry and Korth2007, and Sciurion Skwara, Reference Skwara1986, are small, rare “flying squirrels” with crenulated enamel on the surfaces of the cheek teeth and heretofore known primarily from the northern Great Plains, although Sciurion has also been reported from the Cabbage Patch beds within the Rocky Mountains of Montana (Calede, Reference Calede2020). Originally, three species of Hesperopetes were described, H. thoringtoni, H. jamesi Emry and Korth, Reference Emry and Korth2007, and H. blacki Emry and Korth, Reference Emry and Korth2007, ranging from the Chadronian of Wyoming, the Orellan of Nebraska, through the Whitneyan of North and South Dakota and Saskatchewan (Korth, Reference Korth2017; Korth et al., Reference Korth, Emry, Boyd and Person2019; Bell et al., Reference Bell, Meyer and Storer2023). Recent work by Bell et al. (Reference Bell, Meyer and Storer2023) resulted in reassignment of H. jamesi and H. blacki to Sciurion, thus Sciurion jamesi (Emry and Korth, Reference Emry and Korth2007) and S. blacki (Emry and Korth, Reference Emry and Korth2007).
Bell et al., Reference Bell, Meyer and Storer2023, additionally described two new species from the Orellan of Saskatchewan, S. oligcaenicus Bell, Meyer, and Storer, Reference Bell, Meyer and Storer2023, and S. ikimekooyensis Bell, Meyer, and Storer, Reference Bell, Meyer and Storer2023, as well as a new species of Hesperopetes, H. mccorquodalei Bell, Meyer, and Storer, Reference Bell, Meyer and Storer2023, from material originally referred by Storer (Reference Storer2002) to Nototamias and Protosciurus from the early Arikareean Kealy Springs LF. According to Bell et al. (Reference Bell, Meyer and Storer2023, p. 87; also see Korth, Reference Korth2009a), Sciurion has an open trigonid basin, an anteroconid, and a distinct mesostylid separated from the entoconid, whereas Hesperopetes has “consistently more robust and rounded features,” the absence of an anteroconid (in the type species), and “a mesostylid confluent with the entoconid.” Contrasting with this characterization, however, Bell et al. (Reference Bell, Meyer and Storer2023, p. 92) described the p4 of H. mccorquodalei as having a mesostylid “separated from the entoconid and weakly connected to the metaconid,” and m1 or m2 with a “small, rounded trigonid basin.”
The sciurid teeth from Jones Branch differ somewhat from those described from the more northern localities, primarily in their larger size (although a single M3 from the Orellan of Nebraska referred to Hesperopetes sp. [CM89325] by Korth, Reference Korth2017, is of similar size) and in the structure of the anterior lophid of the m2 (absence of metalophulid II and the consequent absence of an enclosed trigonid basin). But they also share features with both Sciurion and Hesperopetes. Like Sciurion, the m2 has an anteroconid and a distinct mesostylid separated from the entoconid; but like Hesperopetes, it has more robust and rounded cusps. Of these two genera, apparently only Hesperopetes ranges into the Arikareean as H. mccorquodalei.
Until further material is collected from Jones Branch, we provisionally refer these teeth to Hesperopetes. Although assignment to Sciurion is not inadmissible, it would represent a temporal (and geographic) range extension for the genus. The presence of Hesperopetes (or Sciurion) in the Jones Branch LF represents the only known Paleogene record of a “flying squirrel” beyond the Great Plains and northern Rocky Mountains region, although Pratt and Morgan (Reference Pratt and Morgan1989) reported Petauristodon from the Neogene (Hemingfordian) of Florida. The Jones Branch record provides further documentation of the faunal link between the Midcontinent and the Gulf Coastal Plain during the Oligocene.
Aplodontiidae Brandt, Reference Brandt1855
Downsimus Macdonald, Reference Macdonald1970
Holotype
MMNS VP-7334, right m2.
Diagnosis
Differs from the type species, Downsimus chadwicki Macdonald, Reference Macdonald1970, and D. montanus Rasmussen, Reference Rasmussen1977, from Montana, in smaller size, more labially shifted metaconid, much less prominent mesostylid, less robust principal cuspids, absence of lophid extending anteriorly from hypoconulid, and more circular posterior labial fossettid; differs from Haplomys in smaller size, in having a labially shifted metaconid, in lacking a hypolophid and a triangularly shaped hypoconulid and mesoconid, and in having a circular and enclosed posterior fossettid versus a transversely elongate and often open one; differs from Disallomys (originally Allomys storeri Tedrow and Korth, Reference Tedrow and Korth1997; see also Korth, Reference Korth2009b, and discussion below) in its smaller size, more nearly rectangular occlusal shape versus the “parallelogram” shape of Disallomys (Tedrow and Korth, Reference Tedrow and Korth1997, p. 87), in having a labially shifted metaconid versus the anteroposteriorly compressed metaconid positioned at the antero-lingual corner of the tooth in Disallomys, in its diminutive mesostylid and absence of a metastylid crest, in its absence of a hypolophid, and in having a round versus transversely elongate-shaped posterior labial fossettid; differs from Ansomys, Allomys, and Parallomys in its smaller size, in lacking a prominent, blade-like metastylid crest, in lacking an antero-posteriorly compressed hypoconulid, and in lacking a prominent hypolophid and other internal crests.
Description
Aplodontiid tooth morphology follows Bell (Reference Bell2004). This single, small lower molar measures 1.72 mm AP × 1.44 mm TR, somewhat smaller than the m2 of the genoholotype, LACM 17031, at 1.90 mm × 1.70 mm, and that of LACM 9374, at 2.03 mm × 1.50 mm (Macdonald, Reference Macdonald1970). Downsimus montanus is also larger; two m2s measure 2.33 mm and 2.06 mm AP × 1.93 mm and 1.88 mm TR, respectively (Rasmussen, Reference Rasmussen1977, table 13). The metaconid is the largest cusp, occupying the entire anterolingual corner of the tooth, but extending labially approximately half-way across the anterior surface (i.e., the peak of the cusp is not at the antero-lingual corner but has been shifted labially). There is a short anterior cingulid (metalophulid I) between the metaconid and protoconid, and a small, but distinct protrusion on its anterior surface. This protrusion is not an anteroconid, because it is not within the anterior cingulid—it is anterior to the metalophulid I on the anterior surface of the tooth. It may be the structure that Rensberger (Reference Rensberger1975, p. 9) referred to as “a cingulid on the anterior face of the protoconid” as well as what Korth (Reference Korth1989, p. 401) noted as “a basal anterior cingulid below the protoconid.” Based on examination of two p4s assigned to Downsimus from UNSM locality Dw-121 (UNSM 48505 and 48512), the site from which the Ridgeview LF is derived (Bailey, Reference Bailey2004), this protrusion appears to be what was originally a paraconid. Posterior to the metaconid, and at a level just slightly posterior to the peak of the protoconid, is a low, diminutive, barely noticeable mesostylid (Fig. 5.8); too small, in fact, to result in the prominent metastylid crest that forms upon wear and merger of the metaconid and mesostylid so diagnostic of Allomys (including “Alwoodia,” see Hopkins, Reference Hopkins2008) and Parallomys.
The entoconid, a bit smaller than the protoconid, is positioned slightly anterior to the posterolingual corner of the tooth. It is connected to a prominent, but smaller, very slightly anteroposteriorly compressed hypoconulid by a small, subtle, cingular segment; but this cingulid is not so prominent as to block an open inflection between these two cusps. There is a small, short, subdued lophid extending anteriorly from the labial portion of the hypoconulid, but there is no hypolophid so prominent in most other aplodontiids. Another cingular segment (the posterior cingulid or posterolophid) connects the hypoconulid to the slightly larger hypoconid located at the posterolabial corner of the tooth and situated well posterior to the entoconid. Extending anteriorly from the hypoconid are two lophids, one anterolingually and one anterolabially, that connect to the mesoconid, but which also enclose the prominent circular fossettid that lies between the hypoconid and mesoconid (the “posterior labial fossettid” of Rensberger, Reference Rensberger1983, fig. 2, and “posterior buccal fossettid” of Bell, Reference Bell2004, fig. 5.2). The valley between the fossettid and the protoconid is blocked from the interior basin by the mesoconid.
Etymology
Named for Roger Rains of Waynesboro, MS, who, together with Andy Weller, collected and made available to the MMNS so many of the specimens reported herein.
Remarks
The tooth of Downsimus rainsi n. sp. with its brachydont crown height, absence of prominent internal crests or lophids (particularly the hypolophid), and diminutive mesostylid with a consequent absence of a metastylid crest, appears less derived than those of other aplodontiids, such as Haplomys, Ansomys, Allomys, Disallomys, or Parallomys. In these features and others, it most closely resembles Downsimus chadwicki Macdonald, Reference Macdonald1970, and Allomys sharpi Macdonald, Reference Macdonald1970, from the Ar1-aged Sharps Formation of South Dakota and D. montanus from the early Arikareean-aged lower Cabbage Patch beds (Renova Formation) of Montana (Rasmussen, Reference Rasmussen1977; Calede, Reference Calede2020). Allomys sharpi, however, was excluded from that genus by Rensberger (Reference Rensberger1975, Reference Rensberger1983), who considered this species likely congeneric with Downsimus, then later reassigned by Storer (Reference Storer2002) to Downsimus chadwicki on the basis of several specimens from the Ar2 Kealey Springs LF, Saskatchewan. Hopkins (Reference Hopkins2008) also considered Allomys sharpi congeneric with Downsimus, with a consequent referral of the Sharps material to Downsimus sharpi. Downsimus is also recorded from the (late?) Whitneyan-aged Blue Ash LF of South Dakota (Korth, Reference Korth2009b, Reference Korth2010) and the Ar1-aged Ridgeview and Wagner Quarry local faunas, Nebraska (Bailey, Reference Bailey2004; Hayes, Reference Hayes2007, respectively).
Noted in Macdonald’s (Reference Macdonald1970) description of specimens of D. chadwicki (including Allomys sharpi) is the presence of a distinctive mesostylid. Of the m2, he stated “mesostylid larger [than on m1], partially separated from metaconid by notch” (Macdonald, Reference Macdonald1970, p. 29). Several teeth assigned to Downsimus from UNSM locality Dw-121, the site from which the Ridgeview LF is derived (Bailey, Reference Bailey2004), match this description, suggesting that the specimens from Nebraska may be referrable to D. chadwicki. This is further supported by the matching age and geographic proximity of site Dw-121 and Macdonald’s (Reference Macdonald1970) Sharps Formation localities. The specimen from Jones Branch, as noted previously, represents a slightly smaller species with a much smaller, more subtle mesostylid, supporting referral to the new species D. rainsi.
Variably and questionably considered a prosciurine (Macdonald, Reference Macdonald1970; Korth, Reference Korth1989, Reference Korth2009b) or an allomyine (Rensberger, Reference Rensberger1975), the subfamily to which Downsimus belongs is still unresolved. Rensberger (Reference Rensberger1983, p. 35) noted that Downsimus (and ‘Allomys’ sharpi) “were probably not members of a lineage leading toward the allomyines.” Similarly, Storer (Reference Storer2002, p. 112) concluded that Downsimus could not be assigned to the Prosciurinae or the Allomyinae. Hopkins (Reference Hopkins2008, p. 784, 785, 789, 792) considered the Prosciurinae a paraphyletic group encompassing “the species at the base of the aplodontiid radiation,” but noted that Downsimus apparently constitutes a monophyletic clade “placed either just before or just after the divergence of Ansomys” (species of which she placed in a monophyletic Ansomyinae; also see Hopkins, Reference Hopkins2004).
Morphologically the tooth from Jones Branch is less derived than those of any described species of Allomys in its near absence of prominent internal crests or lophids and in the absence of a metastylid crest that forms upon wear and merger of the metaconid and mesostylid. The internal crests of the Jones Branch tooth include only a short, poorly developed lophid off the entoconid that trends posteriorly toward (but not connecting with) the small poorly developed lophid extending anteriorly from the labial portion of the hypoconulid noted above; but there is no true hypolophid between the entoconid and mesoconid seen so prominently in nearly every other species, nor is there a prominent crest extending anteriorly from the hypoconulid, a defining character for Allomys according to Hopkins (Reference Hopkins2008).
Hopkins (Reference Hopkins2008) considered the following species as members of a monophyletic Allomys: A. nitens Marsh, Reference Marsh1877; A. simplicidens Rensberger, Reference Rensberger1983; A. reticulatus Rensberger, Reference Rensberger1983; A. tessellatus Rensberger, Reference Rensberger1983; A. magnus (Rensberger, Reference Rensberger1983); A. harkseni Macdonald, Reference Macdonald1963; and the poorly known A. cristabrevis Barnosky, Reference Barnosky1986—all but the latter two are from various levels within the John Day Formation, Oregon (Rensberger, Reference Rensberger1983). Allomys harkseni is known from the Monroe Creek Formation of South Dakota (R. Macdonald, Reference Macdonald1963, Reference Macdonald1970; L. Macdonald, Reference Macdonald1972) and the Harrison Formation of Nebraska (Korth, Reference Korth1992); A. cristabrevis from the early Arikareean Emerald Lake Fauna, Colter Formation, Jackson Hole, Wyoming (Barnosky, Reference Barnosky1986). All are larger than D. rainsi n. sp., and they have a highly complex occlusal morphology due to “extensive development of accessory crests” (Hopkins, Reference Hopkins2008, p. 790), including the prominent metastylid crest, hypolophid, and anterior extension off the hypoconulid. The same is true for A. stirtoni Klingener, Reference Klingener1968, from the Barstovian-age Norden Bridge LF, Valentine Formation, Nebraska, which, together with Allomys cavatus (Cope, Reference Cope1881a), Hopkins (Reference Hopkins2008) placed in a paraphyletic “Parallomys.”
Allomys storeri, considered ancestral “to all later North American allomyines” by Tedrow and Korth (Reference Tedrow and Korth1997, p. 87) on the basis of its Whitneyan age and on its less-derived morphology, was placed outside the Allomyinae by Hopkins (Reference Hopkins2008) and within a putative monophyletic Ansomyinae. She did not, however, reassign the species to Ansomys, noting that it was “distinct enough from the species previously placed in Ansomys by Hopkins (Reference Hopkins2004) to merit a genus of its own” (Hopkins, Reference Hopkins2008, p. 793). Korth (Reference Korth2009b) apparently agreed with Hopkins’ (2008) assessment and, citing several features distinguishing it from Ansomys, erected Disallomys within the subfamily Prosciurinae for the species—hence Disallomys storeri. The lower molar of the Jones Branch species differs from those of D. storeri in several features including: (1) its smaller size; (2) its more nearly rectangular shape versus the “parallelogram” shape in Disallomys; (3) having a metaconid that is not anteroposteriorly compressed and that has been shifted somewhat labially versus a position at the antero-lingual corner of the tooth; (4) its diminutive mesostylid; (5) its lack of a hypolophid; and (6) having a round versus transversely elongate-shaped posterior labial fossettid.
The appearance of Downsimus in the Jones Branch LF, a form previously known only from the Great Plains and northern Rocky Mountains regions, provides the first record of a North American aplodontiid in the southeastern United States.
Eutypomyidae Miller and Gidley, Reference Miller and Gidley1918
Eutypomys Matthew, Reference Matthew1905
Referred specimens
MMNS VP-8319, left P3; MMNS VP-8326, right P3; MMNS VP-8705, right P3; MMNS VP-8632, left dP4; MMNS VP-6943, right ?P4; MMNS VP-7480, right maxilla fragment with M1–2; MMNS VP-7481, left maxilla fragment with M1; MMNS VP-7782, left M1 or M2; MMNS VP-6584, left p4; MMNS VP-7482, left m1; MMNS VP-6945, right m2; MMNS VP-7283, left m3; SC2013.28.1, left m3.
Description
The dP4 (MMNS VP-8632) is similar to P4 and M1, but much smaller (Fig. 5.10; Table 1), and it closely resembles the dP4 illustrated for E. parvus by Kihm (Reference Kihm2011, fig. 1). As in the P4, there is no labial valley between the metacone and posterior cingulum. The labial terminus of the posterior cingulum joins the posterior surface of the metacone; the M1 has a prominent valley separating the posterior cingulum from the metacone. The valley between the labial terminus of the anterior cingulum and the paracone is narrow relative to that of M1s. There is a small but prominent style anterior to the mesostyle and appressed to the posterior surface of the paracone.
Table 1. Measurements of teeth (in mm) of Eutypomys sp.

The P3s are identified as such on the basis of their matching resemblance to specimens of Eutypomys from Florida’s I-75 LF labeled as P3s (UF 209963, UF 209964, UF 209965), although the I-75 specimens are smaller. P3s are much smaller than the P4s–M2s (Table 1), and they show only a single prominent cusp, here considered the paracone, in an antero-labial position (Fig. 5.11, 5.12). MMNS VP-6943, thought to be a P4, is distinctly elongate anteroposteriorly and shows unilateral hypsodonty, but not nearly to the extent seen in the castorid Microtheriomys brevirhinus Korth and Samuels, Reference Korth and Samuels2015. The elongation and unilateral hypsodonty are traits not seen in other species of Eutypomys, nor in other teeth of this species from Jones Branch. The anterior cingulum, or anteroloph, is widely separated from the paracone by a prominent labial valley that extends approximately halfway across the occlusal surface (Fig. 5.13). At the stage of wear of MMNS VP-6943, there are four enamel lakes, or fossettes, within the anteroloph, and another larger fossette immediately lingual to the valley separating the anteroloph from the paracone. The paracone is slightly anterior to the protocone. Labial to the anterolabially angled lingual valley (the hypoflexus; terminology follows Wu et al., Reference Wu, Meng, Ye and Ni2004) that separates the protocone from the hypocone is a prominent enamel lake, larger than the four anterior to it. Extending lingually from the labial surface of the tooth toward the hypoflexus is a narrow mesoflexus, and between it and the hypoflexus is a mure (= endoloph of Wu et al., Reference Wu, Meng, Ye and Ni2004, fig. 1) that connects the anterior and posterior parts of the tooth. The mesoflexus separates the paracone from the mesostyle, which is found at the labial termination of a mesoloph that originates from the posterior portion of the mure. The mesostyle and mesoloph are entirely isolated from the paracone and metacone by the mesoflexus anteriorly and by a postmesoflexus posteriorly. Between the mesoflexus and the paracone are two prominent fossettes in line with and labial to the prominent fossette noted above anterolabial to the hypoflexus. The mesoloph is parallel to the anterior and posterior surfaces of the tooth. There is no metaloph to connect the hypocone to the metacone, and there is no labial valley separating the metacone from the posterior cingulum. The labial terminus of the posterior cingulum joins the posterior surface of the metacone.
On the M1, the two labial cusps, the paracone and metacone, are the most prominent and are of similar height and size. They are also higher than the rest of the occlusal surface of the tooth, which is essentially flattened from wear. There is a prominent anterior and posterior cingulum with distinct labial valleys separating them from the paracone and metacone, respectively. The prominence of the anterior cingulum (e.g., MMNS VP-7782, Fig. 5.14) results in a greater tooth length than width, suggestive of a P4. The anteroloph connects to the flatly worn protocone, the latter of which is situated slightly posterior to the paracone. Between the paracone and metacone is the mesostyle. Small labial valleys isolate the mesostyle from the paracone anteriorly and metacone posteriorly. Extending lingually from the mesostyle is a convoluted mesoloph, due to fossettes, that attaches to a short mure formed by a posterior extension of the protocone that connects with an anterior extension of the hypocone. The hypocone, also flatly worn, is directly lingual to the metacone. The metaloph extends labially from the hypocone to connect to the metacone, although a transversely elongate fossette splits the metaloph into anterior and posterior segments. Branching off the posterior segment is the posteroloph. In MMNS VP-7782, the labial termination of the posteroloph is separated from the metacone by another small but prominent labial valley. In the more worn MMNS VP-7481 (Fig. 5.9), this labial valley has been obliterated and there is no separation; a single posteroloph connects the hypocone to the metacone. Thus, in relatively unworn teeth there are four labial valleys: the two separating the paracone and metacone from the anterior and posterior cingula, respectively, and the two that are anterior and posterior to the mesostyle. The pattern of the enamel lakes, or fossettes, caused by wear resembles that of E. inexpectatus Wood, Reference Wood1974, which is less complicated than those seen in E. parvus Lambe, Reference Lambe1908, E. hibernodus Korth, Reference Korth2000, E. thomsoni Matthew, Reference Matthew1905, and even the comparably aged E. montanensis Wood and Konizeski, Reference Wood and Konizeski1965.
Regarding the lower teeth, as in other species of Eutypomys, the posterior portion of the p4 is broader transversely than the anterior portion (Fig. 5.15). The lingual cusps, the metaconid and entoconid, and the anterolabially situated protoconid, are somewhat elongated anteroposteriorly. The anterior cingulid is divided into a labial and lingual portion. Extending anterolabially from the metaconid is what would be considered the anterior cingulid proper, and this terminates in an anteroconid. The labial portion is formed by an anterolingually directed extension of the worn protoconid, which is tightly appressed to the lingual surface of the anteroconid. Between this anterolophid and the metalophid is an enclosed enamel lake, or fossettid. The hypoconid is positioned a bit labial to the protoconid and is separated from the latter by the transverse valley, which angles posterolingually from its labial entrance. There is a robust, but isolated, triangular mesostylid and a second prominent stylid posterior to it that is closely appressed to the anterolingual surface of the entoconid. Two lophids extend lingually from the hypoconid: the anterior hypolophid connects to the anterior surface of the entoconid, and the posterolophid, or posterior cingulid, connects to the posterior surface of the entoconid. There is an enamel lake between these two lophids.
The m1s and m2s show a ‘three-lobed’ morphology (Figs. 5.16, 5.17). As described for lower molars of E. inexpectatus by Wood (Reference Wood1974, p. 92), “the metaconid is united with the anterior cingulid, which has a lingual enlargement that might be called an anteroconid, although it does not occupy the typical position of that cusp.” This anterior cingulid plus the metaconid comprise the first, or anterior, lobe, which is isolated from the rest of the tooth by a transverse valley that opens labially anterior to the protoconid and terminates lingually at the posterior surface of the metaconid, seen prominently in MMNS VP-6945 (Fig. 5.17). A prominent metalophid connects the protoconid to the posterior surface of the metaconid, and a lingually open valley immediately posterior to this connection isolates a prominent metastylid. Projecting posteriorly off the metalophid is a ‘spur’ from which another complex lophid extends lingually (?mesolophid), terminating in a small mesostylid located between the metastylid and entoconid. A second transverse valley opens labially between the protoconid and hypoconid and isolates the middle lobe (protoconid, mesolophid, metastylid, and mesostylid) from a third posterior lobe. The third lobe consists of the hypoconid, entoconid, and a bifurcated hypolophid. The anterior bifurcation connects the hypoconid to the entoconid. The posterior lophid is the posterior cingulid or posterolophid. In MMNS VP-6945 there is a lingual valley separating the entoconid from the lingual terminus of the posterior cingulid. In MMNS VP-7482 there is no valley, and the entoconid and lingual terminus of the posterior cingulid are connected (Fig. 5.16).
The m3s are smaller than the m1s and m2s, but morphologically similar (Fig. 5.18, Table 1). They, too, are divided by transverse valleys into three lobes, similar to that seen in Kihm (Reference Kihm2011, fig. 1E), which is an m3 of E. parvus from the Chadronian of North Dakota. The first lobe consists of the metaconid and two lophids that join labially to make a ‘V’ shape, as in the m1s and m2s. The anterior arm of the ‘V’ forms the anterior cingulid and the posterior arm connects to the metaconid. The anterior lobe is separated from the middle lobe by a transverse valley that extends across the tooth and terminates lingually at the posterior surface of the metaconid. As in MMNS VP-6945, a bifurcated metalophid extends lingually from the protoconid. The anterior bifurcation splits again lingually into an anterior section that terminates at a small metastylid appressed to the posterior surface of the metaconid, and the posterior bifurcation terminates at a more robust mesostylid. The posterior bifurcation of the metalophid terminates lingually at the entoconid. Thus, the middle lobe is made up of the protoconid labially and the metastylid, mesostylid, and entoconid lingually. Posterior to this complex is another transverse valley that isolates the third lobe, which consists only of a prominent, labially situated hypoconid and a posterior cingulid extending lingually from it.
Remarks
Eight described species of Eutypomys, comprising a family that Wahlert (Reference Wahlert1977) considered the sister taxon to the Castoridae, range across five NALMAs beginning with E. acares Storer, Reference Storer1988, and E. obliquidens Storer, Reference Storer1988, from the Duchesnean-aged (late Eocene) Lac Pelletier Lower Fauna of Saskatchewan. These are followed by E. inexpectatus from the latest Duchesnean Porvenir and earliest Chadronian Little Egypt local faunas from the Chambers Tuff Formation, Texas (Wood, Reference Wood1974); E. parvus from the Chadronian Cypress Hills Formation, Saskatchewan (Lambe, Reference Lambe1908; Russell, Reference Russell1972; Storer, Reference Storer1978), and Medicine Pole Hills LF, North Dakota (Kihm, Reference Kihm2011); E. hibernodus from the Orellan aged (early Oligocene) Scenic Member of the Brule Formation, South Dakota (Korth, Reference Korth2000; and from the Brule Formation of North Dakota); E. thomsoni Matthew, Reference Matthew1905, from the late Orellan of South Dakota; and E. wilsoni Korth, Reference Korth2007, from the Blue Ash LF of South Dakota considered to be near the Whitneyan–Arikareean boundary (Korth, Reference Korth2007). The last member of the lineage is apparently E. montanensis, recorded from the early Arikareean Tavenner Ranch LF and at other sites in the lower Cabbage Patch beds, Montana (Wood and Konizeski, Reference Wood and Konizeski1965; Calede, Reference Calede2020), the Ar1-aged Wounded Knee-Sharps fauna of South Dakota (Macdonald, Reference Macdonald1970), and the Ar2-aged Kealey Springs LF, Saskatchewan (Storer, Reference Storer2002). The stratigraphically highest known occurrence of E. montanensis is based on a single m3 from the Monroe Creek Formation (Ar2), South Dakota (L.J. Macdonald, Reference Macdonald1972). The small Eutypomys tilliei Storer, Reference Storer1988, from the Lac Pellatier Lower fauna, was moved to Microeutypomys by Walton (Reference Walton1993), and Eutypomys magnus Wood, Reference Wood1937, also from the Scenic Member of the Brule Formation, was moved to ?Oligotheriomys magnus by Korth (Reference Korth2000), citing its combination of Eutypomys and Neatocastor features.
The Jones Branch species is much larger than the late Eocene species from the Lac Pellatier Lower fauna, and larger than E. parvus and E. wilsoni. It is similar in size to E. inexpectatus, E. hibernodus, E. thomsoni, and E. montanensis, and similar in age to the latter. The Jones Branch species has a less complex occlusal enamel pattern than that of E. hibernodus and E. thomsoni (but see discussion below), which both have a very similar, complex pattern. Eutypomys montanensis is also described as having very complex crenulations (Wood and Konizeski, Reference Wood and Konizeski1965). Eutypomys hibernodus is apparently higher crowned than all others, and although Korth (Reference Korth2000) noted no distinguishable lophs on the lowers, the illustration of the m3 in Korth (Reference Korth2000, fig. 1A) very closely resembles that from Jones Branch, and clearly shows anterior, middle, and posterior lobes, as does the m3 of E. parvus, as noted above. Allotypomys pictus Korth and Samuels, Reference Korth and Samuels2015, from Ar1 levels of the John Day Formation, is similar in size to the Jones Branch species, and it too shows a relatively less complicated occlusal pattern than in most species of Eutypomys. But lower cheekteeth of A. pictus have a distinct and prominent mesoconid that is entirely lacking in the Jones Branch species.
One character common to all species of Eutypomys is the highly variable morphology of the teeth, due mainly to the numerous fossettes and fossettids, making comparisons between species (especially those of similar size) particularly difficult. Strongly contributing to this variability is the degree of wear. Because of the complicated pattern of the fossettes and fossettids, the occlusal pattern varies with wear in a non-predictive manner. Although the literature notes a very generalized less-complex to more-complex occlusal pattern from geologically older species to younger (e.g., Korth, Reference Korth2000), this seems to fall apart upon examination of the Jones Branch specimens, which, at the youngest end of the taxon’s range, are relatively uncomplicated. Because of this highly variable morphology within and between the many named species, the possibility that fewer species are represented than described is a possibility (i.e., that the group has been over-split). For example: that E. hibernodus, E. wilsoni, and E. thomsoni, all of similar age, may actually represent a single species cannot be discounted. Thus, the establishment of concrete characters that might differentiate the Jones Branch species from the others (of similar size) is an exercise fraught with potential problems. Additionally, low sample sizes may preclude any meaningful evaluation of intraspecific variability, which, with such complicated teeth, is likely quite high.
Setting the above aside, it might be argued that occurrence of the Jones Branch taxon in the Gulf Coastal Plain, contrary to the fact that all the other species are found almost exclusively in the Great Plains and northern Rocky Mountains (with the exception of E. inexpectatus from west Texas), suggests the possibility of a new species. Geography alone, however, is not a valid criterion for the establishment of such. On the other hand, an argument to support referral of the Jones Branch species to E. montanensis because of their similar age (and size), plus the fact that other mammalian taxa from the more northern sites also occur at Jones Branch, could be made as well. But trying to establish that the Jones Branch species is new based on a potentially different morphology than certain of the other species is difficult at best. In his tentative referral of material from the Wounded Knee faunas of South Dakota to “Eutypomys cf. montanensis,” Macdonald (Reference Macdonald1970, p. 48–49) commented on degree of wear potentially confounding taxonomic assessment in this group. Therefore, as tempting as it is to establish a new species of Eutypomys for the Jones Branch taxon, it seems most prudent to avoid adding yet another species to an already lengthy list until a detailed review of this group of interesting rodents is undertaken.
The Jones Branch species is one of only two eutypomyids noted from the southeastern United States. In discussing Florida’s late Whitneyan-aged I-75 LF found near Gainesville, Patton (Reference Patton1969) reported the occurrence there of teeth he referred to ?Eutypomyidae. Personal examination of these teeth by the first author found them similar to the Jones Branch species morphologically, but distinctly smaller (= similar size [and age] to E. wilsoni). Whether this smaller size indicates yet another new species is difficult to determine, as a difference in age and/or paleoenvironmental factors could be at play.
Castoridae Hemprich, Reference Hemprich1820
Anchitheriomyinae Korth, Reference Korth2001
Microtheriomys Korth and Samuels, Reference Korth and Samuels2015
Type species
Microtheriomys brevirhinus Korth and Samuels, Reference Korth and Samuels2015.
Microtheriomys brevirhinus Korth and Samuels, Reference Korth and Samuels2015

Figure 6. Microtheriomys brevirhinus from the Jones Branch LF, lower Catahoula Formation, Mississippi: (1–3) MMNS VP-11650, right M1, (1) occlusal view, (2) anterior view, (3) posterior view.
Holotype
JODA 16037, partial skull, from Unit H (early Arikareean), Turtle Cove Member, John Day Formation, John Day Fossil Beds National Monument, Oregon.
Referred specimen
MMNS VP-11650, right M1.
Description
Dental terminology follows Wu et al. (Reference Wu, Meng, Ye and Ni2004, fig. 1). Closely matching material from the Turtle Cove Member of the John Day Formation, Oregon, the M1 from Jones Branch (MMNS VP-11650) is similar in size (2.8 mm AP × 3.4 mm TR vs. 2.84 mm AP × 2.66 mm TR for JODA 15263) (Korth and Samuels, Reference Korth and Samuels2015, p. 28), nearly square in occlusal outline, strongly unilaterally hypsodont, with a pair of small labial roots and a single large lingual root (Korth and Samuels, Reference Korth and Samuels2015). The arrangement of flexi and fossettes is also similar, particularly in the J-shaped mesoflexus.
From midway along the labial surface, the mesoflexus extends lingually a bit more than halfway across the occlusal surface, curves posteriorly to form a J-shape terminating near the posterior margin of the tooth. The lingual hypoflexus extends anterolabially to a point approximately level with the lingual-most extent of the mesoflexus. Immediately beyond the lingual termination of the hypoflexus is a circular premesofossette (not to be confused with the more labially positioned premesofossette), and a smaller circular parafossette. Bordering the labial portion of the mesoflexus both anteriorly and posteriorly are a transversely elongate premesofossette and a postmesofossette, respectively. Posterior to the postmesofossette is a similarly transversely elongate metafossette.
Remarks
Microtheriomys brevirhinus was originally known only from between the A-B Tuff (29.75 ± 0.02 Ma) and Deep Creek Tuff (27.89 ± 0.57 Ma) (i.e., early early Arikareean), in the John Day Formation, Oregon (Korth and Samuels, Reference Korth and Samuels2015). More recently, Calede (Reference Calede2020) reported the species from the oldest fossil-bearing deposits of the Cabbage Patch beds, Montana, pushing its age back to an estimated 29.9 Ma. A second, slightly smaller species, M. articulaquaticus Calede, Reference Calede2022, was also described from early Arikareean levels of the Cabbage Patch beds, (Calede, Reference Calede2022), extending the age of the genus upward into the early part of Ar2 (27.05 ± 0.26 Ma).
The Orellan to early Whitneyan Oligotheriomys Korth, Reference Korth1998, resembles Microtheriomys brevirhinus in having low-crowned, unilaterally hypsodont teeth, but differs in lacking the deep J-shaped mesoflexus and in much larger size. Upper molars of Orellan and Whitneyan species of Agnotocastor differ from M. brevirhinus in larger size, in lacking unilateral hypsodonty, and in having more numerous enamel lakes (i.e., a more complicated occlusal pattern; Korth and Samuels, Reference Korth and Samuels2015). Other Arikareean beavers (excluding the much larger Palaeocastorinae; see Flynn and Jacobs, Reference Flynn, Jacobs, Janis, Gunnell and Uhen2008), including Neatocastor Korth, Reference Korth1996 (= Steneofiber complexus Douglass, Reference Douglass1902, and S. hesperus Douglass, Reference Douglass1902), Priusaulax Korth and Bailey, Reference Korth and Bailey2006, and Migmacastor Korth and Rybczynski, Reference Korth and Rybczynski2003, are also larger and lack the distinctive unilateral hypsodonty. The Jones Branch specimen provides another record of a taxon previously known only from the John Day region of Oregon and the northern Rocky Mountains region of Montana—over 3000 km, 13° of latitude, and 31° of longitude from the Jones Branch locality.
Eomyidae Depéret and Douxami, Reference Depéret and Douxami1902
Paraktioeomys new genus
Type species
Paraktioeomys palmeri new genus new species
Diagnosis
As for species.
Etymology
See below.
Remarks
See below.
Paraktioeomys palmeri new genus new species

Figure 7. Dental nomenclature typical for the Eomyidae: (1) upper molar, (2) lower molar. Modified from Wang and Emry (Reference Wang and Emry1991, fig. 2); Escarguel and Aguilar (Reference Escarguel and Aguilar1997, text-fig. 3, pl. 1–3); Smith et al. (Reference Smith, Cifelli and Czplewski2006, fig. 3); Vianey-Liaud and Schmid (Reference Vianey-Liaud and Schmid2009, fig. 1).

Figure 8. Eomyid rodents and Kirkomys from the Jones Branch LF, lower Catahoula Formation, Mississippi: (1–12) Paraktioeomys palmeri n. gen. n. sp., (1) MMNS VP-8731, right P4 or M1, (2) MMNS VP-7024, left M1 or M2 (holotype), (3) MMNS VP-8050, right M1 or M2, (4) MMNS VP-8730, right M1 or M2, (5) MMNS VP-8735, right M1 or M2, (6) SC2013.28.6, left M1 or M2, (7) SC2013.28.3, right p4, (8) MMNS VP-8861, right m1, (9) MMNS VP-8327, right m2, (10) MMNS VP-7711, left m1 or m2, (11) SC2013.28.5, left m2 (paratype), (12) MMNS VP-7712, left m3; (13) Leptodontomys sp., SC2013.28.2, left m1; (14–16) Apeomys catahoulaensis n. sp. (14) MMNS VP-8764, left M1 or M2 (paratype), (15) MMNS VP-8053, left m1 or m2 (holotype); (16) MMNS VP-7023, left m3 (paratype); (17, 18) Apeomys sp. (17) MMNS VP-6728, left p4, (18) MMNS VP-7056, left m1 or m2; (19) Eomyidae undetermined genus and species 1, MMNS VP-6730, right M1 or M2; (20) Eomyidae undetermined genus and species 2, MMNS VP-8656, right M1 or M2; (21) Eomyidae undetermined genus and species 3, MMNS VP-8778, left m1 or m2; (22–25) Kirkomys nebraskensis, (22) MMNS VP-6848, right P4; (23) SC2013.28.20, left M1 or M2; (24) MMNS VP-7121, right m1; (25) MMNS VP-8664, left m1.
Holotype
MMNS VP-7024, left M1 or M2.
Paratype
SC2013.28.5, left m2.
Referred specimens
MMNS VP-8731, right P4 or M1; MMNS VP-8050, right M1 or M2; MMNS VP-8730, right M1 or M2; MMNS VP-8735, right M1 or M2; SC2013.28.6, left M1 or M2; MMNS VP-7045, worn left M3; SC2013.28.3, right p4; MMNS VP-7534, left p4; MMNS VP-8861, right m1; MMNS VP-7711, left m1 or m2; MMNS VP-7769, left m1 or m2; MMNS VP-8327, right m2; MMNS VP-7712, left m3; MMNS VP-7716, left m3.
Diagnosis
The most diagnostic feature of both the upper and lower cheek teeth is a distinctive, slanted V-shaped structure not seen in other eomyids; but like other eomyids they show “four major cusps joined by thin to strong lophs” (Flynn, Reference Flynn, Janis, Gunnell and Uhen2008, p. 415). In the uppers, the ‘V’ occupies a position posterior to the anteroloph, where it is formed by an anterior arm that connects the hypocone with the paracone and a posterior arm connecting the hypocone with the metacone. In the lowers, it occupies a position anterior to the posterolophid and is formed by an anterior arm that connects the protoconid with the metaconid and a posterior arm connecting the protoconid with the entoconid.
The teeth somewhat resemble those of the strictly European theridomyid Theridomys Jourdan, Reference Jourdan1837, as described by Vianey-Liaud (Reference Vianey-Liaud1972) and Kälin (Reference Kälin2013). They also resemble those of the eomyid Pseudotheridomys Schlosser, Reference Schlosser1926, originally described from Europe, but also known in North America. The Jones Branch teeth differ from both (and from other eomyids) in lacking the prominent mesoloph and mesostyle in the uppers and prominent mesolophid in the lowers, and in lacking the bilobed morphology of the lowers, those lobes typically connected by a longitudinal ridge. Dental nomenclature typical for the Eomyidae (Fig. 7) is modified from Wang and Emry (Reference Wang and Emry1991, fig. 2), Escarguel and Aguilar (Reference Escarguel and Aguilar1997, text-fig. 3, pl. 1–3), Smith et al. (Reference Smith, Cifelli and Czplewski2006, fig. 3), and Vianey-Liaud and Schmid (Reference Vianey-Liaud and Schmid2009, fig. 1).
Description
Table 2 provides measurements for the teeth. In the upper M1–2, the anteroloph, or anterior cingulum, arches labially from the protocone to terminate alongside the anterior surface of the paracone. The internal sinus is continuous with syncline I, and in some specimens there is a small lophule about two-thirds the way along the anterior cingulum that protrudes into the basin formed by the internal sinus and syncline I. Emanating labially from the hypocone are two lophs, the anterior connecting to the paracone and the posterior connecting to the metacone, resulting in the formation of an anteriorly slanted V-shaped structure. The valley between the lophs is likely equivalent to syncline III. About halfway along the anterior arm of the ‘V’ (i.e., the loph connecting the hypocone to the paracone) is a small, short, labially directed mesoloph; but there is no mesostyle, therefore no syncline II. The posterior arm of the ‘V’ splits into an anterior branch that connects the hypocone to the metacone and a short posterior branch, the posteroloph or posterior cingulum, which terminates alongside the posterior surface of the metacone. The space between would be considered syncline IV (Fig. 8.1–8.6).
Table 2. Measurements of teeth (in mm) of Paraktioeomys palmeri n. gen. n. sp.

Mirroring the uppers, the lower teeth also have the V-shaped structure, slanted posteriorly, that connects the protoconid with the metaconid through its anterior arm and to the entoconid via its posterior arm. The ‘V’ morphology is in part due to the somewhat pinched labial surface of the protoconid (Fig. 8.8–8.12), in contrast to the labial surface of the hypoconid, that, with wear, is more rounded—a condition more closely resembling theridomyids than Pseudotheridomys in which the opposite occurs (i.e., in Pseudotheridomys it is the labial surface of the hypoconid that shows a more pinched shape than the protoconid). The fossette between the two arms of the ‘V’ represents synclinid II. A small, short mesolophid extends lingually off the posterior arm between the metaconid and entoconid, but it is not connected to a mesostylid between the two lingual cusps. The small stylid in this position is more likely a metastylid because it is closely appressed to the metaconid. Posterior to the ‘V’ is a robust posterolophid, and between the posterior arm of the ‘V’ and the posterolophid (i.e., synclinid IV) is a small lophulid connecting the two. At the labial termination of the posterolophid is the hypoconid. It should be noted that there is another single right lower molar, SC2013.28.4, that is morphologically identical to those noted above, but it is notably smaller (1.2 mm AP × 1.1 mm TR) and therefore not included in the list of referred specimens.
Etymology
Paráktio, Greek for ‘coastal,’ in reference to the coastal location of the Jones Branch locality during the Oligocene (Fig. 3); eomys in reference to the Eomyidae, the family to which this species belongs; and palmeri, for Mack Palmer, owner of the land adjacent to the Jones Branch site and who negotiated permission for collecting purposes.
Remarks
As noted above, the teeth of Paraktioeomys palmeri n. gen. n. sp. somewhat resemble those of both Theridomys, unknown outside of Europe, and Pseudotheridomys, a mainly European taxon also known in North America. Pseudotheridomys first appears in Europe in the early Oligocene “Stampian” (= Rupelian) as P. schaubi Lavocat, Reference Lavocat1951, followed by the type species, P. parvulus (Schlosser, Reference Schlosser1884), in the Chattian. Wilson (Reference Wilson1960) described the first species from North America as Pseudotheridomys hesperus from the early Hemingfordian Quarry A of the Martin Canyon LF (Galbreath, Reference Galbreath1953; Tedford et al., Reference Tedford, Skinner, Fields, Rensberger, Whistler, Galusha, Taylor, Macdonald, Webb and Woodburne1987) in Logan County, Colorado, which he considered an immigrant from the “Old World” and a possible descendent of P. parvulus. The report of P. hesperus was followed by the description of a second species in North America, P. cuyamensis Lindsay, Reference Lindsay1974, from the similarly aged Vedder fauna of Santa Barbara County, California, and by Martin’s (Reference Martin1976) description of Pseudotheridomys sp. from the Batesland Formation in South Dakota. Pseudotheridomys pagei Shotwell, Reference Shotwell1967, from the Barstovian-aged Quartz Basin and Red Basin quarries of Oregon, appears to be the latest occurring species, but apparently differs very little if at all from the much older P. hesperus according to Engesser (Reference Engesser1979). Records of Pseudotheridomys in the Gulf Coastal Plain were documented by Slaughter (Reference Slaughter1981), who reported two m2s from the early Hemingfordian Hidalgo Bluff LF (Garvin Gully fauna), Washington County, Texas.
A downward extension of Pseudotheridomys into the Oligocene of North America resulted from the discovery of P. hesperus in the Ar2-aged Kealy Springs LF, Cypress Hills Formation, Saskatchewan (Williams and Storer, Reference Williams and Storer1998; Storer, Reference Storer2002) and from the Ar2 Monroe Creek Anthills fauna, Monroe Creek Formation, Nebraska (L.J. Macdonald, Reference Macdonald1972). Although Pseudotheridomys was among other allochthonous taxa whose first appearance Tedford et al. (Reference Tedford, Skinner, Fields, Rensberger, Whistler, Galusha, Taylor, Macdonald, Webb and Woodburne1987, Reference Tedford, Swinehart, Swisher, Prothero, King, Tierney, Prothero and Emry1996, Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004) used to define and characterize Ar2, even older specimens were reported by Calede (Reference Calede2020, p. 13) from “the oldest assemblage of the Cabbage Patch beds (C1708)” considered Ar1 in age. Thus, the earliest occurrence of Pseudotheridomys in Europe and North America is similar.
Whether Paraktioeomys palmeri n. gen. n. sp. is derived from Pseudotheridomys, or from Theridomys (or other theridomyids), or is a Gulf Coastal Plain endemic eomyid somewhat convergent with the latter two is equivocal. Whereas the appearance of Paraktioeomys n. gen. at Jones Branch shortly following the first North American appearance of Pseudotheridomys supports the Ar1 immigration into North America of taxa with Eurasian affinities noted by Calede (Reference Calede2020), it seems unlikely that the former is derived from the latter, particularly given the absence in Paraktioeomys n. gen. of the prominent mesoloph/mesolophid typically seen in Pseudotheridomys, which is likely the derived state. Theridomyids also have the prominent mesoloph/mesolophid, but they have not been reported from North America. On the other hand, the morphology of the lower molars in Paraktioeomys (i.e., the pinched labial surface of the protoconid vs. hypoconid) more closely resembles that in theridomyids than in Pseudotheridomys, again precluding the latter from Paraktioeomys ancestry.
It is important to note that the rodent material Patton (Reference Patton1969) referred to “Eomyidae” from the I-75 LF of Florida also appears to represent Paraktioeomys n. gen. To the extent that I-75 is considered late Whitneyan in age, here considered slightly older than Jones Branch, this occurrence would support the hypothesis that Paraktioeomys was a Gulf Coastal Plain endemic well established there prior to the arrival of Pseudotheridomys into North America from Europe, and therefore not closely related. Currently, there does not appear to be any taxon known from North America from which Paraktioeomys may be derived. Given this, Paraktioeomys n. gen. may come to provide biochronologic utility, at least regionally, as a taxon that helps to characterize Ar1 (or the late Whitneyan, depending on the accuracy of the age of I-75).
Eomyinae Winge, Reference Winge1887
Leptodontomys Shotwell, Reference Shotwell1956
Referred specimen
SC2013.28.2, left m1.
Remarks
Smith et al. (Reference Smith, Cifelli and Czplewski2006, p. 386) discussed four distinct variations in cheek teeth patterns of eomyids, and it is their variation 4, exemplified by Leptodontomys Shotwell, Reference Shotwell1956, and Kansasimys Wood, Reference Wood1936a, within which SC2013.28.2 falls: “four to five lophs (-ids), three transverse valleys (syncline 1, middle syncline = synclines II–III, and syncline 4), entoloph and ectolophid present, primary cusps distinct, and low crowned.” Originally described from the Late Miocene of Oregon, but also known from the late Arikareean there, Leptodontomys was later found in the early Arikareean of North Dakota, South Dakota, Nebraska, Montana, California, and Saskatchewan, and apparently ranges from that time to the Hemphillian (Korth and Bailey, Reference Korth and Bailey1992; Storer, Reference Storer2002; Korth, Reference Korth2008a; Korth and Samuels, Reference Korth and Samuels2015; Calede, Reference Calede2020). Its appearance at Jones Branch, therefore, is not temporally anomalous, although it does provide the first record of this taxon beyond the United States and Canadian Great Plains, the Rocky Mountains, and Oregon.
The size of SC2013.28.2 (1.0 mm AP × 0.85 mm TR) closely matches that of L. douglassi (Burke, Reference Burke1934) based on measurements of specimens from UNSM site Dw-121 in Nebraska (the Ridgeview LF; see Korth, Reference Korth2008a). But the worn nature of the tooth makes referral to a particular species equivocal. Furthermore, in describing the Ridgeview material of L. douglassi, Korth and Bailey (Reference Korth and Bailey1992) noted that the lower molars were three-rooted, as are those from the late Arikareean of Oregon (J. Samuels, pers. commun., 2024). The Jones Branch tooth has two roots.
Apeomyinae Fejfar, Rummel, and Tomida, Reference Fejfar, Rummel, Tomida, Tomida, Flynn and Jacobs1998
Apeomys Fahlbusch, Reference Fahlbusch1968
Type species
Apeomys tuerkheimae Fahlbusch, Reference Fahlbusch1968.
Apeomys catahoulaensis new species
Holotype
MMNS VP-8053, left m1 or m2.
Paratypes
MMNS VP-8764, left M1 or M2; MMNS VP-7023, left m3.
Referred specimens
MMNS VP-6734, left M1; MMNS VP-8777, left M1 or M2; MMNS VP-8779, left M1 or M2; MMNS VP-8663, right p4; MMNS VP-7714, left p4; MMNS VP-7713, left m1 or m2.
Diagnosis
Bilobed lower molars similar in size and morphology to Apeomys whistleri Korth and Samuels, Reference Korth and Samuels2015, from the John Day Formation, Oregon; differs from lower molars of Apeomys whistleri in possessing two roots rather than three; upper molars more closely resemble those of the early Barstovian Apeomyoides savagei Smith, Cifelli, and Czplewski, Reference Smith, Cifelli and Czplewski2006, than those of A. whistleri in being distinctly wider transversely than long anteroposteriorly, although A. savagei is much larger.
Description
Diagnostic of this subfamily are the distinctly bilobed lower cheekteeth (Fahlbusch, Reference Fahlbusch1968; Fejfar et al., Reference Fejfar, Rummel, Tomida, Tomida, Flynn and Jacobs1998; Morea and Korth, Reference Morea and Korth2002; Smith et al., Reference Smith, Cifelli and Czplewski2006). The anterior lobe is formed by a connected metalophid and mesolophid; the posterior by a connected hypolophid and posterolophid (see Fig. 7 for dental terminology). The anterior and posterior lobes are separated by a continuous transverse valley (syncline III), and within each is an elongate syncline—syncline II in the anterior lobe and syncline IV in the posterior. In these features, and in size (Table 3), the lower cheek teeth of Apeomys catahoulaensis n. sp. most closely resemble those of A. whistleri from Ar3 levels in the John Day Formation (first noted as “cf. Apeomys n. sp.” in Fremd and Whistler, Reference Fremd, Whistler and Albright2009). However, slight differences indicate that the Oregon and Mississippi species are not the same. Most notably, lower molars of the Jones Branch species have two roots, whereas Apeomys from both Europe and Oregon have three (Fejfar et al., Reference Fejfar, Rummel, Tomida, Tomida, Flynn and Jacobs1998; Korth and Samuels, Reference Korth and Samuels2015). Note, however, that the tip of the anterior root of the lower molars in A. catahoulaensis n. sp. is slightly bifurcated.
Table 3. Measurements of teeth (in mm) of Apeomys catahoulaensis n. sp.

Regarding the upper molars, those assigned here to A. catahoulaensis n. sp. are distinctly wider than long (Table 3), more closely resembling upper teeth of the much larger early Barstovian Apeomyoides savagei than those of A. whistleri, which are approximately as long as they are wide. Unlike the condition in the Jones Branch species, however, in both Apeomyoides and other species of Apeomys, the internal sinus between the protocone and hypocone connects with syncline III, resulting in an uninterrupted, although offset, transverse valley across the occlusal surface. In the Jones Branch species, the internal sinus traverses labially across the tooth nearly as far as syncline III extends lingually, but they do not connect; syncline III is offset posteriorly to the internal sinus (Fig. 8.14). Also, in Apeomyoides and previously described species of Apeomys, the protoloph and mesoloph are separated by a labially open syncline II, and the metaloph and posteroloph are separated by a labially open syncline IV. In the Jones Branch teeth, these synclines are enclosed.
Because the upper teeth from Jones Branch differ from those of previously described species of Apeomys and Apeomyoides, referral of them to this new species is somewhat equivocal. However, there are no other upper teeth from the site that can be more suitably identified as belonging to this taxon.
Etymology
Named for the Catahoula Formation from which the Jones Branch LF was recovered.
Remarks
Apeomyines range from the early Oligocene to Early Miocene of Eurasia, Japan, and North America (Fahlbusch, Reference Fahlbusch1968; Fejfar et al., Reference Fejfar, Rummel, Tomida, Tomida, Flynn and Jacobs1998; Morea and Korth, Reference Morea and Korth2002; Smith et al., Reference Smith, Cifelli and Czplewski2006; Korth, Reference Korth2007, Reference Korth2008b; Korth and Samuels, Reference Korth and Samuels2015; Mörs and Flink, Reference Mörs and Flink2017; Zhu-Ding, Reference Zhu-Ding2017). The oldest known specimens of the first described species, Apeomys tuerkheimae Fahlbusch, Reference Fahlbusch1968, are from European Mammal Zones MP30 and MN1, which straddle the Oligocene–Miocene boundary (Speijer et al., Reference Speijer, Pälike, Hollis, Hooker, Ogg, Gradstein, Ogg, Schmitz and Ogg2020). Older European/Eurasian species have yet to be found.
In North America the oldest known apeomyine, Zophoapeomys indicum Korth, Reference Korth2007 (erroneously referred to Z. indicus in Korth, Reference Korth2008b), is from the late Whitneyan Blue Ash Fauna, South Dakota. Although similar in size to A. catahoulaensis n. sp. (CM 76136, an m1 or m2 of Z. indicum, measures 0.92 mm AP × 1.02 mm TR; Korth, Reference Korth2007), it differs from the latter in having a lower molar in which the anterior loop is not complete, in having distinguishable cusps, and in having a lingually blocked transverse valley. But like the Jones Branch species, Zophoapeomys has only two roots. Zophoapeomys is also known from the Ar1-aged Ridgeview LF, Nebraska (Korth, Reference Korth2008b), here considered comparable in age to Jones Branch.
Another taxon older than those known from outside North America, and comparable in age to A. catahoulaensis n. sp., is Proapeomys condoni Korth and Samuels, Reference Korth and Samuels2015, from Ar1 levels in the John Day Formation, which is a bit younger than Zophoapeomys indicum and older than Apeomys whistleri. Slightly larger than the Jones Branch species, the lower molars of P. condoni (1.29–1.33 mm AP × 1.44–1.49 mm TR; Korth and Samuels, Reference Korth and Samuels2015; table 10), like those of Zophoapeomys, also differ in having distinguishable cusps and in having incomplete transverse loops.
Apeomys sp.
Referred specimens
MMNS VP-6728, left p4; MMNS VP-7056, left m1 or m2.
Remarks
These teeth are nearly identical to those described above for Apeomys catahoulaensis n. sp., but distinctly larger. The p4 measures 1.3 mm AP × 1.1 mm TR (vs. 1.1 × 0.85 for A. catahoulaensis; see Table 3) and the m1 or m2 measures 1.25 mm × 1.15 mm TR (vs. an average of 1.0 × 1.0 for A. catahoulaensis n. sp.). Whether these teeth are evidence of a second, larger species of Apeomys at Jones Branch, or of sexual dimorphism, or are within a range of variation that is not determinable at this time due to the few specimens so far known, they are separated out to note the distinction.
Eomyidae undetermined genus and species 1
Referred specimen
MMNS VP-6730, right M1 or M2.
Description
In this M1 or M2, the transverse valley is continuous across the upper molar; it is not interrupted by a longitudinal ridge as in the upper molars assigned to A. catahoulaensis n. sp. The anterior lobe consists of the anteroloph and protoloph, which merge labially and entirely enclose an anterior sinus (syncline I). The posterior lobe is formed from the merger of the metaloph and posteroloph, which entirely enclose a posterior sinus (syncline IV). The tooth has robust roots and is much larger (1.55 mm AP × 1.55 mm TR) than A. catahoulaensis n. sp. and Apeomys sp. noted above.
Remarks
This tooth somewhat resembles the M1 or M2 of Apeomys in having anterior and posterior lobes (e.g., Korth and Samuels, Reference Korth and Samuels2015, fig. 14), but differs in its much larger size, in having a less transversely compressed protocone and hypocone, and in having a small but distinctive cusp located within and midway across the transverse valley.
Eomyidae undetermined genus and species 2
Referred specimen
MMNS VP-8656, right M1 or M2.
Description
This tiny upper molar (0.95 mm AP × 1.03 mm TR) is also bilobed, but its transverse valley is blocked by a prominent longitudinal crest (see Figs. 7 and 8.20) that extends anteriorly from the metaloph, thus resulting in an internal sinus between the protocone and hypocone and a syncline III between the paracone and metacone. Although this resembles the condition of the upper teeth assigned to Apeomys catahoulaensis n. sp. (compare with Fig. 8.14), MMNS VP-8656 is nearly square in occlusal outline, whereas the former is shorter anteroposteriorly than it is transversely. A short anteroloph separates a small syncline I from the protoloph, and the anterior lobe is formed from the merger of the protoloph and mesoloph, which enclose syncline II. The posterior lobe consists of a merged metaloph and posteroloph, enclosing syncline IV.
Remarks
To the extent that this tooth closely resembles in size and morphology those upper teeth assigned to Apeomys catahoulaensis n. sp., with the exception of slightly different AP/TR dimensions, it may eventually be determined, if additional material can be recovered, that it belongs to that species.
Eomyidae undetermined genus and species 3
Referred specimen
MMNS VP-8778, left m1 or m2.
Description
In its distinctly bilobed morphology and tiny size (1.15 mm × 1.0 mm), this tooth somewhat resembles lower molars of Apeomys, and like A. catahoulaensis n. sp. it has only two roots. It differs, however, and most closely resembles the European theridomyid Blainvillimys Gervais, Reference Gervais1848–1852, in having the two lobes connected by a longitudinal ridge (positioned more lingually than labially), thus blocking the transverse valley, and in having the lobes not entirely closed lingually (i.e., synclinids II and IV are open lingually). Although the Jones Branch tooth differs from lower molars of Blainvillimys in the absence of synclinid I, this feature appears to be variably expressed in theridomyids (Vianey-Liaud, Reference Vianey-Liaud1972; Kälin, Reference Kälin2013, fig. 5b). Another distinction, however, is that in lower molars of theridomyids the labial surface of the protoconid is typically pinched into a ‘V’ shape. In the Jones Branch tooth, it is the hypoconid that shows this morphology, with the protoconid being more rounded (Fig. 8.21).
Remarks
Although theridomyids are not known from North America, the eomyid Pseudotheridomys, originally described from Europe, does occur here. MMNS VP-8778 is somewhat reminiscent of certain lower molars of this taxon, such as Pseudotheridomys parvulus and P. bouziguensis Escarguel and Aguilar, Reference Escarguel and Aguilar1997, from the Lower Miocene of the South of France (see Escarguel and Aguilar, Reference Escarguel and Aguilar1997, pl. 1, fig. 9; pl. 2, fig. 11; pl. 3, fig. 14). This is particularly apparent in the Jones Branch tooth having a labially pinched hypoconid and a more rounded protoconid, as is the condition in Pseudotheridomys but opposite the condition in theridomyids noted above. But in Pseudotheridomys, synclinid IV is always closed lingually and the longitudinal ridge is positioned more labially than lingually. In the Jones Branch species, the longitudinal ridge is positioned more lingually than labially. MMNS VP-8778 does not resemble lower molars of Paraktioeomys n. gen., as described above; it is much smaller, distinctly bilobed, and lacks the V-shaped morphology diagnostic of the latter.
Although most similar to lower molars of theridomyids, which have never been recorded beyond Europe, and to Pseudotheridomys, known from Europe and North America, to which taxon beyond “Eomyidae” this tooth belongs is equivocal. Differences noted above preclude referral of MMNS VP-8778 to either of those taxa. Thus, this tiny tooth from Jones Branch likely belongs to a new genus and species of eomyid endemic to the Gulf Coastal Plain Oligocene. Although its tiny size and presence of a longitudinal ridge suggests that it and MMNS VP-8656, the tiny upper molar noted above, may belong to the same species, we maintain the two specimens as representative of two different species until additional material can provide clarification.
Florentiamyidae Wood, Reference Wood1936b
Kirkomys Wahlert, Reference Wahlert1984
Type species
Kirkomys milleri Wahlert, Reference Wahlert1984
Kirkomys nebraskensis (Wood, Reference Wood1937)
Reference Wood1937 Proheteromys nebraskensis Wood, p. 215, figs. 35, 36.
Reference Wahlert1984 Kirkomys milleri Wahlert, p. 2, figs. 1, 2.
Reference Hayes2007 Proheteromys cf. P. nebraskensis; Hayes, p. 24, fig. 13A.
Reference Korth and Branciforte2007 Kirkomys nebraskensis Korth and Branciforte, p. 189, figs. 4, 5.
Holotype
F:AM 105337, partial skull, from “the Whitneyan of Sioux County, Nebraska” (Korth and Branciforte, Reference Korth and Branciforte2007, p. 188).
Referred specimens
MMNS VP-6848, right P4; SC2013.28.20, left M1 or M2; MMNS VP-7121, right m1; MMNS VP-8706, right m1; MMNS VP-8664, left m1; SC2013.28.19, left m1; MMNS VP-6815, right m3.
Description
Cusp terminology follows Lindsay (Reference Lindsay1974) and Barnosky (Reference Barnosky1986); measurements of the teeth are provided in Table 4. The P4 is simple in that it has a round protocone with only a tiny paracone appressed to its lingual surface, but no protostyle, posterocone, or other accessory cusps, and no posterior cingulum except for a small segment posterior to the metacone (Fig. 8.22). The metacone is angled a bit anteriorly relative to the transverse trend of the metaloph, the hypocone is the largest cusp of the metaloph, and the entostyle (= hypostyle) is transversely compressed, anteroposteriorly elongate, with an anteroposteriorly oriented valley separating it from the hypocone. It also extends slightly anterior to the hypocone. In the upper molar, an M1 or M2, neither the protostyle nor entostyle are expressed as distinctive cusps. Instead, they are apparently subsumed into a continuous lingual cingulum which is highest at the central point where the anterior and posterior cingula meet, completely blocking entrance to the transverse valley (Fig. 8.23). The transverse valley is open labially, however, between the paracone and metacone, and the protoloph is only slightly offset labially from the metaloph.
Table 4. Measurements of teeth (in mm) of Kirkomys nebraskensis

The lower molars are typically geomyoid with six distinct cusps. The metaconid, protoconid, and protostylid makeup the metalophid and the entoconid, hypoconid, and hypostylid makeup the hypolophid. The transverse valley separating the metalophid from the hypolophid is prominent across the entire occlusal surface. The metaconid and protoconid are larger than the entoconid and hypoconid, and the protostylid and hypostylid are diminutive. A prominent cingular segment protects the metaconid and continues lingually anterior to the protoconid to connect with the protostylid. In MMNS VP-7121, a right m1, there is a small cuspid (posteroconid?) between the entoconid and hypoconid (Fig. 8.24). The degree of expression of the cingular segments and cuspids varies among the teeth. Like the m1–2, the m3 also has a small anterolabial cingulid and a small cingular segment at the metaconid. The hypolophid is transversely narrower than the metalophid and, although worn, all three cusps (of the hypolophid) are discernable.
Remarks
Identifying heliscomyids, florentiamyids, and heteromyids definitively to species, and often even to genus, is fraught with difficulty because the general morphology of their cheek teeth is very similar, and the teeth can vary considerably between species in the same genus. As Samuels et al. (Reference Samuels, Calede and Hunt2023, p. 2) noted in their study on the phylogenetic relationships of geomorph rodents, “Parallelism in dental morphology is certainly common in geomyoids.” But the Jones Branch species, with its simple P4 and continuous lingual cingulum in the upper molar, matches the emended diagnosis of dental features for Kirkomys in which “…cheek teeth submesodont, similar in crown height to Proheteromys but cusps more bunodont; premolars simple; P4 with simple protoloph (consisting of circular protocone and occasional minute cuspules); P4 consists of four main, subequal cusps (no protoconid) with occasional minute accessory cuspules; transverse valley of molars relatively shallow, lophs uniting centrally after only moderate wear; lingual cingulum on upper molars continuous (not interrupted by transverse valley)” Korth and Branciforte (Reference Korth and Branciforte2007, p. 187).
But Kirkomys also exemplifies variation across its species. For example, P4s of Kirkomys parvus (Troxell, Reference Troxell1923b), K. martintau (Korth, Reference Korth2014), and K. miriamae Korth et al., Reference Korth, Boyd, Person and Anderson2022, have a posterior cingulum (or posterocone), whereas that feature is absent in K. nebraskensis (Korth, Reference Korth2008b); hence referral of the Jones Branch specimens to the latter species. Kirkomys nebraskensis is known from the Whitneyan and Ar1 of South Dakota and Nebraska (Korth, Reference Korth2008b), with several specimens from UNSM locality Dw-121, the site from which the Ridgeview LF is derived and from which several specimens of Downsimus chadwicki were recovered (Bailey, Reference Bailey2004).
Other florentiamyids that span this interval of time, such as Florentiamys Wood, Reference Wood1936b, Hitonkala Macdonald, Reference Macdonald1963, and Sanctimus Macdonald, Reference Macdonald1970, also have a continuous lingual cingulum on the upper molars, but they differ from K. nebraskensis and the Jones Branch species in the more complicated P4 with its variety of accessory cusps and cingula (see Wood, Reference Wood1936b, fig. 5; Wahlert, Reference Wahlert1983). The heteromyids Mookomys Wood, Reference Wood1931, and Proheteromys Wood, Reference Wood1932, can also have simple P4s (although several named species of Proheteromys show accessory cuspules), but neither has a continuous lingual cingulum on upper molars. Lindsay (Reference Lindsay1974), however, noted that a lingual cingulum can form on upper molars of Mookomys after wear. The appearance of Kirkomys in the Jones Branch LF provides yet another unexpected record of a taxon in the Gulf Coastal Plain that was previously known only from the more northern regions of the continent.
Carnivora Bowdich, Reference Bowdich1821
Mustelidae Fischer von Waldheim, Reference Fischer von Waldheim1817
Corumictis Paterson et al., Reference Paterson, Samuels, Rybczynski, Ryan and Madden2019
Type species
Corumictis wolsani Paterson et al., Reference Paterson, Samuels, Rybczynski, Ryan and Madden2019.
Corumictis sp.

Figure 9. Carnivores from the Jones Branch LF, lower Catahoula Formation, Mississippi. All figures except (4–8) are microCT scans: (1–5) Corumictis sp., MMNS VP-6617, left dentary with m1, (1) occlusal view, (2) labial view, (3) lingual view, (4) labial view (standard photograph), (5) lingual view (standard photograph); (6–8) Promartes sp., MMNS VP-6613, left proximal ulna, (6) dorsal view, (7) medial view, (8) lateral view; (9–11) Phlaocyon taylori, MMNS VP-7444, right m1, (9) lingual view, (10) occlusal view, (11) labial view; (12) ?Phlaocyon sp., MMNS VP-6606, left M2.
Referred specimen
MMNS VP-6617, left dentary with m1.
Description
The m1 measures 7.31 mm AP × 3.59 mm TR, with a crown height of 5.15 mm. The depth of the ramus below m1 is 10 mm. The m1 of the holotype, JODA 8167, at 5.9 mm AP × 2.4 mm TR, is slightly smaller; but in all other features it very closely matches the Jones Branch specimen. The trigonid is more than twice the length of the talonid, but not three times as long as noted in Wolsan (Reference Wolsan1993, fig. 6, character 22b); the metaconid, if it was complete, would be approximately equal in height to the paraconid (Wolsan, Reference Wolsan1993, character 23b); the entoconid and entoconulid are poorly differentiated, if at all, as they are subsumed within the very low, ridge-like, lingual border of the talonid; the anterior half of the lingual surface of the talonid is distinctly lower than the posterior half (Wolsan, Reference Wolsan1993, character 24b); the posterior base of the protoconid is separated from the labial surface of the talonid by a small notch; posterior to this notch the labial, ridge-like margin of the talonid ascends to its highest point at the posterolabial corner of the tooth where the hypoconid is manifested as a swelling rather than as a distinct cusp. Additionally, in both the holotype and the Jones Branch species, the m2 is double-rooted (Wolsan, Reference Wolsan1993, character 25a).
Remarks
According to Baskin (Reference Baskin, Janis, Scott and Jacobs1998), there are three musteloids in North America that bracket or span the interval of time over which the Jones Branch LF is considered to range. The first is the “archaic,” “stem,” or “basal” musteloid, Mustelavus (see Baskin, Reference Baskin, Janis, Scott and Jacobs1998, p. 153; Wang et al., Reference Wang, McKenna and Dashzeveg2005, p. 44, 50; Sato et al., Reference Sato, Wolsan, Prevosti, Guillermo, Begg, Begg, Hosoda, Campbell and Suzuki2012, p. 753), known from the late Chadronian through Orellan of the Great Plains. Based on the illustration of Mustelavus in Scott and Jepsen (Reference Scott and Jepsen1936, plate XIV) and on the measurements of m1s in Baskin (Reference Baskin, Janis, Scott and Jacobs1998, p. 154; average m1 length = 5.6 mm), this taxon is smaller than the Jones Branch species and it has a more gracile ramus.
The second is Plesictis, originally described from Europe, but apparently known from isolated specimens tentatively referred to that taxon from the late early Arikareean (Ar2) Wounded Knee-Monroe Creek fauna of South Dakota (Macdonald, Reference Macdonald1970; Baskin, Reference Baskin, Janis, Scott and Jacobs1998). The Jones Branch m1 is similar in size to that of Plesictis genettoides Pomel, Reference Pomel1846, the genoholotype from Europe, but the trigonid of the former is relatively longer than in the latter (the metaconid is more posteriorly placed relative to the protoconid in the Jones Branch specimen), the talonid of Plesictis is smaller (shorter and narrower) relative to the trigonid than it is in the Jones Branch specimen, and the notch between the posterior base of the protoconid and the labial surface of the talonid is not as prominent in P. genettoides as in the Jones Branch specimen (Fig. 9.1). It also appears, based on root alveoli, that the m2 in the Jones Branch species would have been distinctly more elongate than in P. genettoides (based on observations of photos of P. genettoides, AMNH 11001).
The third, Promartes, spans late early Arikareean through early Hemingfordian faunas of the Great Plains, and is also known from California (Baskin, Reference Baskin, Janis, Scott and Jacobs1998; Tedford et al., Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004). Promartes is much larger than this Jones Branch species (average length of m1 = 8.3–10.4 mm; Baskin, Reference Baskin, Janis, Scott and Jacobs1998, p. 155), although it may be present as a second mustelid in the fauna. A fourth musteloid-like taxon, the tiny Palaeogale von Meyer, Reference von Meyer1846, also apparently spans this temporal interval. Previously considered a primitive mustelid and/or a taxon closely related to the Viverravidae, Baskin and Tedford (Reference Baskin, Tedford, Prothero and Emry1996, p. 495) noted that it shared no “unambiguous synapomorphies with either the Feliformia or Caniformia.” More recently, however, Famoso and Orcutt (Reference Famoso and Orcutt2022), based on new specimens from the John Day Formation, placed the genus within Feliformia. Palaeogale is smaller than the Jones Branch species and, according to Baskin and Tedford (Reference Baskin, Tedford, Prothero and Emry1996), its m1 lacks a metaconid.
In addition to the presence of Palaeogale in Florida’s Ar2-aged Brooksville 2 LF, Hayes (Reference Hayes2000) described two additional species of musteloids from there, noting a diversity in North America not previously recognized. Only a partial m1 (the trigonid only; UF163703) is known for Acheronictis webbi Hayes, Reference Hayes2000, and it appears to have been a smaller taxon than the Jones Branch species. The second, Arikarictis chapini Hayes, Reference Hayes2000, is only known from an upper carnassial and molars and, therefore, cannot be directly compared with the Jones Branch species. But measurements of the P4 (UF163695; 7.4 mm AP × 4.3 mm TR) suggest that they were similar in size.
Important, however, is a recently described new genus and species from the John Day Formation, Corumictis wolsani Paterson et al., Reference Paterson, Samuels, Rybczynski, Ryan and Madden2019, which spans the early Arikareean and appears to provide the oldest record of Mustelidae in North America (Paterson et al., Reference Paterson, Samuels, Rybczynski, Ryan and Madden2019). Variably referred to Plesictis and to Palaeogale prior to detailed study (J. Samuels, pers. commun., 2018), the specimens include a nearly complete cranium and a couple of jaw fragments broadly distributed across the Turtle Cove and overlying Kimberly members. The dentary fragment with m1 (JODA 396) was collected in unit F, which is bracketed by the Blue Basin Tuff below, dated at 28.8 Ma, and the Picture Gorge Ignimbrite above, at 28.7 ± 0.07 Ma (i.e., Ar1) (Albright et al., Reference Albright, Woodburne, Fremd, Swisher, MacFadden and Scott2008). The cranium (JODA 8167) was recovered from unit L of the Kimberly Member “just above the Tin Roof Tuff” (Paterson et al., Reference Paterson, Samuels, Rybczynski, Ryan and Madden2019) dated to 25.9 ± 0.3 Ma, near the Ar2–Ar3 boundary. Another specimen that includes a partial dentary with m1 (JDBLM14-8a) closely resembles that from unit F, but this specimen was not included in material referred to Corumictis wolsani by Paterson et al. (Reference Paterson, Samuels, Rybczynski, Ryan and Madden2019). This specimen was found even higher in the John Day sequence, in the Balm Creek Member, below which is a tuff dated to 23.9 ± 0.18 Ma (Albright et al., Reference Albright, Woodburne, Fremd, Swisher, MacFadden and Scott2008), considered late Ar3. Although the claim that C. wolsani is apparently North America’s oldest mustelid is supported and well constrained by the dates on the tuffs that bracket its stratigraphic occurrences, Paterson et al. (Reference Paterson, Samuels, Rybczynski, Ryan and Madden2019) apparently overlooked the two slightly younger species from Florida described by Hayes (Reference Hayes2000).
In summary, the m1 from Jones Branch most closely resembles that of C. wolsani, the only notable difference being the slightly smaller size of the latter and the deeper, more robust dentary of the former. With only one specimen known, we refrain from referring the Jones Branch taxon to a specific species at this time.
Promartes Riggs, Reference Riggs1942
Referred specimen
MMNS VP-6613, fragment of proximal left ulna.
Description
The olecranon process of this fragment of ulna is broken and missing, as is the distal portion of the shaft. Most distinctive, however, is the highly angled trochlear notch relative to the shaft (Fig. 9.6), a morphology strongly suggestive of a fossorial taxon (Kilbourne, Reference Kilbourne2017) and seen in Promartes, Zodiolestes, and Megalictis. Based on measurements of the ulnae of extant mustelids (e.g., Meles meles Linnaeus, Reference Linnaeus1758), the Jones Branch specimen would have measured approximately 11 cm long if complete.
Remarks
This element belonged to a species of mustelid much larger than Corumictis. The giant terrestrial mustelid Megalictis ferox Matthew, Reference Matthew1907, known almost exclusively from the late Arikareean ‘Upper Harrison beds’ (= Anderson Ranch Formation) of Nebraska and Wyoming (Hunt and Skolnick, Reference Hunt and Skolnick1996), has a highly angled trochlear notch (Riggs, Reference Riggs1945, fig. 38a, b), but is a much larger species than the one represented by the Jones Branch element. An ulna figured by Hunt and Skolnick (Reference Hunt and Skolnick1996, fig. 8) is approximately 15 cm long. Also different, in the Jones Branch specimen the dorsal and ventral surfaces of the shaft immediately distal to the trochlear notch are parallel; in Megalictis they converge. Zodiolestes daimonelixensis Riggs, Reference Riggs1942, known only from the Harrison Formation, has an ulna estimated to be about 109 mm long, which is similar to that estimated for the Jones Branch specimen. However, a comparison of MMNS VP-6613 with a recent photo of the holotype, FM 12032, does not support referral of the former to Zodiolestes. In the Jones Branch specimen, the lateral margins of the trochlear notch are distinctly concave due to the broader distal portion of the notch relative to the narrower proximal (Fig. 9.6). Also, the distal portion of the notch extends medially beyond the margin of the shaft—features not seen in FM 12032.
More closely resembling the morphology of the Jones Branch element is the holotype ulna, FM 15178, of Promartes olcotti Riggs, Reference Riggs1942. With an overall length of 70 mm (Riggs, Reference Riggs1945), P. olcotti was smaller than the Jones Branch species, and recent photographs of the holotype confirm this. But the highly angled trochlear notch, the relatively shallow nature of this notch, the concave lateral margins, and the medial extension of the distal portion, are similar. An ulna of P. gemmarosae (Loomis, Reference Loomis1932) from the Monroe Creek or Harrison formations in the collections at Amherst College (ACM VP 13133; Macdonald, Reference Macdonald1963), at approximately 90 mm long, is closer in size to the Jones Branch specimen. Although the degree to which the trochlear notch is angled relative to the shaft is hidden because the forelimb is still entombed in its matrix, what can be observed, such as the shallowness of the notch and the prominence of the distal portion of the notch above the shaft (Fig. 9.7, 9.8), is very similar to that of P. olcotti and to the Jones Branch specimen. Hence our referral of MMNS VP-6613 to Promartes.
This single fragment is the only record of a large mustelid in the southeastern United States during the Oligocene. Oligobunis floridanus White, Reference White1947, may have been of similar size, but it is known from the Hemingfordian-aged (Early Miocene) Thomas Farm Fauna, Florida. Whether the Jones Branch specimen represents a new coastal species cannot be confirmed until additional material is recovered, if ever, from the site.
Canidae Fischer von Waldheim, Reference Fischer von Waldheim1817
Borophaginae Simpson, Reference Simpson1945
Phlaocyon Matthew, Reference Matthew1899
Type species
Phlaocyon leucosteus Matthew, Reference Matthew1899.
Phlaocyon taylori Hayes, Reference Hayes2000
Holotype
UF 163524, left m1, from Brooksville 2 LF, late early Arikareean, Hernando County, FL.
Referred specimens
MMNS VP-7444, right m1.
Description
Although the labial surface of the paraconid of the Jones Branch tooth is broken and missing, there appears to have been a basal cingulid (Fig. 9.9). The labial surface of the protoconid, the tallest cusp of the trigonid, lacks any indication of a protostylid. The metaconid is posterior to the protoconid and may have been slightly taller than the paraconid if unworn. At the posterior base of the metaconid is a prominent entoconulid (terminology of Wang et al., Reference Wang, Tedford and Taylor1999, fig. 4; mesoconid of Hayes, Reference Hayes2000, p. 34, 35) with a notch posterior to it separating it from the entoconid. The talonid is as broad as the trigonid with a prominent entoconid and hypoconid. In the current stage of wear, the entoconid is taller than the hypoconid, but they may have been of nearly equal height prior to wear. The opposing surfaces of these two posterior cusps are broadly separated, with a small, but distinct, hypoconulid between them. Between the protoconid and hypoconid, labial to the cristid obliqua, is a short cingulid. The m1 measures 7.40 mm AP × 3.64 mm TR.
Remarks
One of the key features that supports referral of this tooth to a borophagine canid is the basined, bicuspid talonid, with its prominent entoconid and hypoconid. Both of these cusps show apical wear facets, which provide evidence for what Wang et al. (Reference Wang, Tedford and Taylor1999, p. 23) noted was the “first step toward an increased grinding function for the dentition.” This function (due to the enclosed basined talonid), in turn, enabled the early borophagines to avoid competition with hypercarnivorous canids, like the hesperocyonines, by filling the hypocarnivorous small canid niche (Wang et al., Reference Wang, Tedford and Taylor1999).
Another character diagnostic of the tribe Phlaocyonini, into which Wang et al. (Reference Wang, Tedford and Taylor1999) placed Cynarctoides and Phlaocyon, is the presence of a protostylid on m1. It is important to point out, however, that several studies on the various species of these two borophagine groups, including those of Wang et al. (Reference Wang, Tedford and Taylor1999), Wang and Tedford (Reference Wang and Tedford2008), and Hayes (Reference Hayes2000), have found this feature to be variably present. The absence of this character, therefore, from an isolated tooth such as that noted here, does not necessarily preclude its identification as a member of this tribe.
Early Arikareean borophagines include Archaeocyon, Rhizocyon, Oxetocyon, Otarocyon, Cynarctoides, Phlaocyon, Cormocyon, and Desmocyon (Wang et al., Reference Wang, Tedford and Taylor1999). Of the basalmost borophagines, Archaeocyon pavidus (Stock, Reference Stock1933a) is similar in size to the Jones Branch species, and A. leptodus (Schlaikjer, Reference Schlaikjer1935) is slightly larger; but they differ from MMNS VP-7444 in having a more elongate m1 trigonid, crest-like rather than distinctly conical talonid cusps, and a much less prominent entoconulid. The m1 of Rhizocyon oregonensis (Merriam, Reference Merriam1906), which Wang et al. (Reference Wang, Tedford and Taylor1999) noted was similar to A. leptodus, is larger than that from Jones Branch (Wang et al., Reference Wang, Tedford and Taylor1999, appendix 3) with a slightly more elongate trigonid, but is similar in the presence of an entoconulid immediately posterior to the metaconid. Oxetocyon cuspidatus Green, Reference Green1954, is also of similar size to the Jones Branch species, and lower molars assigned to this species by Wang et al. (Reference Wang, Tedford and Taylor1999) show rounded talonid cusps and no protostylid. Their figured specimen, however, differs from the Jones Branch tooth in having a more elongate trigonid and in lacking an entoconulid. Measurements of Otarocyon in Wang et al. (Reference Wang, Tedford and Taylor1999, appendix 3) indicate a smaller animal than the Jones Branch species, and this taxon also lacks the entoconulid between the metaconid and entoconid. The m1s of Cynarctoides lemur (Cope, Reference Cope1879a) and C. luskensis Wang, Tedford, and Taylor, Reference Wang, Tedford and Taylor1999, are also of similar size to the Jones Branch species, although JODA 363 (assigned to the former, J. Samuels, pers. commun., 2024) has a much more prominent metaconid and protostylid. Cynarctoides luskensis is similar in the presence of relatively high-crowned and conical talonid cusps, but there is no indication that it had the prominent entoconulid seen in the Jones Branch tooth. The m1s of Cormocyon haydeni Wang, Tedford, and Taylor, Reference Wang, Tedford and Taylor1999, and C. copei Wang and Tedford, Reference Wang and Tedford1992, are similar in having an entoconulid, but are more elongate than the m1 from Jones Branch (Wang et al., Reference Wang, Tedford and Taylor1999, figs. 38, 41). Desmocyon was a much larger taxon than the Jones Branch species.
Referral of the Jones Branch species to Phlaocyon is based primarily on the very close resemblance of the m1 to that of P. taylori from the late early Arikareean-aged Brooksville 2 LF of Florida and from early Arikareean sites in California (Hayes, Reference Hayes2000; Wang and Tedford, Reference Wang and Tedford2008), to P. latidens (Cope, Reference Cope1881b) from the early Arikareean of Oregon, and to P. minor (Matthew, Reference Matthew1907) from the early to late Arikareean of, primarily, the Great Plains (Wang et al., Reference Wang, Tedford and Taylor1999). Phlaocyon latidens and P. minor average slightly larger than P. taylori (Wang et al., Reference Wang, Tedford and Taylor1999, appendix 3), but the m1s of all have a very similar morphology in the conical talonid cusps, the distinct entoconulid, and the variably expressed to absent protostylid, although Wang et al. (Reference Wang, Tedford and Taylor1999, p. 67) noted that the protostylid on the m1 of P. latidens is “usually well-developed.” The Jones Branch m1 is approximately the same size as the holotype m1 of P. taylori from Florida (UF 163524; 7.0 mm AP × 3.4 mm TR), and resembles it in the straight alignment of the paraconid–protoconid shearing blade and the in presence of a distinct, but small, hypoconulid; features also seen in the California specimens (Wang and Tedford, Reference Wang and Tedford2008, p. 264). Additionally supporting referral of the Jones Branch taxon to P. taylori is the proximity of the Jones Branch site to Florida (and their Gulf Coastal Plain paleoenvironment).
?Phlaocyon sp.
Referred specimen
MMNS VP-6606, left M2.
Description
The most diagnostic feature of this M2 is the antero-posteriorly broader lingual half than labial due to a highly bulbous lingual cingulum. The rounded paracone is the largest cusp and is protected labially and anteriorly by a prominent cingulum at the corner of which is a thickening that would otherwise be the parastyle; but no distinct conule exists in this position (Fig. 9.12). There is a subtle paraconule located anteriorly between the paracone and protocone, the protocone is compressed transversely, and the metacone is slightly elongate transversely. The posteriorly extending ‘arm’ of the protocone terminates at a small, subtle metaconule that is distinctly separated from the metacone and weakly separated from a swelling at the posterolingual corner of the highly robust lingual cingulum where the hypocone would typically be located. This cingulum continues anteriorly to about the point of the paraconule.
Remarks
Measuring 4.79 mm AP × 7.36 mm TR, the M2 from Jones Branch is larger than specimens of P. taylori from Florida and California (3.6–3.8 mm AP × 5.1–5.4 mm TR, n = 4, from Hayes, Reference Hayes2000, and Wang and Tedford, Reference Wang and Tedford2008) and somewhat more similar in size to that of P. achoros (Frailey, Reference Frailey1979) from Florida’s ‘middle’ Arikareean Buda LF (UF171361: 4.1 mm AP × 6.0 mm TR). It also differs from P. taylori and most other early Arikareean borophagines in its broader lingual half as a result of the highly bulbous lingual cingulum. In this feature it somewhat resembles that illustrated for P. minor and P. latidens in Wang et al. (Reference Wang, Tedford and Taylor1999, figs. 25, 26). Importantly, however, Hayes (Reference Hayes2000) and Wang and Tedford (Reference Wang and Tedford2008) noted that P. taylori, P. achoros (Frailey, Reference Frailey1979), and P. multicuspus (Romer and Sutton, Reference Romer and Sutton1927), from the northern Great Plains, all share a unique feature in the upper molars not found in other species of the genus, this being an accessory cuspule next to the metaconule. MMNS VP-6606 appears to have this cuspule, albeit subtle, between the metaconule and metacone, thus supporting referral not only to Phlaocyon, but perhaps to P. achoros. Additional material from Jones Branch will be required to confirm this.
Perissodactyla Owen, Reference Owen1848
Equidae Gray, Reference Gray1821
Miohippus Marsh, Reference Marsh1874
Referred specimens
MMNS VP-6559, right M2; MMNS VP-8358, left M3; MMNS VP-7485, right dentary fragment with p4–m3.
Description
All teeth, upper and lower, are low-crowned and lack cement. MMNS VP-6559 is considered to be an M2 on the basis of the greater angle of the labial surface of the metacone relative to that of the paracone and on its larger size (17.6 mm AP × 19.5 mm TR) than MMNS VP-8358, an M3 (15.4 mm AP × 19.1 mm TR). In addition to its smaller size, MMNS VP-8358 also has a highly angled ectoloph and no interstitial wear on its posterior surface. Both teeth are little worn. They have a prominent anterior cingulum that merges labially with the parastyle, no lingual cingulum, a metaloph with no crochet or plications that does not reach the ectoloph, and a “type 3” hypostyle (Prothero and Shubin, Reference Prothero, Shubin, Prothero and Schoch1989, fig. 10), although Famoso (Reference Famoso2017) concluded that the hypostyle condition is dependent on wear stage, not necessarily taxonomy.
The p4, m1, and m2 are similar in size and in lacking a lingual cingulid, but they have a prominent and continuous anterior, labial, and posterior cingulid. This cingulid begins at the parastylid and ends at the hypoconulid, the latter of which is slightly less prominent than the closely appressed entoconid. The metastylid and metaconid are very closely appressed and merge in early wear. The m3 has a similarly prominent and continuous anterior and labial cingulid, but it does not continue posteriorly beyond the metaconid (i.e., the posterior heel, an apparently expanded hypoconulid, has no cingulid). Respective AP measurements, in mm, for the p4, m1, m2, and m3 are: 13.2, 13.1, 13.15, and 15.33, respectively.
Remarks
In North America during the Oligocene, two equid genera, Mesohippus and Miohippus, are recognized, for which several species have been described. Although work over the last few decades has addressed this over-splitting issue, resulting in synonymy of several species, nearly all workers agree that a detailed taxonomic study of all the species assigned to both genera is greatly needed (e.g., Prothero and Shubin, Reference Prothero, Shubin, Prothero and Schoch1989; MacFadden, Reference MacFadden1992, Reference MacFadden, Janis, Scott and Jacobs1998; Hayes, Reference Hayes2007; Famoso, Reference Famoso2017). According to Prothero and Shubin (Reference Prothero, Shubin, Prothero and Schoch1989; also see Emry et al., Reference Emry, Bjork, Russell and Woodburne1987), Mesohippus spans the Chadronian and Orellan NALMAs and Miohippus occurs primarily in the Whitneyan to early Arikareean, but also includes some species that temporally overlap with Mesohippus. Particularly difficult to distinguish on the basis of teeth alone, Prothero and Shubin (Reference Prothero, Shubin, Prothero and Schoch1989) determined that the most useful feature for distinguishing between the two genera is the articulation between the cuboid and third metatarsal of Miohippus—a feature absent in Mesohippus. Unfortunately, this requires the requisite postcranial material, which is typically rare relative to teeth, and currently unknown from Jones Branch. Given, however, that Mesohippus apparently goes extinct at the end of the Orellan (Emry et al., Reference Emry, Bjork, Russell and Woodburne1987; Prothero and Shubin, Reference Prothero, Shubin, Prothero and Schoch1989), the dental remains of the small, low-crowned horse from Jones Branch are here assigned to Miohippus. We hesitate to assign these remains to a known species pending further review of this widely distributed and currently over-split genus.
Most specimens of Miohippus are known from sites in the Great Plains and from the John Day Formation, Oregon, although specimens are also known from the northern Rocky Mountains region. Exceptionally rare from the Gulf Coastal Plain, teeth of Miohippus have been noted from Florida’s latest Whitneyan I-75 and early Arikareean aged Brooksville 2 and Cow House Slough local faunas (Morgan et al., Reference Morgan, Czaplewski and Simmons2019), and from the Ar3-aged Toledo Bend Fauna of easternmost Texas (Albright, Reference Albright1999).
Tapiridae Gray, Reference Gray1821
Protapirus Filhol, Reference Filhol1877
Type species
Protapirus priscus (Filhol, Reference Filhol1874).
Protapirus simplex Wortman and Earle, Reference Wortman and Earle1893

Figure 10. Perissodactyls from the Jones Branch LF, lower Catahoula Formation, Mississippi: (1–4) Miohippus sp., (1) MMNS VP-6559, right M2, (2) MMNS VP-8358, left M3, (3, 4) MMNS VP-7485, partial right ramus with p4–m3, (3) labial view, (4) occlusal view; (5, 6) Protapirus simplex, (5) MMNS VP-6621, left P3, stereo view, (6) MMNS VP-6599, right ramal fragment with p3, m1–2; (7–11) Diceratherium sp., (7, 8) MMNS VP-7484, incisor (left i1 or i2), (7) lingual view, (8) labial view; (9, 10) MMNS VP-7489, right p2, (9) lingual view, (10) labial view, (11) MMNS VP-6579, right m3 (cast); (12) MMNS VP-6592, left patella. 3-cm scale bar for (3, 4, 6–10).
Reference Wortman and Earle1893 Protapirus simplex Wortman and Earle, p. 168, Fig. 1A.
Reference Marsh1894a Tanyops undens Marsh, p. 348.
Reference Hatcher1896 Protapirus validus Hatcher, p. 162, pl. 2.
Reference Scott1941 Protapirus simplex; Scott, p. 758.
Reference Scott1941 Protapirus obliquidens; Scott, p. 759, pl. 80, fig. 2.
Reference Scott1941 Protapirus validus; Scott, p. 761, pl. 79, fig. 1, 1b, pl. 80, fig. 1.
Reference Schoch1983 Protapirus obliquidens; Schoch, p. 2, fig. 1.
Reference Albright1998b Protapirus simplex; Albright, p. 202, figs. 2, 3.
Holotype
AMNH 660, incomplete right maxilla with P2–4, from the Scenic Member of the Brule Formation, Orellan, South Dakota.
Referred specimens
MMNS VP-6621, left P3; MMNS VP-6318, right M3; MMNS VP-6599, right dentary fragment with p3, m1–2, m3 (m3 originally in crypt, but subsequently removed for measurement); MMNS VP-12249, distal end of ?left Mc II; MMNS VP-12247a, proximal end of left Mc III; MMNS VP-12247b, distal end of left Mc III; MMNS VP-8336, distal end of left Mc V (or right Mt II).
Description
Although the jaw fragment from Jones Branch (MMNS VP-6599) with m1–2 is separated from that with the p3, the two specimens belong to the same individual, with the intervening part of the jaw containing p4 missing. The unerupted m3 is also associated with the same jaw, but it was removed from the crypt. The two upper teeth, however, are from different individuals based on the degree of wear and nature of preservation. The teeth identically match those of other specimens of P. simplex discussed in Albright (Reference Albright1998b) including the non- to submolariform upper premolars, obliqueness of the ectoloph in upper molars, and simple bilophodont morphology of the lower molars; they lack the interloph dental complexities that define Nexuotapirus, so abundant in the Toledo Bend Fauna. Measurements for the teeth are provided in Table 5.
Table 5. Measurements of teeth (in mm) of Protapirus simplex; *ectoloph broken, actual width would have been slightly greater

Remarks
In a review of North American Oligocene tapirs, Albright (Reference Albright1998b) concluded that Protapirus validus Hatcher, Reference Hatcher1896, and Tanyops undens Marsh, 1894a, were junior subjective synonyms of Protapirus simplex Wortman and Earle, Reference Wortman and Earle1893. Protapirus obliquidens Wortman and Earle, Reference Wortman and Earle1893, was tentatively retained as a separate species, due to its slightly larger size and more derived upper premolar morphology, but Albright (Reference Albright1998b) left open the possibility that it too might eventually be found to represent P. simplex, primarily because of its recovery from the same beds as nearly all the specimens referred to P. simplex (i.e., the “Protoceras beds” of South Dakota—the Whitneyan-aged Poleside Member of the Brule Formation). Along these lines, Albright (Reference Albright1998b) concluded that a specimen (SDSM 2829) originally referred to P. obliquidens by Scott (Reference Scott1941) was more representative of P. simplex, as was the type specimen of Marsh’s (Reference Marsh1894a) Tanyops undens, which Schoch (Reference Schoch1983) had synonymized with P. obliquidens. Over 20 years after Albright (Reference Albright1998b), this seems the most parsimonious solution to the ‘P. obliquidens’ problem, given that different species of tapir are rarely found together in the same stratigraphic horizon (especially in the same geographic area) and given that premolar variation is known intra-specifically in Protapirus.
As noted above, Protapirus is known primarily from the Whitneyan-aged Poleside Member of the Brule Formation. Prothero and Emry (Reference Prothero, Emry and Woodburne2004) listed the last occurrence of this taxon, among others, as marking the end of the Whitneyan. Albright (Reference Albright1998b), however, noted two specimens found in Arikareean-aged deposits: one from the earliest Arikareean Brown Siltstone Beds of Wildcat Ridge, Nebraska, which led Tedford et al. (Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004) to extend its last occurrence into the early early Arikareean, and the other from “the type Rosebud Fm. of Gidley” (Albright, Reference Albright1998b, p. 203). The other North American species of tapir referred to Protapirus, P. robustus Sinclair, Reference Sinclair1901, from the upper John Day Formation (Haystack Valley Member), was removed from that genus and placed into Nexuotapirus by Albright (Reference Albright1998b). The presence of Protapirus simplex in the Jones Branch LF represents the first record of this species outside the Great Plains region, thus providing further documentation of the faunal link between the Midcontinent and the Gulf Coastal Plain during the early Arikareean.
Rhinocerotidae Owen, Reference Owen1845
Diceratherium Marsh, Reference Marsh1875
Description
Teeth of Diceratherium have been extensively described in the literature (e.g., Peterson, Reference Peterson1920), thereby precluding detailed description here. Briefly, however, the crown of the incisor is small and cap-like, smooth labially, with a V-shaped lingual cingulum. The relatively straight root is over 5× the length of the crown (Fig. 10.7, 10.8). The p2 has a posterolingual invagination between the entoconid and hypoconulid and another recess labially between the protoconid and hypoconid. A prominent basal cingulum completely encircles the crown (Fig. 10.9, 10.10). The m1 and m3 have the typical rhinoceros lower molar pattern, although the cingulum of the m1 extends only from the base of the metacone around the anterior surface of the tooth to the anterolabial corner. That of the m3 is essentially continuous around the entire base of the crown, although it is briefly interrupted at the posterolingual corner (Fig. 10.11). Measurements are provided in Table 6.
Table 6. Measurements of teeth (in mm) of Diceratherium sp.

Referred specimens
MMNS VP-7484, incisor (left i1 or i2); MMNS VP-6616, left p2; MMNS VP-7489, right p2; MMNS VP-6600, left m1; MMNS VP-6579, right m3 (cast); MMNS VP-4575, fragment of left distal femur; MMNS VP-6592, left patella; MMNS VP-8332, proximal end right Mt III; MMNS VP-6934, left Mt IV (cast); MMNS VP-7562, lateral medial phalanx.
Remarks
Although Subhyracodon, the common diceratheriine rhinoceros of the Chadronian and Orellen, and Diceratherium, which ranges from the Whitneyan to Hemingfordian, somewhat overlap in time, the only species of the former that apparently survived into the Arikareean was Subhyracodon kewi Stock, Reference Stock1933b. As Prothero noted, however (Reference Prothero2005, p. 48), S. kewi is known exclusively from the early Arikareean of California and apparently remained there as a primitive endemic “long after its relatives had been replaced by Diceratherium” in the High Plains and Oregon. The only records of Subhyracodon from east of the Mississippi River include (1) a nearly complete jaw from the Byram Formation in Mississippi that Manning (Reference Manning1997) considered Orellen in age and (2) a palate with premolars and molars from the upper Eocene (late Chadronian) Harleyville Formation, South Carolina, referred to S. mitis (Cope, Reference Cope1875) by Albright et al. (Reference Albright, Sanders, Weems, Cicimurri and Knight2019).
The large size of some of the elements from Jones Branch (particularly the femur fragment, MMNS VP-4575, and the m3, MMNS VP-6579) supports referral of that species to Diceratherium rather than Subhyracodon. For example, Prothero (Reference Prothero2005, table 4.3) provided measurements for Subhyracodon mitis and S. occidentalis Leidy, Reference Leidy1850a, showing an average length and width for the m3 of 27.5 mm and 19 mm, respectively. The m3 from Jones Branch measures nearly 48 mm by 29 mm (Table 6), intermediate in size between that of D. armatum and D. annectens. The p2 and m1 from Jones Branch are also similar in size to Diceratherium (Prothero, Reference Prothero2005, table 4.4). Additionally, the morphology of the patella from Jones Branch (Fig. 10.12) identically matches the same element illustrated for Diceratherium by Prothero (Reference Prothero2005, fig. 5.30).
Artiodactyla Owen, Reference Owen1848
Leptochoeridae Marsh, Reference Marsh1894b
Leptochoerus Leidy, Reference Leidy1856
Type species
Leptochoerus spectabilis Leidy, Reference Leidy1856.
Leptochoerus sp.

Figure 11. Artiodactyla from the Jones Branch LF, lower Catahoula Formation, Mississippi: (1) Leptochoerus sp., MMNS VP-6944, right M1; (2–6) ?Daeodon sp., (2, 3) MMNS VP-8335, right i2, (2) labial view, (3) lingual view, (4) MMNS VP-7483, fragment of right P1 or P2, (5, 6) MMNS VP-6876, right patella, (5) dorsal view, (6) ventral view; (7–12) Elomeryx sp., (7) MMNS VP-6457, left M2, (8–10) MMNS VP-6605, partial right dentary with p4–m2, (8) labial view, (9) lingual view, (10) occlusal view, (11, 12) MMNS VP-6596, right m3, (11) occlusal view; (12) labial view; (13) Prosynthetoceras orthrionanus, MMNS VP-7488, left M3; (14, 15) Hypertragulus minutus, (14) MMNS VP-6614, right M3; (15) MMNS VP-7490, right dentary fragment with root of p3, p4–m2; (16, 17) ?Leptomeryx sp., (16) MMNS VP-8338, right M3; (17) MMNS VP-7487, left m3. Vertical scale bar for (8–10); 1-cm scale bar for (13–17).
Referred specimen
MMNS VP-6944, right M1.
Description
The M1 from Jones Branch (MMNS VP-6944), at 4.1 mm AP × 5.5 mm TR, is smaller and less transversely elongate than all four currently recognized species: the type species, Leptochoerus spectabilis Leidy, Reference Leidy1856; L. elegans (Macdonald, Reference Macdonald1955); L. supremus Macdonald, Reference Macdonald1955; and L. emilyae Edwards, Reference Edwards1976. Like teeth referred to Leptochoerus by Macdonald (Reference Macdonald1955) and Edwards (Reference Edwards1976), the paracone is slightly larger than the metacone and it extends farther lingually (as seen in Macdonald, Reference Macdonald1955, fig. 2). A tiny cusp at the posterior base of the paracone may be considered the mesostyle, but it does not block the central valley between the paracone and metacone. Extending from the anterior surface of the paracone to the anterior cingulum is a small transversely compressed cristid, and a similar structure extends off the posterior surface of the metacone to the posterior cingulum. A matching cristid extends off the anterior surface of the metacone toward the mesostyle, but it is not connected and therefore does not block the central valley. The paraconule is slightly smaller than the metaconule and both are entirely separated from the paracone and metacone, respectively. They are also slightly farther apart than the paracone and metacone. At the degree of wear seen in the Jones Branch tooth, the paraconule is just beginning to merge with an anterolabial extension (preprotocrista?) of the protocone, whereas the metaconule remains well separated from the protocone (Fig. 11.1). The protocone is the largest of the three principal cusps and includes the anterolabial extension noted above, as well as a posterior extension that Macdonald (Reference Macdonald1955) referred to as the hypoconal spur. All but the lingual surface of the tooth is surrounded by a prominent cingulum. On the anterior surface, it is shelf-like and extends to a point even with the lingual border of the protocone. On the posterior surface it extends lingually to a point where it abuts the hypoconal spur.
Remarks
Edwards (Reference Edwards1976), followed by Stucky (Reference Stucky, Janis, Scott and Jacobs1998), recognized four species of Leptochoerus as valid: the early Chadronian to middle Whitneyan L. elegans, the early to late Orellan L. emilyae, the early Orellan to late Whitneyan L. spectabilis, and the late Whitneyan L. supremus. Based on measurements in Macdonald (Reference Macdonald1955) and Edwards (Reference Edwards1976), all of these species are larger than that from Jones Branch, particularly L. supremus, which is the largest, but from which only lower teeth are known. Of the two recognized genera of leptochoerids with ranges that extend into the Oligocene, Stibarus and Leptochoerus, referral of the Jones Branch upper molar to the latter is based on its rounded, or blunt, rather than sharp lingual apex, resulting in a “less triangular appearance” than those of Stibarus (Edwards, Reference Edwards1976, p. 100).
Although both genera are known from the Chadronian through Whitneyan, until recently it was thought that only Leptochoerus ranged into the early Arikareean (i.e., the Wounded Knee-Sharps fauna of southwestern South Dakota) (Macdonald, Reference Macdonald1957, Reference Macdonald1963, Reference Macdonald1970; Stucky, Reference Stucky, Janis, Scott and Jacobs1998; Tedford et al., Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004). Samuels et al. (Reference Samuels, MacKenzie and Fremd2013), however, reported leptochoerid material from both Whitneyan and early Arikareean levels of the Turtle Cove Member, John Day Formation, Oregon, which was also the first record of leptochoerids west of the Rocky Mountains. The Arikareean specimen from the John Day, a single upper molar, was provisionally referred to Stibarus quadricuspus (Hatcher, Reference Hatcher1901), thus providing the first record of that genus in this NALMA. It was recovered from unit D, bracketed between the Blue Basin tuff above, at approximately 28.8 Ma, and the AB tuff below at about 29.75 Ma, thus Ar1 in age (Albright et al., Reference Albright, Woodburne, Fremd, Swisher, MacFadden and Scott2008; Samuels et al., Reference Samuels, MacKenzie and Fremd2013). The additional cranial and post-cranial material provisionally referred by Samuels et al. (Reference Samuels, MacKenzie and Fremd2013) to Leptochoerus elegans was recovered from unit A, below the AB tuff, of Whitneyan age.
An M1 from Whitneyan-aged strata of the John Day Formation referred provisionally to Leptochoerus elegans (JODA 15850) is also larger (data from poster associated with Samuels et al., Reference Samuels, MacKenzie and Fremd2013), with additional differences including the following: (1) the paracone of the Jones Branch tooth is more transversely elongate than in the John Day tooth; (2) the paraconule of the Jones Branch tooth is more anteroposteriorly compressed (i.e., less conical) than in the John Day tooth, and (3) the Jones Branch tooth is broader anteroposteriorly, both labially and lingually, than the John Day tooth (i.e., the paracone and metacone are relatively farther apart and the hypoconal spur of the protocone extends farther posteriorly). This feature also appears to differentiate the Jones Branch species from L. spectabilis, L. emilyae, and L. elegans, as TR/AP ratios calculated from measurements of those species in Macdonald (Reference Macdonald1955), Edwards (Reference Edwards1976), and Samuels et al. (Reference Samuels, MacKenzie and Fremd2013) provide a range from 1.4 to nearly 1.6, versus 1.28 for MMNS VP-6944 (i.e., the Jones Branch tooth is less transversely elongated than the other species of Leptochoerus).
The Jones Branch specimen of Leptochoerus provides another very rare Arikareean occurrence of the already rare Leptochoeridae, the only record of Leptochoerus outside the central and northern Great Plains and Pacific Northwest (aside from a rare exception from Mexico), and the only record of a leptochoerid east of the Mississippi River. Prior to discovery of the tooth from Jones Branch, the only known leptochoerid material from anywhere near the Gulf Coastal Plain was that of Laredochoerus edwardsi Westgate, Reference Westgate1994, from the late Uintan-aged Casa Blanca LF near Laredo, Texas—a taxon from which Westgate (Reference Westgate1994) hypothesized Leptochoerus may have derived. It is important to note, however, the report of Leptochoerus sp. from the Iniyoo LF, Oaxaca state, southern Mexico (Jiménez-Hidalgo et al., Reference Jiménez-Hidalgo, Smith, Guerrero-Arenas and Alvarado-Ortega2015), originally considered Chadronian in age, but later determined to be earliest Arikareean (Jiménez-Hidalgo et al., Reference Jiménez-Hidalgo, Lander, Israde-Alcántara, Rodríguez-Caballero and Guerrero-Arenas2021).
Despite a difference in size and subtle differences in morphology between the Jones Branch species and those previously described, its Arikareean age, and the seemingly anomalous occurrence along the Gulf Coast in a paleoenvironment that would have been much different from that where the other species are found, we defer naming a new species until a comprehensive comparison of the Jones Branch tooth can be conducted with the other species and/or additional material is recovered allowing further characterization.
Entelodontidae Lydekker, Reference Lydekker1883
Daeodon Cope, Reference Cope1878
Referred specimens
MMNS VP-8335, right i2; MMNS VP-7483, fragment of right P1 or P2; MMNS VP-7053, fragment of distal sternum element?; MMNS VP-6876, right patella.
Description
The incisor (MMNS VP-8335) is a large, robust tooth with a stout root; the crown of the tooth measures about 23.3 mm AP × 17.5 mm TR. These measurements are closest to those of the i2 for Daeodon hollandi (Peterson, Reference Peterson1905b) in Peterson (Reference Peterson1909, p. 83). The crown consists of very thick enamel and is well worn from either occlusion with the opposing upper incisor or from processing coarse food, or both (Fig. 11.2, 11.3). The other tooth fragment (MMNS VP-7483) most closely resembles the posterior portion of an upper premolar 1 or 2, with its thick enamel, prominent ridge extending from the base of the crown toward the apex, and the pustulose texturing along the posterolabial region (Fig. 11.4). Its size, based on comparisons in Peterson (Reference Peterson1909, p. 80), suggests that it is a P2. The patella (MMNS VP-6876) very closely resembles in size and morphology that illustrated for D. hollandi by Peterson (Reference Peterson1909, p. 130) and to specimens of this species from Agate Fossil Beds National Monument, Nebraska, in the collections at the UNSM (e.g., UNSM 1150). It measures 96 mm long × 65 mm wide (Fig. 11.5, 11.6).
Remarks
The giant entelodont Daeodon hollandi (Peterson, Reference Peterson1905a, Reference Petersonb), known primarily from late Arikareean levels of the John Day Formation, Oregon, (Foss, Reference Foss2001; Albright et al., Reference Albright, Woodburne, Fremd, Swisher, MacFadden and Scott2008) and the similarly aged Agate quarries of northwestern Nebraska (Peterson, Reference Peterson1909), has also been found in South Dakota, Wyoming, Montana, and along the Gulf and Atlantic coastal plains, including Texas, Alabama, Florida, South Carolina, and New Jersey (Marsh, Reference Marsh1893; Allen, Reference Allen1926; Simpson, Reference Simpson1930; Wilson, Reference Wilson1957; Westgate, Reference Westgate1992, Reference Westgate1993; Albright, Reference Albright1999; Foss, Reference Foss2001; Calede, Reference Calede2020).
In addition to Daeodon hollandi, which is primarily a late Arikareean taxon (although Effinger, Reference Effinger, Janis, Scott and Jacobs1998, noted the first appearance of Daeodon in the early Arikareean), there were at least two other large entelodonts that apparently last occurred in the early Arikareean. One of these, Archaeotherium trippensis Skinner, Skinner, and Gooris, Reference Skinner, Skinner and Gooris1968, was originally described from the late early Arikareean Wewela Fauna, South Dakota, in sediments considered equivalent to the Monroe Creek Formation. Whereas the type specimen of A. trippensis is that of a juvenile, another specimen, UNSM 1098, is thought to represent an adult of that species (R. Hunt, personal communication, July 2022), and includes a partial cranium (primarily the rostrum) with the upper dentition and a partial left ramus with p3–m3. This specimen was recovered from strata in Morrill County, Nebraska, also considered equivalent in age to the Monroe Creek Formation. Although considerably larger than those species of Archaeotherium from the stratigraphically lower White River Group, UNSM 1098 is still smaller and much less robust than the massive adult Daeodon hollandi.
The other, Archaeotherium calkinsi (Sinclair, Reference Sinclair1905), is known only from early Arikareean levels in the John Day Formation. A third large species, A. caninus (Troxell, Reference Troxell1920), is also known exclusively from the John Day Formation, although Foss (Reference Foss2001) implied that the two may represent a single species. It is possible, therefore, that the entelodont material from Jones Branch could represent one of the large Arikareean species of Archaeotherium rather than Daeodon; hence the “?” in the systematic heading. But it is the close similarity of the teeth and patella from Jones Branch to the latter taxon in both size and morphology that results in our tentative referral of this material to Daeodon.
Detailed discussions regarding which generic name should be applied to North America’s largest Arikareean–Hemingfordian entelodont, Daeodon Cope, Reference Cope1878, or Dinohyus Peterson, Reference Peterson1905b, can be found in Foss and Fremd (Reference Foss and Fremd1998), Lucas et al. (Reference Lucas, Emry and Foss1998), Albright (Reference Albright1999), and Foss (Reference Foss, Prothero and Foss2007).
Anthracotheriidae Leidy, Reference Leidy1869
Bothriodontinae Scott, Reference Scott1940
Elomeryx Marsh, Reference Marsh1894c
Referred specimens
MMNS VP-6459, incisor (?right I2); MMNS VP-6528, incisor; MMNS VP-7051, incisor (?left i2); MMNS VP-7571, incisor (?right i3); MMNS VP-7654, canine fragment; MMNS VP-6457, left M2; MMNS VP-7569, right p2; MMNS VP-6458, left p2 or p3; MMNS VP-6454, left m2; MMNS VP-6456, right partial m2; MMNS VP-6596, right m3; MMNS VP-6455, left partial m3; MMNS VP-6605, right dentary fragment with p4–m2; MMNS VP-4576, axis vertebra; MMNS VP-6631, left partial radius; MMNS VP-6512, right proximal ulna; MMNS VP-7665, right pisiform; MMNS VP-6873, left navicular; MMNS VP-8337, ?right Mc I; MMNS VP-6572, proximal left Mc IV; MMNS VP-7563, pes medial phalanx, digit 3 or 4; MMNS VP-6630, left partial tibia; MMNS VP-6461, right calcaneum; MMNS VP-7545, left Mt III.
Remarks
Although the single upper molar (MMNS VP-6457) thus far recovered from Jones Branch is somewhat worn (Fig. 11.7), the presence of a protoconule is still evident, therefore precluding referral to the last occurring North American anthracothere, Arretotherium Douglass, Reference Douglass1902, in which the protoconule is lost. As Macdonald (Reference Macdonald1956, table 1) noted for Aepinacodon Troxell, Reference Troxell1921, Elomeryx, and Arretotherium, the mesostyle of the Jones Branch tooth is “invaded” by the transverse valley (although highly variable in Elomeryx). Measurements of teeth and selected postcranial material are provided in Table 7.
Table 7. Measurements of specimens (in mm) of Elomeryx sp.

In addition to the presence of the protoconule, referral to Elomeryx is also supported by its age, as Aepinacodon americanus (Leidy, Reference Leidy1856) is apparently confined to the Chadronian, whereas Elomeryx spans the Orellan through the early early Arikareean (Kron and Manning, Reference Kron, Manning, Janis, Scott and Jacobs1998). Elomeryx is best known from the Whitneyan-aged Poleside and Whitney members of the Brule Formation of South Dakota, but also occurs as a relic of the White River Chronofauna in the earliest Arikareean Gering Formation of Nebraska, the Kew Quarry LF from the Sespe Formation, California, and the Ar2-aged Kealey Springs LF, Saskatchewan (Storer, Reference Storer, Prothero and Emry1996; Tedford et al., Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004). The first report of substantial anthracothere material from the Gulf Coastal Plain was by Albright (Reference Albright1999) on the last North American member of the group, Arretotherium, from the early late Arikareean (Ar3) Toledo Bend LF in which it was one of the most commonly occurring mammals. This material was assigned to Arretotherium acridens Douglass, Reference Douglass1902. Thus, the presence of Elomeryx at Jones Branch is less surprising than might otherwise be the case.
As in most anthracotheres, the tooth shows rugosely textured enamel and shelf-like anterior and posterior cingula. However, MMNS VP-6457 differs from upper molars of Elomeryx armatus (Marsh, Reference Marsh1894d) in its absence of the lingual extension of the anterior cingulum around the protocone and the complete ‘invasion’ of the mesostyle (Fig. 11.7). The lingual extension of the anterior cingulum around the protocone is expressed prominently in Marsh’s (Reference Marsh1894c) figures 4 and 5 of E. armatus, in Marsh’s (Reference Marsh1894d) figure 2 in his initial description of “Heptacodon” armatus, and in Lambe’s (Reference Lambe1908) plate II, figure 1 for “Ancodus brachyrhyncus (Osborne and Wortman, Reference Osborn and Wortman1894)” considered a synonym of E. armatus by Macdonald (Reference Macdonald1956). It is also highly prominent in the type specimen (LACM 9515) of E. garbanii Macdonald (Reference Macdonald1970). The Jones Branch tooth lacks this morphology, instead having a short cingular segment at the lingual entrance to the deep transverse valley. In this feature it closely resembles upper molars of Arretotherium, although the latter lacks a protoconule.
It is important to note that another anthracothere tooth (discussed in more detail in a following section on fossils from Mississippi that are not part of the Jones Branch LF) was recovered from the underlying Byram Formation at a level thought to be late Orellan to early Whitneyan in age (Fig. 4). It is mentioned here, however, because its morphology much more closely matches Elomeryx armatus than does the Jones Branch tooth in that it has the prominent lingual extension of the anterior cingulum around the protocone. In fact, the Byram tooth is almost identical to that figured by Lambe (Reference Lambe1908), leaving little doubt as to its identity. With the close resemblance of the Jones Branch tooth to Arretotherium except for the presence of the protoconule, perhaps it represents a transitional species derived relative to E. armatus, but not yet having lost the protoconule that characterizes Arretotherium; hence referral of the Jones Branch material to Elomeryx sp. at this time rather than E. armatus.
Additionally noteworthy regarding the presence of anthracotheres along the Gulf Coastal Plain is the mention by Albright (Reference Albright1999, p. 56) of a personal communication with, and unpublished manuscript by, C. D. Frailey who reported the presence of the even older taxon, Bothriodon Aymard, Reference Aymard1846, from a site in Pasco County, Florida. This material is in the collections at the FLMNH, and, although still unpublished, has been examined by the first author with the conclusion that the Florida material indeed represents a different, much smaller species than that referred to Elomeryx at Jones Branch. The presence in the Gulf Coastal Plain of at least three, possibly four, temporally successive forms of anthracothere, as with other taxa, further supports the existence of a corridor of dispersal between the Great Plains, which were becoming more arid as the Oligocene progressed, and the Gulf Coastal Plain, which maintained a subtropical to warm temperate environmental setting—that corridor most likely existing in the form of broad riparian environments that followed major river systems from the Midcontinent to the Gulf Coast.
Protoceratidae Marsh, Reference Marsh1891
Synthetoceratinae Frick, Reference Frick1937
Prosynthetoceras Frick, Reference Frick1937
Type species
Prosynthetoceras francisi Frick, Reference Frick1937.
Prosynthetoceras orthrionanus Albright, Reference Albright1999
Reference Wood and Wood1937 Blastomeryx texanus Wood and Wood, p. 137, pl. 1, figs. 5, 6.
Reference Albright1991 Protoceras neatodelpha; Albright, p. 232.
Reference Albright, Terry, LaGarry and Hunt1998a ?Protoceras sp. Albright, table 2, p. 176.
Reference Albright1999 Prosynthetoceras orthrionanus Albright, p. 60.
Holotype
LSUMG V-2619, right orbital horn tip, Toledo Bend LF, early late Arikareean, Newton County, Texas.
Referred specimens
MMNS VP-6946, right dP3; MMNS VP-8241, left P4; MMNS VP-6645, left M1 or 2; MMNS VP-9128, right M2; MMNS VP-7488, left M3; MMNS VP-7573, left p2; MMNS VP-6646, left m1; MMNS VP-6648, right m2; MMNS VP-6647, right m3; MMNS VP-8369, ?right scaphoid; MMNS VP-6936, right Mc IV; MMNS VP-6597, partial right tibia; MMNS VP-6511, left calcaneum; MMNS VP-6942, left astragalus; MMNS VP-7570, left astragalus; MMNS VP-8238, right ?ecto-mesocuneiform; MMNS VP-6937, right Mt IV.
Description
Albright (Reference Albright1999) thoroughly described the abundant material from the Toledo Bend Fauna, Texas, and compared this species with the similar-sized Protoceras and Paratoceras, thus precluding further detailed description of the specimens from Jones Branch. Table 8 provides measurements of the teeth from Jones Branch.
Table 8. Measurements of teeth (in mm) of Prosynthetoceras orthrionanus

Remarks
The oldest and smallest species of Prosynthetoceras, P. orthrionanus Albright, Reference Albright1999, was originally described from Toledo Bend. It is by far the most abundant taxon from that locality, accounting for nearly 22% of the total number of mammalian remains recovered, 40.7% of all artiodactyl remains recovered, with a relative abundance of 59.3% based on the minimum number of each artiodactyl taxon relative to the minimum number of artiodactyls in the fauna (Albright, Reference Albright1991, p. 256–260). Similar in size to Protoceras from the Whitneyan to Arikareean of the Great Plains (Patton and Taylor, Reference Patton and Taylor1973) and to Paratoceras from the late Arikareean of southern Mexico (Webb et al., Reference Webb, Beatty and Poinar2003), from the late Arikareean and early Hemingfordian of Panama (MacFadden et al., Reference MacFadden, Bloch, Evans, Foster, Morgan, Rincon and Wood2014; Rincon et al., Reference Rincon, Bloch, MacFadden and Jaramillo2015), and from the early Barstovian through Clarendonian of Texas (Patton and Taylor, Reference Patton and Taylor1973), it is to Protoceras that Albright (Reference Albright1991) originally referred the Toledo Bend material. However, with the recovery of additional material, particularly that of the cranial armature (horns), Albright (Reference Albright1999) concluded that the material was representative of a smaller, older species of Prosynthetoceras—a genus previously known from the Texas Coastal Plain through a succession of species, which include the Hemingfordian through Barstovian P. texanus (Wood and Wood, Reference Wood1937) and P. francisi, the Clarendonian and Hemphillian Synthetoceras tricornatus (Patton and Taylor, Reference Patton and Taylor1971), eventually culminating in the latest Hemphillian Kyptoceras amatorum Webb, Reference Webb1981, all attaining larger size through time.
Although horns have yet to be recovered from Jones Branch, the size (Table 8) and morphology of the teeth and other elements recovered matches that of Prosynthetoceras orthrionanus (Albright, Reference Albright1999, table 10). The thorough description of the various elements recovered from Toledo Bend precludes further description of the Jones Branch material. The Jones Branch record does, however, provide a temporal range extension of the genus down into the early Arikareean from the early late Arikareean, and further support for the southern North American affinities and distribution of this genus.
MMNS VP-6936, a small right Mc IV, resembles that of a protoceratid on the basis of its size (79 mm), its non-fusion to Mc III, and on the presence of a scar indicating articulation with a Mc V. However, referral of this element to Leptomeryx cannot be excluded because that taxon also has unfused Mc II–V. On the other hand, MMNS VP-6937, a small Mt IV (72 mm long), is considered to be that of a protoceratid because there is no indication that it was fused to Mt III; in Leptomeryx, Mt III and IV are co-ossified into a cannon bone. MMNS VP-6937 also has a scar indicating articulation with a Mt V. Both of these elements are much larger than metapodials of Hypertragulus. MMNS VP-8708, a slightly larger Mt III (85 mm long) is also not fused to Mt IV and therefore not referrable to Leptomeryx. However, it does not appear to belong to a protoceratid either because it shows no scar or facet for articulation with Mt II. Therefore, MMNS VP-8708 indicates the possibility of another small artiodactyl at Jones Branch that can be assigned to neither Prosynthetoceras, Hypertragulus, nor Leptomeryx.
Hypertragulidae Cope, Reference Cope1879b
Hypertragulus Cope, Reference Cope1874
Type species
Hypertragulus calcaratus (Cope, Reference Cope1873).
Hypertragulus minutus Lull, Reference Lull1922
Holotype
YPM 10545, “fragments of upper and lower jaws and teeth” (Lull, Reference Lull1922, p. 115) from the Arikareean-aged John Day Formation, Oregon.
Referred specimens
MMNS VP-6614, right M3; MMNS VP-6794, left m2; MMNS VP-7490, right dentary fragment with root of p3, p4–m2; MMNS VP-6460, left astragalus.
Description
M3 has an anterior cingulum, a prominent style between the protocone and hypocone, and, diagnostic of Hypertragulus, no mesostyle; but the labial surface of the paracone and metacone is prominently ribbed. The most characteristic feature of this tooth is a distinctly prominent metastyle ‘heel’ projecting posteriorly from the metacone (Fig. 11.14). On the lower molars, the labial surfaces of the protoconid and hypoconid are relatively sharp (i.e., V-shaped), with a small stylid between them (Fig. 11.15). They have an anterior and posterior cingular segment with the anterior a bit more prominent, and they have no ribs ascending the lingual surface of the metaconid and entoconid. Table 9 provides measurements of the teeth. In addition, the ramus measures 7.4 mm deep below the m2. The astragalus (MMNS VP-6460) measures 13.7 mm long × 7.1 mm wide, and it has the “bent” morphology that Webb (Reference Webb, Janis, Scott and Jacobs1998, p. 464) noted is diagnostic of the Hypertragulidae.
Table 9. Measurements of teeth (in mm) of Hypertragulus minutus

Remarks
Webb (Reference Webb, Janis, Scott and Jacobs1998) considered four species of Hypertragulus valid: the type species, H. calcaratus (Cope, Reference Cope1873), from the Great Plains (see Scott, Reference Scott1940); H. hesperius Hay, Reference Hay1902, and H. minutus Lull, Reference Lull1922, from the John Day Formation; and H. heikeni Ferrusquía-Villafranca, Reference Ferrusquía-Villafranca1969, from the early Chadronian Rancho Gaitan LF of northernmost Mexico. Ferrusquía-Villafranca (Reference Ferrusquía-Villafranca1969, p. 131) considered H. heikeni the most primitive species and “an excellent geologic and morphologic bridge between the latest Eocene Simimeryx and the Orellan–Arikareean Hypertragulus.” Unlike H. heikeni and H. calcaratus, however, both of which are adequately described, H. hesperius and H. minutus are very poorly described. As Lull (Reference Lull1922) noted, Hay (Reference Hay1902) gave no definition of H. hesperius, nor did he designate a type specimen or note the level from which the material was collected. Yet Lull (Reference Lull1922) did little better in his ‘description’ of H. minutus, also from the John Day beds, reproduced in its entirety as follows: “A very small form, apparently Hypertragulus. Distinguishable from H. hesperius mainly by its small size. Cingula well developed, but metastyle of M3 much reduced, not forming the conspicuous ‘heel’ of hesperius.” He followed this with minimal comparative measurements of teeth of H. minutus and H. hesperius, but he did designate a type specimen, YPM 10545, consisting of “fragments of upper and lower jaws and teeth” (Lull, Reference Lull1922, p. 115). Although H. hesperius had not yet been named when Cope (Reference Cope1873, Reference Cope1874) described H. calcaratus, he later noted no difference between the Great Plains and John Day forms except for a larger size of the latter in some cases (Cope, Reference Cope1884). Thus, the validity of H. hesperius remains equivocal pending a thorough review of, at least, these two species.
In a much more recent study, Jewell (Reference Jewell2019) statistically analyzed the known hypertragulid specimens from the John Day Formation in order to either support or refute the presence there of more than one species. As noted above, the John Day species include H. minutus and H. hesperius, but Jewell (Reference Jewell2019) also included Nannotragulus (= Allomeryx) planiceps (Sinclair, Reference Sinclair1905) in her analysis. She concluded that only one species is present in the John Day. However, Jewell (Reference Jewell2019) did not address the question of which species is taxonomically valid. Although Hay named H. hesperius in 1902, suggesting that it should have priority at least among the three John Day ‘species,’ the absence of a description or designation of a type specimen supports H. minutus as the valid single John Day taxon, particularly considering that a type specimen was designated and that there is at least somewhat of a description; hence, our referral of the Jones Branch species to H. minutus. On the other hand, as noted above, future study may determine that H. calcaratus may be the single valid taxon (excluding H. heikeni).
The teeth from Jones Branch are distinctly small and nearly identical to those of H. minutus, based on comparisons with JODA 1168. The John Day specimen was collected from Unit F of the Turtle Cove Member (N. Famoso, personal communication, 2018), which is sandwiched between the Blue Basin Tuff below and the Picture Gorge Ignimbrite above. These volcanic horizons have respective dates of 28.8 Ma and 28.7 ± 0.07 Ma, thus placing the specimen in the early early Arikareean (Albright et al., Reference Albright, Woodburne, Fremd, Swisher, MacFadden and Scott2008). As noted above, Lull (Reference Lull1922) described the metastyle of M3 in H. minutus as much reduced relative to the “conspicuous ‘heel’ of [H.] hesperius.” This reduced condition differs from the prominent metastyle of the Jones Branch specimen, although expression of the metastyle is likely a variable feature.
The Jones Branch occurrence of H. minutus, previously known only from the John Day region of Oregon, represents a geographic range extension of nearly 3000 km. Although the environmental setting during the early Arikareean of the Pacific Northwest would be expected to have been much different than that of the Gulf Coastal Plain, the similarity of the specimens from both regions precludes any reason to assign the Mississippi material to a new species until additional specimens are recovered that might alter this conclusion. Badly needed is a review of all hypertragulids.
As this manuscript was in review, Famoso and Jewell (Reference Famoso and Jewell2024) published an update of Jewell’s Reference Jewell2019 honor’s thesis work. In that publication, Famoso and Jewell (Reference Famoso and Jewell2024) recommended that the John Day specimens all be assigned to H. hesperius, given its first mention by Hay in 1902; they also suggested a lectotype, AMNH 7918. But they, too, suggested that a more in-depth review of all hypertragulids was needed before formally synonymizing the John Day specimens as one species. Until such a review is conducted, we maintain the view that, as currently understood, H. minutus is a valid taxon to which we assign the Jones Branch species.
Leptomerycidae Zittel, Reference Zittel1893
Leptomeryx Leidy, Reference Leidy1853
Referred specimens
MMNS VP-8338, right M3; MMNS VP-7487, left m3.
Description
The M3 is slightly wider transversely than it is long anteroposteriorly (11.62 mm AP × 13.16 mm TR) and it has crenulated enamel. The paracone and metacone are ribbed and the parastyle and metastyle are prominent, particularly the latter. There is a small but relatively robust labial cingular segment between the paracone and metacone, a prominent anterior cingulum on the anterior surface of the protocone, and a weaker posterior cingulum on the posterior surface of the hypocone. There is also a prominent cingular segment at the lingual entrance to the transverse valley, between the protocone and hypocone, which is style-like on the anterior surface of the latter cusp (Fig. 11.16). This tooth is very reminiscent of the upper molars of protoceratids, but much smaller than even the smallest species, Prosynthetoceras orthrionanus.
The m3 (13.02 mm AP × 7.28 mm TR) has no lingual ribs on the metaconid and entoconid, but it has small stylids between the protoconid and hypoconid and between the hypoconid and hypoconulid. There is also a tiny cingular segment on the anterior surface of the protoconid. The enamel is weakly crenulated, best seen on the lingual surface, and somewhat weaker than on the upper molar. There is no Palaeomeryx fold as seen in various species of Leptomeryx, particularly the post-Chadronian species. The entoconulid is not as tall as the metaconid or entoconid, and a posterior extension of this cuspid meets a lingually directed extension of the hypoconulid. At this juncture on the lingual surface of the tooth is a prominent lingual rib not seen in other species of Leptomeryx (Fig. 11.17).
Remarks
Based on these two teeth, the Jones Branch species is larger than all known species of Leptomeryx from the Great Plains except the Whitneyan L. obliquidens Lull, Reference Lull1922. It is close in size to Pseudoprotoceras longinarus Cook, Reference Cook1934, from the Chadronian of Nebraska, but Pseudoprotoceras has a robust, almost cusp-like stylid on the anterior surface of the hypocone of the upper molars at the lingual entrance to the transverse valley. As in Leptomeryx, the much smaller Hypertragulus, and protoceratids, the Jones Branch m3 has no lingual ribs on the metaconid and entoconid, and it has small stylids between the protoconid and hypoconid and between the hypoconid and hypoconulid. But there are also important differences.
Heaton and Emry (Reference Heaton, Emry, Prothero and Emry1996), followed by Korth and Diamond (Reference Korth and Diamond2002), distinguished species of Leptomeryx by the morphology of the entoconulid on the m3. In the early Chadronian species, L. yoderi Schlaikjer, Reference Schlaikjer1935, and in its slightly larger middle to late Chadronian descendent, L. mammifer Cope, Reference Cope1885, the entoconulid is long and narrow, and lower than the entoconid and hypoconulid. Another probable descendent of L. yoderi, the late Chadronian L. speciosus Lambe, Reference Lambe1908, plus its apparent descendent, the very abundantly represented Orellan species L. evansi Leidy, Reference Leidy1853, have an entirely different m3 entoconulid morphology in which that cusp “is much more rounded and pronounced, and it slopes steeply downward posteriorly” (Heaton and Emry, Reference Heaton, Emry, Prothero and Emry1996, p. 599). Korth and Diamond (Reference Korth and Diamond2002) added that in the speciosus/evansi morphology, the entoconulid is as tall as the metaconid and entoconid.
In this regard, the entoconulid of the Jones Branch tooth more closely resembles the yoderi/mammifer style than the speciosus/evansi morphology in that it is triangular in lingual view, it is not cylindrical, it is not nearly as tall as the metaconid or entoconid, nor is it separated from the hypoconulid by a deep valley (Korth and Diamond, Reference Korth and Diamond2002, p. 127). It further resembles the Chadronian L. yoderi and L. mammifer, and differs from later occurring species, particularly L. evansi, in having weakly crenulated enamel and in lacking a Palaeomeryx fold. These features are consistent with the findings of Mathis and MacFadden (Reference Mathis and MacFadden2010, p. 683) who statistically analyzed the change in enamel sculpturing in Leptomeryx species across the Eocene–Oligocene boundary. They found that “In general, the Chadronian specimens have simple enamel, little to no crenulations, and lack a Paleomeryx [sic] fold. In contrast, the Orellan specimens have more complex enamel, are more strongly crenulated, and most often exhibit the presence of a Paleomeryx [sic] fold.” A feature of the Jones Branch m3 not seen in any known species of Leptomeryx, however, is the nature of the hypoconulid; it continues around to the lingual surface where it meets a posterior extension of the entoconulid resulting in a prominent lingual rib (Fig. 11.17).
In addition to L. evansi, Korth and Diamond (Reference Korth and Diamond2002) recognized two other species from the Orellan of the northern Great Plains. One of these, L. elissae Korth and Diamond, Reference Korth and Diamond2002, has the speciosus/evansi entoconulid morphology, but is smaller than L. evansi, has smooth rather than crenulated enamel, and lacks the Palaeomeryx fold. The second species, L. exilis Cook, Reference Cook1934, is even smaller than L. elissae, and it too has smooth rather than crenulated enamel and lacks the Palaeomeryx fold; but it has the yoderi/mammifer style entoconulid.
Korth and Diamond (Reference Korth and Diamond2002) also discussed Whitneyan and Arikareean species of Leptomeryx from the Great Plains. The Whitneyan Leptomeryx lentis Cook, Reference Cook1934, with its highly crenulated enamel, presence of a Palaeomeryx fold, and similar size, they synonymized with L. evansi. Likewise, similarities between L. minimus Frick, Reference Frick1937, and L. exilis resulted in synonymy of the former with the latter. Another Whitneyan species is L. obliquidens, which the Jones Branch taxon most closely resembles. Much larger than all other known species of Leptomeryx, L. obliquidens is the same size as that from Jones Branch, it has the yoderi/mammifer style entoconulid, and it lacks a Palaeomeryx fold. It differs from the Jones Branch species, however, in having highly crenulated enamel and in lacking the prominent lingual rib where the posterior extension of the entoconulid meets a lingually directed extension of the hypoconulid.
Of the two Arikareean species, L. agatensis Cook, Reference Cook1934, is known only from a maxilla with teeth found in Sioux County, Nebraska (Korth and Diamond, Reference Korth and Diamond2002). The other, Leptomeryx transmontanus Douglass, Reference Douglass1903, known from Montana and South Dakota, was referred to Pronodens Koerner, Reference Koerner1940, by Rasmussen, Reference Rasmussen1977; hence, Pronodens transmontanus (also see Taylor and Webb, Reference Taylor and Webb1976; Webb, Reference Webb, Janis, Scott and Jacobs1998; Korth and Diamond, Reference Korth and Diamond2002; Calede, Reference Calede2020). This assignment was further confirmed by Calede et al. (Reference Calede, Constenius, Famoso and Kehl2024) in their study of new material recovered from Arikareean-aged outcrops of the Kishenehn Formation within Glacier National Park, northwestern Montana. Although noted as larger than Leptomeryx, Pronodens sliberlingi Koerner, Reference Koerner1940, cannot be compared with that from Jones Branch, as there is no complete m3 available. Pronodens transmontanus can be compared, however, and it is distinctly smaller (measurements in Rasmussen, Reference Rasmussen1977, and Calede et al., Reference Calede, Constenius, Famoso and Kehl2024).
In summary, the Jones Branch species is larger than all the known species of Leptomeryx except L. obliquidens, the species it most closely resembles. The only substantive difference from the latter is the weakly crenulated enamel and the prominent lingual rib—a feature not seen in any known species of Leptomeryx. The questionable referral of the Jones Branch taxon to this genus, however, is based on its general similarity and because there are no known artiodactyls spanning the early Arikareean that it more closely resembles. An hypothesis could be put forth whereby late Chadronian species ranged across a much broader geographic expanse than just the Great Plains prior to the subhumid to semiarid savanna woodlands that would form there during the Orellan as climate became drier due to the major climatic changes that occurred across the Eocene–Oligocene boundary (Retallack, Reference Retallack1983). This might account for a refuge population along the Gulf Coastal Plain with an entoconulid morphology closest to the yoderi/mammifer type, and which also maintained the weaker crenulations than those that developed in the Whitneyan L. obliquidens. As noted by Mathis and MacFadden (Reference Mathis and MacFadden2010, p. 686), increased enamel crenulations, which increases the wear resistance of enamel, and the Palaeomeryx fold, which increases the area of enamel, “would have resulted in an artiodactyl better adapted to new environmental conditions [in the Great Plains], most probably resulting from the dramatic climate change at the Eocene–Oligocene boundary.” In a Gulf Coastal Plain population, neither highly crenulated enamel nor a Palaeomeryx fold would be necessary because the vegetation there over this interval was not changing to more arid-adapted, more abrasive forms as was happening in the Great Plains and northern Midcontinent. More material of this enigmatic small artiodactyl will be required to further clarify its identity.
As noted above in the section on Prosynthetoceras, MMNS VP-6936, a small Mc IV, not only resembles that of a protoceratid, but also that of Leptomeryx because both of these taxa have unfused Mc II-V. Also noted was MMNS VP-8708, a Mt III that does not appear referrable to either Prosynthetoceras or Leptomeryx. Thus, if this single metatarsal belongs to the same taxon as do the two teeth, MMNS VP-8338 and MMNS VP-7487, then they cannot belong to Leptomeryx, again hinting that there may be another small protoceratid-like artiodactyl in the Jones Branch fauna.
Sirenia Illiger, Reference Illiger1811
Dugongidae Gray, Reference Gray1821
Crenatosiren Domning, Reference Domning1991
Type species
Halitherium olseni Reinhart, Reference Reinhart1976.
Crenatosiren olseni (Reinhart, Reference Reinhart1976)

Figure 12. Crenatosiren olseni from the Jones Branch LF, lower Catahoula Formation, Mississippi: (1) MMNS VP-6560, right M1, (2) MMNS VP-6618, right m1, (3) MMNS VP-7047, left m2, (4) MMNS VP-6935, right m2, (5) MMNS VP-7486, right m3, (6) MMNS VP-7597, supraorbital process of left frontal, (7) MMNS VP-6577, left jugal, (8) MMNS VP-7736, left periotic, (9) MMNS VP-6947, juvenile basioccipital, (10) MMNS VP-6632, manubrium, (11) MMNS VP-8359, parietal skullcap, (12) MMNS VP-6598, parietal-supraoccipital skullcap. Upper scale bar for (1–5); center scale bar for (6–9); bottom scale bar for (10–12).
Holotype
UF/FGS V6094, cranium and mandible, vertebrae and postcranial elements, from east bank of the Suwanee River, “approximately 1.6 mi. below White Springs….Hamilton Co., Florida” (Reinhart, Reference Reinhart1976, p. 238); early late Arikareean-aged Porters Landing Member of the Parachucla Formation (see discussion of type locality in Albright et al., Reference Albright, Sanders, Weems, Cicimurri and Knight2019, p. 132).
Referred specimens
MMNS VP-6560, right M1; MMNS VP-9050, partial right edentulous dentary; MMNS VP-6618, right m1; MMNS VP-6935, right m2; MMNS VP-7047, left m2; MMNS VP-7486, right m3; MMNS VP-7597, supraorbital process of the frontal; MMNS VP-6577, left jugal; MMNS VP-7596, ?left squamosal; MMNS VP-7658, left exoccipital with paroccipital process and occipital condyle; MMNS VP-7736, left periotic; MMNS VP-6527, juvenile parietal skullcap; MMNS VP-8359, parietal skullcap; MMNS VP-6598, parietal-supraoccipital skullcap; MMNS VP-6947, juvenile basioccipital; MMNS VP-7054, right portion of atlas vertebra; MMNS VP-6632, manubrium; MMNS VP-7543, ?juvenile left humerus.
Description
The tiny M1 (MMNS VP-6560, Fig. 12.1) closely matches the description of Crenatosiren olseni and Nanosiren garciae Domning and Aguilera, Reference Domning and Aguilera2008, in size and in having “two transverse lophs formed by the main cusps, with narrow anterior and posterior basins encircled by pre- and postcingulae that are respectively connected to the lophs at both ends or only at the lingual ends” (Domning, Reference Domning1997, p. 404–405; also see Domning and Aguilera, Reference Domning and Aguilera2008, p. 485). The m1 (MMNS VP-6618, Fig. 12.2) is bilobed with a cristid obliqua joining, at this wear stage, the anterior and posterior lophids, and there is a prominent hypoconulid lophule. The tooth has a long posterior root, nearly twice the length as the height of the crown. The m2s are similar to the m1, just slightly larger (Fig. 12.3, 12.4). The m3 (MMNS VP-7486) also matches Domning’s (Reference Domning1991, Reference Domning1997) description of that tooth for Crenatosiren olseni, but the Jones Branch tooth differs in having a “centrally located posterior cuspule” posterior to the hypoconulid lophule (Fig. 12.5), as noted by Domning and Aguilera (Reference Domning and Aguilera2008, p. 486) for Nanosiren garciae. How variable this feature might be is unknown. Measurements of the teeth are provided in Table 10.
Table 10. Measurements of teeth (in mm) of Crenatosiren olseni

The supraorbital process of the frontal (MMNS VP-7597, Fig. 12.6) is similar in size and morphology to that illustrated for Crenatosiren olseni in Domning (Reference Domning1997, fig. 2) and to ChM PV 5473, ChM PV 7217, CCNHM-200.1 (all from the Chattian-aged Chandler Bridge Formation near Charleston, SC), and ChM PV 4888 (a cast of USNM 425488 from the late Rupelian-aged Ashley Formation also near Charleston). As described for Crenatosiren by Domning (Reference Domning1997, p. 401), the dorsal surface of MMNS VP-7597 has “a prominent, thick ridge (a continuation of the temporal crest) that curves toward, but does not reach, the posterolateral corner of the supraorbital process.” The antero-posterior length of the process is 40 mm compared with 41 mm in USNM 425488, 40 mm in SC 89.243.2 (Domning, Reference Domning1997, fig. 2), and 41 mm in ChM PV 7217. The supraorbital process of the frontal is missing in both specimens of both species of Nanosiren, N. garciae and N. sanchezi (Domning and Aguilera, Reference Domning and Aguilera2008).
The jugal (MMNS VP-6577, Fig. 12.7) more closely resembles that of Crenatosiren than Nanosiren when compared with figures of this element in Domning (Reference Domning1997) and Domning and Aguilera (Reference Domning and Aguilera2008), respectively, and when compared with ChM PV 5473 and CCNHM-200.1. As in Crenatosiren, the anterior end of the preorbital process extends anterolabially to posteromedially, is about 21 mm wide, and has a distinct groove into which the lacrimal articulates. This process is about 14 mm wide at the base of the orbit, and on its ventral surface, from its anterior end to the point where the orbit begins to ascend, is a prominent, somewhat laterally positioned ridge resulting in a triangular cross-section. Also similar to Crenatosiren, the posterior orbit ascends rather abruptly, resulting in a nearly vertical posterior orbital wall, with its dorsalmost point about 30 mm above the base of the orbit. In Nanosiren, the orbit is shallower, and the posterior orbital wall does not become vertical. The surface for articulation with the zygomatic process of the squamosal is 36 mm long, and it has an elongated, teardrop-shaped cross section. At 86 mm long, the jugal from Jones Branch is smaller than in previously noted specimens of Crenatosiren (avg. 125 mm, n = 3; Domning, Reference Domning1997) and Nanosiren (103+ mm; Domning and Aguilera, Reference Domning and Aguilera2008), but this may be a function of ontogeny.
The periotic (MMNS VP-7736, Fig. 12.8) is nearly identical in morphology and size to that of ChM PV 5473. It measures 31.2 mm across the tegmen tympani × 38.9 mm antero-posteriorly; the same measurements for ChM PV 5473 are 32 mm × 36.8 mm. The Jones Branch specimen also resembles that of Metaxytherium albifontanum Vélez-Juarbe and Domning, 2014, (UF 49051; see Vélez-Juarbe and Domning, 2014, fig. 5), but differs in its smaller size (UF 49051 measures 36 mm × 40 mm), in its less elongate tegmen tympani, and in the absence of the pars cochlearis, although absence of the latter feature may be a result of breakage.
The Y-shaped basioccipital (MMNS VP-6947) is that of a juvenile, as there is no fusion to the occipital condyles, nor to the basisphenoid (Fig. 12.9). Total anteroposterior length is 37 mm.
The manubrium (MMNS VP-6632) measures 79.7 mm antero-posteriorly and its maximum width at the spatulate posterior portion is 46.1 mm (Fig. 12.10). Left and right lateral bosses (articular surfaces for costal cartilages per Domning, Reference Domning1997, p. 405) begin about 15 mm posterior to the anteriormost surface of the element and are each about 15 mm long. On the ventral surface, a keel extends from midway between the two bosses to the posterior end. The Jones Branch specimen closely matches Domning’s (Reference Domning1997) description of the sternum of the holotype specimen of C. olseni, UF/FGS V6094.
It should be noted that the skull caps from Jones Branch (MMNS VP-6527, MMNS VP-8359, and MMNS VP-6598) may belong to a taxon other than C. olseni. Although their size falls within the range of the latter (Domning, Reference Domning1997, table 1), they differ in lacking the prominent temporal crests and the consequent deeply concave invagination between them (Fig. 12.11, 12.12). Although subtle, these crests are slightly more prominent in MMNS VP-8359 than in MMNS VP-6598, but they are nowhere near as prominent as in the specimens illustrated for C. olseni (Domning, Reference Domning1997, fig. 2), nor do they extend as far anteriorly as in those specimens. MMNS VP-6527 represents that of a juvenile and it too shows only very subtle crests.
Remarks
Six species of dugongids are known from the western Atlantic region during the Oligocene. Metaxytherium albifontanum, Dioplotherium manigaulti Cope, Reference Cope1883, and Callistosiren boriquensis Vélez-Juarbe and Domning, Reference Vélez-Juarbe and Domning2015, are known from the late Oligocene (Vélez-Juarbe et al., Reference Vélez-Juarbe, Domning and Pyenson2012; Vélez-Juarbe and Domning, Reference Vélez-Juarbe and Domning2014a, Reference Vélez-Juarbe and Domning2015). Priscosiren atlantica Vélez-Juarbe and Domning, Reference Vélez-Juarbe and Domning2014b, and Stegosiren macei Domning and Beatty, Reference Domning and Beatty2019, are found in the early Oligocene (both occur in the Ashley Formation). The sixth species, C. olseni, which spans both the early and late Oligocene, is smaller than all of the others.
The only dugong other than C. olseni from this region as diminutive as the Jones Branch taxon is Nanosiren. The two species of Nanosiren, however, are known only from the Early Miocene to early Pliocene, including the latest Hemphillian of Florida (Domning and Aguilera, Reference Domning and Aguilera2008). Referral of the Jones Branch dugong to Crenatosiren olseni, therefore, is supported by its very small size, similar morphological traits, southeastern United States distribution, and its Oligocene age. As temporally distant as Crenatosiren and Nanosiren are, their similarity supported Domning’s and Aguilera’s (Reference Domning and Aguilera2008) conclusion that these two taxa constitute the basalmost clade of the Dugonginae.
Notes on two specimens recovered from older strata in Mississippi
Two specimens brought to our attention and donated to the MMNS during preparation of this report warrant mention, although neither was recovered from the Jones Branch locality. The first, MMNS VP-9129, is a left I3 of a large entelodont from the Bucatunna Clay and the second, MMNS VP-8707, is a right M3 of an anthracothere recovered from the underlying Byram Formation. These formations represent the upper two units of the Vicksburg Group that unconformably underlie the Chickasawhay Limestone in southeastern Mississippi (Poag, Reference Poag1974; Miller et al., Reference Miller, Thompson and Kent1993; Dockery and Thompson, Reference Dockery and Thompson2016). Both have been correlated to Calcareous Nannofossil Zone NP23 and to Planktonic Foraminiferal zones P19–20 of the Rupelian Stage (Fig. 4).
As noted earlier in this report, Miller et al. (Reference Miller, Thompson and Kent1993) correlated the base of the Bucatunna with the top of chron C12n, with most of the unit residing within C11r. This provides a range from about 30.6 Ma to 30.0 Ma, with a consequent correlation to the later part of the Whitneyan (Fig. 4). The underlying Byram Formation was corelated to C12n in the core that Miller et al. (Reference Miller, Thompson and Kent1993) analyzed from the St. Stephens Quarry, Alabama, but this was extended downward into C12r upon analysis of the core they collected at Bay Minette. Although Siesser (Reference Siesser1983) placed the Byram Formation in NP22, Hall (Reference Hall2003) placed both the Byram Formation type section and the section at Big Black River in NP23. As shown in Figure 4, NP23 spans the Whitneyan and latest part of the preceding Orellan NALMAs. Two rhinoceros genera, Metamynodon and Subhyracodon, reported by Manning et al. (Reference Manning, Dockery and Schiebout1985) and Manning (Reference Manning1997), respectively, from the Big Black River section further support this correlation. Together, the C12n/C12r magnetostratigraphic data, the NP23 nannoplankton data, and the occurrence of Metamynodon and Subhyracodon strongly support correlation of the Byram Formation to the later part of the Orellan and early part of the Whitneyan.
Siesser’s (Reference Siesser1983) account of the calcareous nannofossil Reticulofenestra umbilicus in the Bucatunna Formation at St. Stephens Quarry is problematic. According to Agnini et al. (Reference Agnini, Fornaciari, Raffi, Catanzariti, Pälike, Backman and Rio2014) and Coccioni et al. (Reference Coccioni, Montanari, Bice, Brinkhuis and Deino2018), and as shown in GTS2020 (Speijer et al., Reference Speijer, Pälike, Hollis, Hooker, Ogg, Gradstein, Ogg, Schmitz and Ogg2020, fig. 28.10), R. umbilicus does not range into NP23; its HO is at the NP22/23 boundary, which occurs in C12r at about 32.3 Ma (Coccioni et al., Reference Coccioni, Montanari, Bice, Brinkhuis and Deino2018) and which is also very near the Orellan/Whitneyan boundary (Fig. 4). Miller et al.’s (Reference Miller, Thompson and Kent1993) magnetostratigraphic correlation of the Bucatunna to C11r, strongly supported by their finding the HO of Turborotalia ampliapertura in that chronozone at the St. Stephens Quarry, which marks the P19–P20 and the O2–O3 planktonic foraminifera zone boundaries, places that formation considerably above the HO of Reticulofenestra umbilicus (Fig. 4).
Archaeotherium Leidy, Reference Leidy1850b
Type species
Archaeotherium mortoni Leidy, Reference Leidy1850b.
Archaeotherium sp.

Figure 13. Archaeotherium and Elomeryx from the Bucatunna and Byram formations, respectively: (1–4) Archaeotherium sp., MMNS VP-9129, left I3, (1) posterior surface, (2) anterior surface, (3) occlusal surface showing labial and lingual orientation and the rounded 90° angle noted in text, (4) view showing measured dimensions as noted in text; (5) Elomeryx armatus, MMNS VP-8707, right M3, stereo occlusal view.
Referred specimen
MMNS VP-9129, left I3, from exposures of Bucatunna Clay along Buckatunna Creek south of Waynesboro, Wayne County, Mississippi, collected by Mr. Allen Austin.
Description
Morphologically this tooth (MMNS VP-9129) closely resembles the I3 of UNSM 1098, a large entelodont identified as the early Arikareean Archaeotherium trippensis (noted above in the section on Daeodon). Diagnostic of these teeth are (1) the somewhat pinched ridges or carinae that ascend both sides of the crown separating the lingual from the labial surfaces, (2) a lingual cingulum that is nearly shelf-like medially, and (3) the rounded 90° angle formed where the anterior surface of the crown curves into the labial surface (Fig. 13.1–13.3). Although MMNS VP-9129 is considerably larger than UNSM 1098 (35.5 mm × 31.6 mm vs. 27.3 mm × 26.9 mm, respectively; see Fig. 13.4 for dimensions measured) and more similar in size (only) to the upper I2 of D. hollandi, it differs from the latter in lacking the extremely robust, purported bone-crunching, columnar-like morphology and in the presence of the characteristics noted above.
Remarks
The Whitneyan age of the Bucatunna tooth further supports its referral to a large species of Archaeotherium rather than Daeodon given that the former is an autochthonous North American taxon that spans the early Chadronian through early Arikareean, whereas Daeodon is an immigrant that first appears in North America in the early Arikareean according to Effinger (Reference Effinger, Janis, Scott and Jacobs1998, fig. 24.4), although the preponderance of evidence supports a first appearance in the late Arikareean (e.g., Foss, Reference Foss2001). That is, Daeodon would not yet have been in North America during Bucatunna time.
As noted above in the section on ?Daeodon from the Jones Branch locality, there were additional large North America species of Archaeotherium, as well, including A. calkinsi and A. caninus. But whether these two species (possibly one, see Foss, Reference Foss2001) extend down into the early Whitneyan or older, is not known, as both are known only from early Arikareean levels in the John Day Formation, Oregon. Apparently, however, they do not co-occur with D. hollandi in that region (Foss and Fremd, Reference Foss and Fremd1998). The latter is found stratigraphically higher than A. calkinsi and A. caninus.
Elomeryx Marsh, Reference Marsh1894c
Type species
Heptacodon armatus Marsh, Reference Marsh1894d.
Elomeryx armatus (Marsh, Reference Marsh1894d)
Holotype
YPM 10176, right maxilla with P3–M3, from the “Protoceras channels” of the Poleside Member, Brule Formation, Whitneyan, Big Badlands, South Dakota (Macdonald, Reference Macdonald1956, p. 627).
Referred specimen
MMNS VP-8707, right M3, from the type locality of the Byram Formation along the Pearl River, Hinds County, Mississippi, collected by Ms. Sara Yarbrough.
Description
Determination that MMNS VP-8707 is an M3 is based on the presence of a wear facet on its anterior surface, absence of one posteriorly, and on its transversely wider anterior half than posterior. It is very low-crowned and measures 25.8 mm AP × 25 mm TR, although the parastyle is broken (Fig. 13.5). The crests formed by the anterior and posterior ‘arms’ of the paracone and metacone are broadly separated, not narrow, and the transverse valley, which does not invade the prominent mesostyle, is also very broad anteroposteriorly and thus relatively shallow. This latter feature results in wide separation between the paracone and metacone and between the protocone and hypocone (the latter cusp also referred to as the metaconule; see Lihoreau and Ducrocq, Reference Lihoreau, Ducrocq, Prothero and Foss2007, fig. 7.1). The protocone and hypocone are positioned slightly posteriorly to the paracone and metacone, respectively, thus resulting in a weakly convex-anterior trace of the transverse valley. The lingual portion of the transverse valley is slightly blocked from the labial portion by a very low, relatively subtle posterior ‘arm’ of the protocone, which abuts the anterior ‘arm’ of the hypocone. The prominent protoconule (the latter cusp also referred to as the paraconule; see Lihoreau and Ducrocq, Reference Lihoreau, Ducrocq, Prothero and Foss2007, fig. 7.1) is slightly anterior to the protocone and ‘wedged’ between it and the paracone. The posterior arm of the metacone terminates into a prominent metastyle, which forms the posterolabial corner of the tooth. Because the mesostyle, metastyle, and presumably the missing parastyle are so prominent, the labial outline of the tooth (the ectoloph) is W-shaped (Fig. 13.5). The shelf-like anterior cingulum begins at the protoconule, wraps around the protocone lingually, crosses the transverse valley, and terminates against the anterolingual surface of the hypocone. It remains prominent along its entire path. The posterior cingulum is most prominent between the metastyle and hypocone but continues along the posterior surface of the latter cusp. The transverse valley terminates labially at the mesostyle to form a narrow V, as opposed to a U-shaped terminus noted for the M3s of some taxa (e.g., Russell, Reference Russell1978). Not noted in molars of any other North American anthracothere is a distinct, thin, transversely oriented loph emanating from the labial terminus of the transverse valley at the mesostyle (Fig. 13.5). Positioned between the posterior arm of the paracone and the anterior arm of the metacone, this structure extends lingually to a point nearly even with the tips of these two cusps.
Remarks
Three North American anthracotheres span the interval of time (Orellan/Whitneyan) over which deposition of the Byram Formation occurred: Heptacodon, Bothriodon, and Elomeryx. Kron and Manning (Reference Kron, Manning, Janis, Scott and Jacobs1998, fig. 25.6) showed the range of Bothriodon terminating at the Orellan–Whitneyan boundary, that of Heptacodon terminating at the Whitneyan–Ar1 boundary, and Elomeryx ranging from the Orellan through Ar1 (Fig. 4).
The Byram tooth resembles that of Heptacodon in being low-crowned, in having shallow (and weakly ribbed) labial crescents, in having a mesoloph that is not ‘invaded’ by the transverse valley, and in the lingual extension of the anterior cingulum around the protocone; but it differs in its W-shaped ectoloph, due in part to having a prominent metastyle. In Heptacodon, the metastyle is reduced to absent, resulting in a distinct posteromedial slope of the posterolabial corner of the tooth. In general, the teeth of Bothriodon and Elomeryx are higher crowned than the Byram tooth, the labial crests are narrower and V-shaped, the ectoloph is not as strongly W-shaped (in part because Bothriodon and Elomeryx do not have as prominent a metastyle as that in the Byram tooth), and the transverse valley, which typically invades the mesostyle, is not as broad anteroposteriorly. Additionally, in Bothriodon, according to Scott (Reference Scott1940, p. 443), the “external crescents” (paracone and metacone) are shifted lingually to near the “middle of crown,” which is clearly seen in the type specimens of B. rostratus (Scott, Reference Scott1894) (YPM-PU 11172) and B. advena Russell, Reference Russell1978, (ROM 21744; see Russell, Reference Russell1978; Prothero et al., Reference Prothero, Marriott and Welsh2022). These specimens also lack the lingual extension of the anterior cingulum around the protocone expressed so prominently in Elomeryx and the Byram tooth. In Elomeryx, the ‘invasion’ of the mesostyle by the transverse valley varies (i.e., in some specimens it is open across the entire occlusal surface, as in the Jones Branch tooth, MMNS VP-6457, whereas in others it is not).
However, as noted above in the section on the Jones Branch anthracothere, the Byram tooth very closely resembles the M3s of E. armatus illustrated in Marsh’s (Reference Marsh1894c, Reference Marsh1894d) and Lambe’s (Reference Lambe1908) figures, as well as E. garbanii, in the lingual extension of the anterior cingulum around the protocone; but the Marsh teeth and E. garbanii do not have as prominent a metastyle as the Byram tooth, resulting in a less W-shaped ectoloph. On the other hand, the Byram tooth is nearly identical to that illustrated by Lambe (Reference Lambe1908) with the exception of the loph emanating from the labial terminus of the transverse valley, hence our referral of this tooth to E. armatus.
The Byram tooth differs substantially from the Jones Branch upper molar (MMNS VP-6457) referred to the same genus. The Jones Branch tooth is slightly smaller, wider transversely than anteroposteriorly, with narrower V-shaped external crescents and a narrower, deeper, transverse valley that invades the mesostyle; it does not have a W-shaped ectoloph in part because the metastyle is less prominent, and there is no lingual cingulum other than a short segment between the protocone and hypocone at the lingual entrance to the transverse valley. In these features, the Jones Branch tooth closely resembles those of Arretotherium (particularly common in the Ar3 Toledo Bend LF) except for the presence of a protoconule, which is lost in the latter.
The morphology of the Jones Branch tooth is suggestive of a species somewhat transitional between Elomeryx armatus and Arretotherium, and this is supported by the approximately 4-Myr separation between the Byram and Jones Branch occurrences with a similar separation between the Jones Branch and Toledo Bend occurrences. Another consideration, however, is “the great deal of individual variation within this [the Elomeryx] group” that Macdonald (Reference Macdonald1956, p. 628) noted. But until additional material is recovered from the Jones Branch locality, referral to Elomeryx sp. for the species found there is maintained due to the retention of the protoconule.
Discussion
As documented above, nearly all of the mammals that constitute the Jones Branch LF are forms that indicate an early Arikareean (Ar1–2) North American Land Mammal Age (see Tedford et al., Reference Tedford, Skinner, Fields, Rensberger, Whistler, Galusha, Taylor, Macdonald, Webb and Woodburne1987, Reference Tedford, Swinehart, Swisher, Prothero, King, Tierney, Prothero and Emry1996, Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004), with several relicts of the older White River Chronofauna (Chadronian through Whitneyan), and some closely resembling taxa from Florida’s I-75 LF (Patton, Reference Patton1969), as well (Fig. 1). Morgan et al. (Reference Morgan, Czaplewski and Simmons2019) placed the I-75 LF in the late Whitneyan, or slightly older than the Whitneyan/Arikareean boundary at about 30 Ma. Many taxa from Jones Branch are last known from early early Arikareean (Ar1) faunas of the Midcontinent, with a few others last occurring in the late early Arikareean (Ar2). Based on mammalian biochronology (Fig. 4), the Jones Branch LF cannot be younger than Ar2, the top of which is approximately 26 Ma (early Chattian; Albright et al., Reference Albright, Woodburne, Fremd, Swisher, MacFadden and Scott2008). Supporting an Ar1 age is the occurrence of Protapirus and Elomeryx, both of which are last known from that interval (Tedford et al., Reference Tedford, Skinner, Fields, Rensberger, Whistler, Galusha, Taylor, Macdonald, Webb and Woodburne1987, Reference Tedford, Swinehart, Swisher, Prothero, King, Tierney, Prothero and Emry1996, Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004). Holdover elements from the White River Chronofauna, such as Eutypomys, Miohippus, Protapirus, Leptochoerus, Hypertragulus, and Leptomeryx, also support this age, although Eutypomys, Miohippus, and Hypertragulus extend through Ar2. Others do, as well, such as Corumictis, Promartes, and Crenatosiren, in turn supporting that later age (Fig. 4).
Another important mammal fossil from Jones Branch that bears on its age is a tooth of the early odontocete Agorophius pygmaeus (Müller, Reference Müller1849) mentioned by Dockery and Thompson (Reference Dockery and Thompson2016, p. 505). The lost holotype, plus additional specimens (Fordyce, Reference Fordyce1981; Godfrey et al., Reference Godfrey, Uhen, Osborne and Edwards2016; Boessenecker and Geisler, Reference Boessenecker and Geisler2018), were recovered from the Ashley Formation north of Charleston, South Carolina, which, like the Paynes Hammock and Chickasawhay formations, is correlated to NP24 and P21, both of which span the Rupelian/Chattian stage boundary (Weems et al., Reference Weems, Albright, Bybell, Cicimurri, Edwards, Harris, Lewis, Osborne, Sanders and Self-Trail2016; Albright et al., Reference Albright, Sanders, Weems, Cicimurri and Knight2019). The uppermost member of the Ashley Formation, the Givhans Ferry Member, from which Agorophius is known, is dated to between 28.43 and 28.75 Ma (late Rupelian) based on 87Sr/86Sr analysis of mollusks (Weems et al., Reference Weems, Albright, Bybell, Cicimurri, Edwards, Harris, Lewis, Osborne, Sanders and Self-Trail2016).
As discussed previously, the stratigraphic level of the Jones Branch site lies at the very base of the Catahoula Formation, where it rests unconformably on the underlying Paynes Hammock Formation. The boundary between these two formations is thought to very closely approximate the Rupelian/Chattian boundary, at 27.41 Ma. Complimenting the years of bio- and chronostratigraphic work in the Great Plains (e.g., Prothero and Emry, Reference Prothero and Emry1996; Tedford et al., Reference Tedford, Albright, Barnosky, Ferrusquia-Villafranca, Hunt, Storer, Swisher, Voorhies, Webb, Whistler and Woodburne2004) and Albright et al.’s (Reference Albright, Woodburne, Fremd, Swisher, MacFadden and Scott2008) work in the John Day Formation, Calede’s (Reference Calede2020) biostratigraphic study of the Cabbage Patch beds resulted in the conclusion that the Ar1–Ar2 transition was essentially synchronous across the Pacific Northwest–northern Rocky Mountains–Great Plains regions at approximately 28 Ma. Taken together, all of these chronostratigraphic data, as well as the currently understood biochronologic ranges of many of its mammalian constituents, support an estimated age for the Jones Branch LF just slightly younger than the 27.41 Ma Rupelian/Chattian boundary, but likely no younger than approximately 27 Ma (Fig. 4). This places the age of the fauna in Ar2, probably in the early part of this subage given the presence of Protapirus and Elomeryx—taxa that last occur in Ar1 in the more northern regions. It appears, therefore, that these taxa persisted slightly longer in the Gulf Coastal Plain than they did in the Midcontinent. However, the Jones Branch LF is derived from a single site from a single stratigraphic horizon, therefore unfortunately precluding biostratigraphic (and geochronologic) tests that could address Ar1–Ar2 boundary synchroneity between the Gulf Coast and the more northern regions.
One of the most interesting aspects of the Jones Branch LF is the abundance of taxa previously known only from the Great Plains, northern Rocky Mountains, and/or Oregon—regions that would have had entirely different, cooler and drier, paleoenvironments during the mid-Oligocene than the warm, humid, subtropical coastal lowlands of the Gulf Coastal Plain (e.g., Albright, Reference Albright1994; Sheldon, Reference Sheldon2009). The taxonomic similarity with faunas from the John Day Formation of Oregon is particularly perplexing given that region’s distance from the Gulf Coast, its elevation, its location west of the Rocky Mountains, east of the Cascade Range, and adjacent to the southward flowing, cold, California Current, in addition to recent interpretations of the paleoenvironment that existed there during this time (e.g., Retallack and Samuels, Reference Retallack and Samuels2020). Taxa at the generic level that mutually occur at Jones Branch and in the John Day Formation include Herpetotherium, ?Sinclairella, Microtheriomys, Apeomys, Corumictis, Phlaocyon, Miohippus, Leptochoerus, Daeodon, and Hypertragulus (Table 11).
Table 11. Taxa from the Jones Branch locality, lower Catahoula Formation, southeastern Mississippi, that are mutually shared with those found within the John Day Formation, Oregon, from various localities across the Great Plains and northern Rocky Mountains (mainly Nebraska, South Dakota, and Montana), and from Florida. Taxa listed from the Great Plains are from both Whitneyan and Arikareean sites; Florida’s I-75 LF is considered latest Whitneyan and Brooksville 2 is considered late early Arikareean (Morgan et al., Reference Morgan, Czaplewski and Simmons2019)

Based on studies of paleosols in the Turtle Cove Member of the John Day Formation (approximately 31–26 Ma), Retallack and Samuels (Reference Retallack and Samuels2020) determined that the environment there alternated between semiarid shrublands and subhumid woodlands over 41 Kyr Milankovitch obliquity cycles. By 30 Ma (the Whitneyan–Arikareean boundary), central Oregon had already become cooler and drier, due (in part) to the rain shadow cast by an uplifting Cascade Range (Kohn et al., Reference Kohn, Miselis and Fremd2002), than it had been during a wetter phase in the late Eocene (Retallack et al., Reference Retallack, Bestland and Fremd1999; Retallack, Reference Retallack2007; Sheldon, Reference Sheldon2009). This was likely compounded by the rapid decline in global temperatures across the Eocene–Oligocene boundary (the Eocene–Oligocene Transition) upon separation of Antarctica from South America and the consequent formation of the Antarctic Circumpolar Current (e.g., Prothero et al., Reference Prothero, Ivany and Nesbitt2003). Calculating mean annual precipitation levels (MAP) of 471 ± 59 mm for the semiarid shrublands and 879 ± 141 mm for the subhumid woodlands (Retallack and Samuels, Reference Retallack and Samuels2020), regions with comparable MAP today include North and South Dakota for the former and Ohio and Oklahoma for the latter (NCICS, 2022).
By comparison, southern Mississippi’s MAP today is approximately 1600–1700 mm; a level slightly greater than what has been calculated for the Jones Branch region during the early Oligocene. Hou et al. (Reference Hou, Zhuang, Ellwood, Liu and Wu2022), using leaf-wax carbon and hydrogen isotopic records (from a core within 20 km of the JB site), determined that precipitation increased across the Eocene–Oligocene boundary along the Gulf Coast from an average of about 965 mm before 34 Ma to about 1392 mm between 33.70–33.45 Ma (about 5–6 Myr earlier than Jones Branch time). They attributed this increase in precipitation in the Gulf of Mexico to a “northward shift of the Intertropical Convergence Zone, which was driven by the enlarged polar-tropic temperature gradient in the Southern Hemisphere and the strengthened Atlantic Meridional Overturning Circulation (AMOC)” (Hou et al., Reference Hou, Zhuang, Ellwood, Liu and Wu2022, p. 2342–2343). Additional studies by Oboh et al. (Reference Oboh, Jaramillo and Morris1996, Reference Oboh, Jaramillo, Prothero, Ivany and Nesbitt2003) on palynomorphs from the Vicksburg and Jackson groups, and the overlying Chickasawhay Limestone that, together with the overlying and biostratigraphically similar Paynes Hammock Formation, underlies the level low in the Catahoula Formation where the Jones Branch LF occurs (Fig. 4), concluded that the climate during Vicksburgian time (early Rupelian) was warm temperate. This was based on palynomorph assemblages from these units that included both semi-tropical and tropical elements. From the Jones Branch locality, endocarps of Nyssa (Cornaceae) and leaf impressions of Lauraceae, palms, and other undescribed morphotypes with entire or toothed margins provide additional support for a warm temperate to subtropical, estuarine paleoenvironmental setting (Stults et al., Reference Stults, Hermsen and Starnes2024), as does the presence of sciaenid otoliths noted earlier in this report.
The Jones Branch LF also shares several taxa originally known from the Great Plains and the Rocky Mountains region, such as ?Sinclairella, Mesoscalops, Hesperopetes, Downsimus, Eutypomys, Microtheriomys, Leptodontomys, Kirkomys, Promartes, Diceratherium, Protapirus, Leptochoerus, Daeodon, Elomeryx, Hypertragulus, and ?Leptomeryx (Table 11; note also, however, that Eutypomys is recorded from Florida’s I-75 LF, a second species of Sinclairella is known from Florida’s Buda LF, and Leptochoerus has also been reported from Oaxaca, Mexico). Studies of paleosols in the White River Group of Nebraska and South Dakota show that this region also underwent a progressive drying trend “from a predominantly forested habitat in the Late Eocene Chadron Formation to open savannas of the Oligocene Brule Formation” (Benton et al., Reference Benton, Terry, Evanoff, and McDonald and Farlow2015, p. 58). Earlier work by Retallack (Reference Retallack1983) on paleosols in Badlands National Park noted humid, warm temperate to subtropical forests during the Late Eocene culminating in increasingly open woodlands through the Chadronian. Climate continued to become drier, with subhumid to semiarid savanna woodlands during Scenic Member time (Orellan), widespread semiarid savanna with savanna woodlands near streams during Poleside Member time (Whitneyan), and finally semiarid steppe conditions “with severe seasonal drought” during Sharps Formation time (early Arikareean) (Retallack, Reference Retallack1983, p. 61). Like the rain shadow cast over central Oregon by an uplifting Cascade Range, the Rocky Mountains provided a comparable situation over the western Great Plains, contributing to this cooling and (mostly) drying trend (Retallack, Reference Retallack2007; Sheldon, Reference Sheldon2009; Boardman and Secord, Reference Boardman and Secord2013).
Several studies (e.g., Samuels and Hopkins, Reference Samuels and Hopkins2017, p. 39, and references within) have found that small mammals show a more rapid morphological response to environmental changes than do larger species “given the finer scale at which [small mammals] experience the environment.” Importantly, however, nearly all of these studies have focused on changes that occurred across the Columbia Plateau, the northern Rocky Mountains, and the Great Plains—the regions most affected by the cooling and drying trend from the late Eocene through the Oligocene, which resulted in more open environments and the appearance of new niches. Understandably, the paucity of data from the Gulf Coastal Plain in these studies has precluded any consideration of what was occurring there over this same interval of time. Apparently though, as different as Oregon and the Great Plains/northern Rocky Mountains regions must have been from the Gulf Coastal Plain during the early Arikareean, the fact that so many taxa mutually occur in both suggests that the climatic and environmental filters that separated them into distinct physiographic provinces, at least from a biotic perspective, had not yet been strongly emplaced at that time; and/or, that several of these taxa had broader ecological tolerances than what has been traditionally concluded from paleoenvironmental studies of the regions from which they were originally, and are commonly, known. Small taxa such as ?Sinclairella, Mesoscalops, Hesperopetes, Downsimus, Microtheriomys, Apeomys, Leptodontomys, Kirkomys, and Leptochoerus at Jones Branch support these assumptions, as does the presence of the larger horses, rhinos, tapirs, entelodonts, and anthracotheres, although the latter are “buffered” to some extent by such environmental changes due to their ability to range over broader areas (Samuels and Hopkins, Reference Samuels and Hopkins2017).
Although not known from the Gulf Coastal Plain previously, the presence there of Protapirus and Elomeryx is, perhaps, a little less surprising. These two taxa typically have been considered to occupy a semiaquatic niche, based, in part, on comparisons with their modern counterparts. The purported semiaquatic behavior of anthracotheres has been largely confirmed by studies of their skeletal morphology, modifications of their petrosal bone and cochlea, the depositional environmental interpretations of sediments in which their fossils are found, and on phylogenetic analyses of the hippopotamids (Orliac et al., Reference Orliac, Mourlam, Boisserie, Costeur and Lihoreau2023). Additionally, Boardman and Secord (Reference Boardman and Secord2013, p. 46), using stable carbon and oxygen isotope data, determined that by the Orellan, tapirs (i.e., Colodon) “occupied a similar ecological niche [riparian habitat] to Neogene and modern tapirs.” Therefore, it might be expected that Protapirus and Elomeryx would follow riparian corridors to regions more amenable to that niche as the Great Plains cooled and dried, eventually finding their way to the lush coastal environments of what is now the southeastern United States, and where they apparently survived slightly longer than their counterparts in the more northern regions due to a more stable, less changing environment. Nexuotapirus and Arretotherium presumably did just that during the late Arikareean, as these are two of the most common taxa of the Toledo Bend fauna.
With paleoenvironments more amenable to the preservation of vertebrate remains, and a modern landscape much more amenable to erosion and exposure of fossil-bearing strata, the Great Plains, northern Rocky Mountains, and John Day region have been the foci of paleontological studies for well over one hundred years. But along the Gulf and southeastern Atlantic coastal plains, including Florida, the situation is entirely different. These areas are thickly vegetated, of low topographic relief, and the only view of the stratigraphy (other than cores and well logs) with the consequent potential for finding fossils is along riverbanks and roadcuts, in rare commercial quarries, or even beneath the surface of rivers (Albright et al., Reference Albright, Sanders, Weems, Cicimurri and Knight2019). But the lesson learned from sites like Jones Branch, Toledo Bend, Brooksville 2, and I-75 is that the separation of these two regions (i.e., the Great Plains/northern Rockies + Oregon vs. the Gulf Coastal Plain) into distinct physiographic provinces does not necessarily translate into entirely different biogeographic provinces, at least not during the early to middle part of the Oligocene. At the generic level, the two regions are highly comparable, sharing many of the same taxa, although the apparent absence of some groups at Jones Branch, such as nimravids, camels, oreodonts, and (surprisingly) peccaries is particularly notable given that the latter three are found in the Arikareean of Florida (and minimally in the Toledo Bend fauna, easternmost Texas). At the specific level, on the other hand, endemism resulting in different species would be expected along the coast versus more northerly regions of the continent because of adaptations there to a different environmental setting. New species of Mesoscalops, Downsimus, and Apeomys exemplify this, as does the presence of Oligolagus n. gen., Paraktioeomys n. gen., and the small artiodactyl tentatively referred to Leptomeryx.
It appears, therefore, that the Arikareean represents a crucial interval of time for unraveling the level and nature of biotic disparity between the two regions. As Albright (Reference Albright, Terry, LaGarry and Hunt1998a, p. 167) noted in discussing the late Arikareean-aged Toledo Bend Fauna, “On the one hand, the mammalian taxa from Toledo Bend provide evidence for a filtered corridor of dispersal between the rich faunas of the northern Great Plains and the Gulf Coastal Plain, but on the other hand they support recognition of earliest Miocene Gulf Coastal Plain endemism.” Although the Jones Branch LF reinforces that conclusion to some extent, pushing this scenario even farther back in time, it appears that these filters were much weaker during the early Arikareean than later given that the Gulf Coastal Plain and the Great Plains + Oregon + the northern Rocky Mountains regions shared so many taxa then. If the paleoenvironmental reconstructions noted above for Oregon and the Great Plains are accurate for Chadronian through early Arikareean time, then there appears to be a biotic lag between that time and the later Oligocene/Early Miocene when Gulf Coastal Plain endemism was more pronounced.
Another interesting aspect of this fauna involves the presence of a few rodent taxa never described from any other North American site of any age. Although rodents have been heavily studied from Oligocene-aged sites all over North America, with new species being described regularly, nothing like Paraktioeomys n. gen. or the specimens referred to “Eomyidae undetermined genus and species” appears in any of them. Paraktioeomys, for example, more closely resembles the European Theridomyidae than any other group, yet differences preclude its placement into that family. There are also similarities with Pseudotheridomys, known from both Europe and North America, but it does not ‘fit’ into that genus either. As noted earlier, the specimens from Florida’s I-75 LF that Patton (Reference Patton1969) referred to “Eomyidae” also belong to Paraktioeomys n. gen. These new “anomalous” rodents provide further support for the unique nature of the early to middle Oligocene Gulf Coastal Plain noted above. Limited filters between regions separated by thousands of kilometers and under different climatic regimes apparently resulted in unexpected biotic similarity, although the paleoenvironment of the Gulf Coast was likely conducive to a degree of endemism, in addition to serving as a refugium for certain of the large mammals thought to have gone extinct at the end of Ar1 based on their northern Midcontinent records (e.g., Protapirus and Elomeryx).
The rarity of fossil sites with faunas spanning this temporal interval in the Gulf Coastal Plain is in large part due to the nature of the paleo- and current environment there, with its limited potential for the preservation of remains and for exposure of sites that might yield remains that are preserved. This contrasts with the situation in the more northern part of the continent with its hundreds of sites. As noted above, the Gulf Coastal Plain then, as now, was/is a subtropical to warm temperate, humid, heavily vegetated region of low topographic relief, whereas the more northern regions were/are drier and less vegetated, with much greater preservation/erosion/exposure potential. As rare as sites of Oligocene age are across the Gulf and southeastern Atlantic coastal plains, the paradoxical biotic similarity/disparity between this region and the more northerly parts of North America should come into even sharper focus as more localities in the ‘deep South’ are discovered and studied.
Acknowledgments
First and foremost, our gratitude is extended to A. Weller and R. Raines of Waynesboro, MS, who discovered the Jones Branch site, alerted GEP to its fossils, assisted with field work, and donated many of their finds to the MMNS making this report possible. Also assisting with field work were B. Axsmith, J. Axsmith, R. Bonett, D. Ehret, K. Irwin, and additional members of the Weller family—Ashlin, Landon, and Zoey. Others who collected and/or donated fossils and/or processed matrix for micro-vertebrates include C. Ciampaglio, W. Collins, J. Dearman, D. Ehret, B. Hart, R. Horn, K. Irwin, B. Martin, J. McCraw, E. Mooney, L. Ritchie, J. Rushing, K. Shannon, L. Weller, and A. Winters. D. Cicimurri, Curator of Natural History at the SCSM, also processed and sorted matrix from the site, and provided for loans of the material he recovered. M. Ganesan and Q. Song of Johnson and Johnson’s 3-D Printing Laboratory at the University of North Florida generously gave their time and expertise to help produce the CT images of the micro-mammals. Having never before attempted the imaging of fossil material, particularly such tiny specimens, their heroic efforts are greatly appreciated. M. Boyles of UNF’s Center for Instruction and Research Technology is thanked for the help he provided in preparing the figures that included the CT images. LBA thanks R. Hunt for assistance while visiting the UNSM, M. Price for assistance with specimens at the CCNHM, M. Gibson and J. Peragine for similar assistance at the ChM, plus R. Hulbert, J. Bloch, and R. Narducci at the FLMNH. P. Holroyd, UCMP, H. Singleton, Amherst College Museum, B. Simpson, Field Museum, Chicago, and R. Hunt, UNSM, Lincoln, are thanked for providing images of specimens in their care. R. Hunt and M. Woodburne reviewed early versions of the manuscript, and formal reviews were provided by B. MacFadden, G. Morgan, J. Samuels, and an anonymous reviewer. All of their comments and suggestions helped improve the paper, as did additional comments by Journal of Paleontology editor J. Calede and technical assistance from J. Kastigar. Financial support for this project was provided, in part, by the Florida Academy of Sciences.
Competing interests
The authors declare none.