Introduction
GowersReference Gowers 1 was the first to describe cerebral venous thrombosis (CVT) in his seminal work on diseases of the nervous system. Since that time, and in concert with the evolution of novel noninvasive imaging techniques,Reference Poon, Chang, Swarnkar, Johnson and Wasenko 2 , Reference Renowden 3 our knowledge of CVT has greatly expanded. CVT accounts for 0.5% of all strokes,Reference Bousser and Ferro 4 with an annual incidence of roughly 7 cases per million adults.Reference Agnelli and Verso 5 , Reference Stam 6 Of all reported CVT cases, roughly 75% occur in females,Reference Stam 6 , Reference Ferro, Canhao, Stam, Bousser and Barinagarrementeria 7 likely related to such conditions as pregnancy, puerperium, and the use of oral contraceptives.Reference Agnelli and Verso 5 , Reference Dentali, Crowther and Ageno 8 Though a large number of additional CVT risk factors have been proposed, recent multicenter studies have helped clarify the most prevalent and critical risk factors.Reference Agnelli and Verso 5 - Reference Ferro, Canhao, Stam, Bousser and Barinagarrementeria 7 , Reference Wasay, Bakshi and Bobustuc 9 - Reference Masuhr, Mehraein and Einhaupl 12 One of the largest studies to date, the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT), demonstrated that thrombophilia, puerperium, and infection are the most common risk factors in patients with CVT.Reference Ferro, Canhao, Stam, Bousser and Barinagarrementeria 7
The clinical presentation of CVT is highly variable, with the most common presentation being headache.Reference Ferro, Canhao, Stam, Bousser and Barinagarrementeria 7 A wide range of other symptoms—including focal motor deficits, focal sensory deficits, seizure, encephalopathy, and coma—have been well-described throughout the literature, though all of them lack specificity for CVT.Reference Stam 6 While clinical presentation and known CVT risk factors can provide clues to the diagnosis of CVT, neuroimaging studies are key to establishing the diagnosis. A variety of imaging modalities—including computed tomography (CT), magnetic resonance imaging (MRI), CT angiography (CTA) and venography (CTV), MR venography (MRV) and contrast enhanced MRV, and digital-subtraction angiography (DSA)—now exist to help confirm the diagnosis of CVT. Of these, MRI with contrast-enhanced MRV is the most sensitive modality for detection of CVT.Reference Bousser and Ferro 4 - Reference Leach, Fortuna, Jones and Gaskill-Shipley 13 Guidelines now exist for the treatment of CVT,Reference Einhaupl, Bousser and de Bruijn 14 with a focus on early administration of antithrombotic therapy, aggressive management of raised intracranial pressure, and prompt control of seizures.
Classically, the prognosis of CVT has been grim, what with a mortality rate as high as 50%.Reference Barnett and Hyland 15 , Reference Nagpal 16 However, with the evolution of modern neuroimaging techniques and interventions, in addition to the early administration of antithrombotic therapy, the prognosis of CVT is felt to have significantly improved.Reference Ferro, Canhao, Stam, Bousser and Barinagarrementeria 7 , Reference Wasay, Bakshi and Bobustuc 9 , Reference Baumgartner, Studer, Arnold and Georgiadis 17 - Reference Stolz, Trittmacher and Rahimi 20 Surveillance studies describing the trends in the prognosis of CVT over time in defined populations are lacking. In the present study, we compare patients diagnosed with nonseptic CVT at the University of Alberta Hospital (UAH) from two time periods (1988-1998 and 1999-2009) in order to better understand the evolution and outcomes of this condition in a Canadian tertiary-care institution.
Methods
A retrospective study was performed on patients diagnosed with CVT at the UAH during two sequential time periods: 1988-1998 and 1999-2009. The UAH is the only tertiary-care neurological and stroke center in a region with a catchment area of about 1.5 million persons. Patients were included in the study provided they had a diagnosis of nonseptic CVT (with ICD–9 codes 325 and 437.6 and ICD–10 codes I63.6, I67.6, and G08) and were over 18 years of age. From these patients, we included only those with nonseptic cerebral venous sinus thrombosis. All the patients in our study were admitted through the neurology service. The study received ethics approval from the Human Research Ethics Board of the University of Alberta.
Data extraction focused on presenting signs and symptoms; a comprehensive list of risk factors associated with CVT;Reference Ferro, Canhao, Stam, Bousser and Barinagarrementeria 7 imaging findings (including CT, CTA, CTV, MRI, MRA, MRV, and DSA); identified etiologies; treatment modalities; and clinical status at discharge. Discharge-modified Rankin Scale (mRS) score was determined from the allied health (rehabilitation) section on inpatient charts. Severe symptoms at discharge were defined as death, seizures, focal weakness, or language deficit. All data are presented as means±standard deviations or percentages. Univariate analysis was performed with chi-square tests, t-tests, or Wilcoxon’s rank-sum tests where appropriate. Multivariate analysis was undertaken employing logistic regression. Statistical calculations were executed using OriginPro 8® (Origin Lab, Northampton, MA) and SAS 9.3 (SAS Institute, Cary, NC). Results were considered significant when p<0.05.
Results
Twenty-one patients from the 1988-1998 cohort (C1) and 40 patients from the 1999-2009 cohort (C2) were identified as having CVT. There were 19 females (90.5%) and 2 males in C1, whereas there were 22 females (55.0%) and 18 males in C2, and this difference was significant (p=0.009). Age was comparable between the two groups, with the average age of C1=35.2±15.9 and C2=40.7±15.4 years (p=0.195). There was no significant difference in median time to presentation (C1=3 days, interquartile range [IQR]=1, 6; C2=7 days, IQR=1, 10.5; p=0.193) between the groups. Presenting signs and symptoms were similar between the two cohorts, with the most common being headache, nausea/vomiting, focal motor deficit, and seizure (see Table 1). Seizure at presentation was significantly more common in C1, as compared to C2. The most common identified CVT risk factor in both groups was active hormonal contraception (Table 2). Previous DVT was the only risk factor that was different between the two groups, being more frequent in C2.
Table 1 Presenting symptoms/signs

Table 2 Identified risk factors
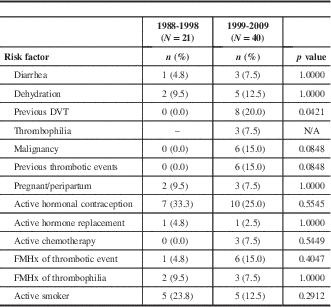
DVT=deep vein thrombosis; FMHx=family history; N/A=not applicable.
Imaging findings were similar between both groups (Table 3). All patients received CT scanning, and in both cohorts the most frequent CT findings were hematoma and hyperdense sinuses. Rare CT findings from C1 included one patient with intraventricular hemorrhage (IVH) and another with a dilated superior orbital vein, whereas temporal metastasis was noted in one patient from C2 (data not shown). There were 90.5% (18/21) of patients from the first cohort who received MRI and 87.5% (35/40) in the second. On MRI, sinus thrombus was detected in roughly three-quarters of patients in each cohort. Intracranial hemorrhage (ICH) was also common among the two groups, with approximately 30% of patients in each group demonstrating this finding. Seven C1 patients (33.3%) underwent DSA, all demonstrating the presence of a venous thrombus. A total of 5 patients (12.5%) from the C2 cohort also had a cerebral angiogram. As with C1, all 5 had demonstrated thrombus on cerebral angiogram.
Table 3 CT and MRI findings
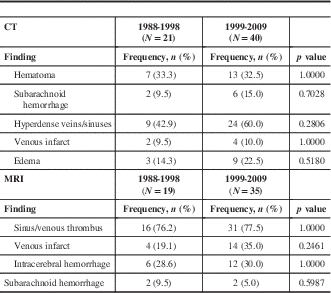
There was no significant difference between the two groups with respect to CVT etiologies (Table 4). The most common etiologies in C1 were thrombophilia and the use of hormonal medications. In C2, thrombophilia (5 patients, 12.5%) and malignancy (5 patients, 12.5%) were common etiologies, though an etiology was not identified in 17 patients (42.5%). The identified malignancies were breast cancer (3 patients), colon cancer (1 patient), and brain cancer (grade IV astrocytoma, 1 patient).
Table 4 Etiologies

* The identified thrombophilias in the 1988-1998 cohort were systemic lupus erythematosus (SLE, one patient); SLE with anti-phospholipid antibody syndrome (antiphospholipid antibody syndrome [APLA], one patient); paroxysmal nocturnal hemoglobinuria (one patient); APLA with protein S deficiency (one patient); factor V Leiden mutation (heterozygous, one patient); and polycythemia rubra vera (one patient). The thrombophilias identified in C2 were protein S deficiency (one patient), APLA (two patients), hyperhomocysteinemia (one patient), and factor V Leiden mutation (one patient).
Anticoagulation with unfractionated heparin or low-molecular-weight heparin was the most common treatment modality between the two cohorts, with 18 patients (85.7%) from C1 and 36 (90.0%) from C2 undergoing this treatment. We found that 71.4% of patients in C1 and 72.5% of C2 patients were also bridged to warfarin during hospitalization. Thrombolytics were rarely used in the treatment of CVT, with only 3 patients (14.3%) in the C1 group and 2 (5.0%) in the C2 group undergoing catheter-based intracranial thrombolysis. Similarly, antiplatelet agents were used in only 9.5% of C1 patients and 15% of C2 patients.
At discharge, 4 patients (19.1%) from the C1 group and 2 patients (5.0%) from the C2 cohort died during the course of hospitalization, but this difference was not statistically significant (Table 5). Ongoing seizure activity at the time of discharge was more common in the C1 cohort, as compared to the C2 cohort. These were continued intermittent seizures, as no patients had partial or generalized status epilepticus. Other individual deficits (including focal motor, focal sensory, and language deficits) were not significantly different between groups at discharge. Patients in the second cohort were significantly less likely to have at least one severe deficit or to have died at discharge, and this difference persisted after adjustment for age and sex (odds ratio [OR]=0.178; 95% confidence interval [CI 95%]=0.051, 0.625). Similarly, patients in the second cohort were considerably more likely to have a favorable mRS score of 0 or 1 at discharge (Table 5). This difference persisted after adjustment for age and sex (OR=7.98; CI 95%=1.79, 35.71). The average length of stay was similar between groups, being 12.0±12.5 days in C1 and 12.5±9.9 days in C2 (p=0.449).
Table 5 Clinical status at discharge

* Severe symptoms at discharge defined as any of death, continuing intermittent seizures, focal weakness, or language deficit.
** This difference persisted after adjustment for age and sex (see Results section).
Discussion
To our knowledge, our study represents the longest duration of surveillance for CVT hospitalizations within a defined catchment area to examine for trends in prognosis over time. Our study differs in purpose from such longitudinal studies as the Cerebral Vein Thrombosis International Study (CEVETIS), which took a single large cohort of patients and followed them over time to determine long-term outcomes.Reference Dentali, Poli and Scoditti 22 Similarly, the ISCVT clarified much in the way of our understanding of CVT, though it did not examine trends over time.Reference Ferro, Canhao, Stam, Bousser and Barinagarrementeria 7
Herein we retrospectively studied two cohorts (C1=1988-1998, C2=1999-2009) of patients diagnosed with CVT at the UAH, so that we might better understand the evolution of presentation and prognosis of this condition at a major Canadian tertiary-care institution. We found diverse etiologies, the most common being thrombophilia, hormonal medications, and malignancy. A number of cases, particularly in the C2 cohort, did not have an identified etiology.
The majority of patients diagnosed with CVT from our study were female. There was a strikingly higher preponderance of female patients in C1 compared to C2. In addition, the number of patients presenting in C2 was double that of C1. The population of Edmonton increased by 16% between the midpoints of the two study periods, which does not account for the discrepancy in numbers. This suggests that the condition may have been underdiagnosed at our institution during the first time period, and particularly among males. Both cohorts had similar rates of CT scan and MRI scan use. Improvements in diagnostic imaging modalities such as contrast-enhanced MRV and better availability of these modalities in the second cohort may have allowed for diagnosis of more patients of lower severity. It is possible that over the time period of the first cohort there were other patients not diagnosed and not included due to false-negative imaging, as a result of sequences that were less advanced than those in the second cohort. This may have produced some degree of ascertainment bias favoring better prognosis in the second cohort.
However, if the differences in outcome were entirely due to ascertainment bias, we would have expected a much greater baseline severity at presentation in the first cohort compared to the second. There did seem to be a trend toward a higher frequency of severe symptoms in the first cohort, who, overall, were 14% more likely to have at least one severe symptom at presentation. However, this difference was not statistically significant. Seizure occurrence was the only presentation variable that was significantly different between the two groups, with seizures being more common in C1. Despite being not significantly different at presentation, patients in C1 had a higher likelihood of being dead or having at least one severe deficit at discharge and were less likely to have a favorable discharge mRS score than those in C2. In contrast to the relatively small 14% discrepancy in severe symptoms at presentation, there was a nearly 40% difference in severe symptoms upon discharge in C1 compared to C2 (Table 5). This implies a greater deterioration over the length of admission of the C1 cohort versus that in the C2 cohort. The reasons for this are not known with certainty, as both cohorts received similar antithrombotic management. We are unable to compare the timing of initiation of anticoagulant therapy between cohorts, as that information was not collected. The C1 cohort patients actually presented an average of 4 days earlier from symptom onset than those in the C2 cohort. Even if we allow for a 1- to 2-day delay in obtaining imaging in C1 compared to C2 due to less timely access, it seems unlikely that anticoagulation would have been initiated longer after presentation in C1 compared to C2. It is likely that other advances in general stroke care at the UAH from 1999 to 2009 may have resulted in improved outcomes.
We found in-hospital mortality to be higher in the C1 group (19.1%) compared to that in the C2 cohort (5%), but this difference was not statistically significant and was based on only 6 deaths. Nonetheless, our combined mortality rate across both cohorts was 10%. The 1988-1998 cohort had a significantly lower proportion of patients with an mRS score of 0 to 1. In contrast, 75% of the patients in the 1999-2009 cohort had an mRS score of 0 to 1. We were not able to detect any differences in investigation, management, and etiology between our two cohorts that could clearly explain the discrepancies in outcome.
Study Limitations and Strengths
There are several limitations to our study to be noted. This research was retrospective in nature and relied on ICD-9 and ICD–10 codes to identify only CVT patients who were admitted to hospital. We may have missed patients with less severe disease seen in neurological clinics. Where inpatients are concerned, studies suggest that ICD codes have reasonable sensitivity and specificity to detect CVT.Reference Liberman, Kamel, Mullen and Messe 21 It is therefore unlikely that we missed CVT patients in our target population of those patients severe enough to be admitted to hospital. The small study size is a limitation, and our sample may not provide adequate power to demonstrate statistically significant differences between the two groups. This is a limitation common to studies examining low-incidence conditions. We still consider it worthwhile to obtain regional data on such uncommon conditions as CVT. We discussed above the possibility that patients with milder symptoms in the first cohort may have been missed, and males in particular. Nonetheless, our conclusions still apply to those patients receiving a confirmed diagnosis of cerebral venous sinus thrombosis at a tertiary-care institution during the time periods in question. Finally, we do not have predmission mRS score available to ensure that differences between mRS score on discharge were not due to preexisting differences. Having said that, the patients in both cohorts were admitted from the community, were quite young on average, and had a similar proportion of severe neurological deficits at presentation, so we think it very likely that they were similar functionally upon preadmission.
Taken together, our data suggest improvements in the number of patients diagnosed, a reduction in severe residual symptoms at discharge, and improved functional status at discharge for patients presenting with CVT from 1999 to 2009 compared to patients from the time period of 1988-1998.
Acknowledgments
The authors acknowledge the medical records department at Alberta Health Services for their assistance in gaining access to patient charts.
Statement of Authorship
TJ designed the study. JK and TJ performed the chart review. DA and TJ performed data analysis and wrote the manuscript.
Disclosures
Drs. Anderson and Kromm contributed equally to this work. They both hereby declare that they have nothing to disclose.
Dr. Jeerakathil reports grants from AIHS, grants from HSFC, grants from the Canadian Stroke Network, grants from the CIHR, grants from The University Hospital Foundation, and grants from Alberta Health Services, outside the submitted work. He also received honoraria from Bayer for two advisory board meetings within the last five years on a topic unrelated to this manuscript.






