Introduction
Hypomania (symptoms include irritability and elevated mood) is a defining feature of bipolar disorder (BD) [1], but subclinical hypomania (i.e., symptoms that do not meet the diagnostic criteria) is common in the general population. Though often transient and benign, subclinical hypomania can have serious consequences, ranging from life dissatisfaction and suicidality to the later development of psychopathology, including BD [Reference Hosang, Cardno, Freeman and Ronald2–Reference Päären, Bohman, Von Knorring, Von Knorring, Olsson and Jonsson4]. For instance, up to 10% of adolescents have been identified as being at high-risk for BD (based on the number, clustering, and impact of hypomanic symptoms) [Reference Hosang, Cardno, Freeman and Ronald2]. The study of subclinical phenotypes (e.g., hypomania) in the general population can be useful for understanding the developmental pathways of associated disorders (e.g., BD), which is critical for advancing prevention and treatment efforts [Reference Martin, Taylor and Lichtenstein5]. Little is known about subclinical hypomania’s etiology, although this is a growing area of investigation [Reference Gonzalez-Calvo, Ronald, Shakoor, Taylor, Eley and Hosang6].
Childhood trauma (e.g., emotional or physical maltreatment) is an important risk factor to consider, given its strong link with BD and other mental illnesses [Reference Palmier-Claus, Berry, Bucci, Mansell and Varese7,Reference Hosang, Fisher, Hodgson, Maughan and Farmer8]. There is emerging evidence that childhood trauma is associated with adolescent subclinical hypomania [Reference Gajwani, Dinkler, Lundström, Lichtenstein, Gillberg and Minnis9]. These findings need to be replicated, especially considering hypomania at ages beyond adolescence, when psychopathology becomes more prevalent at clinical levels [Reference Caspi, Houts, Ambler, Danese, Elliott and Hariri10]. Because BD typically manifests between the ages of 15– 24 years [Reference Kroon, Wohlfarth, Dieleman, Sutterland, Storosum and Denys11], early adulthood constitutes a key stage to focus on the study of hypomania. Beyond associations, it is important to illuminate the etiological influences underlying the childhood trauma–hypomania link to clarify causality and guide better-targeted intervention.
Heritability and gene–environment interplay
While childhood trauma can be considered an environmental risk factor, it is partly genetically influenced, with up to 62% of its variance attributed to genetic factors [Reference Jay Schulz-Heik, Rhee, Silvern, Lessem, Haberstick and Hopfer12–Reference Warrier, Kwong, Luo, Dalvie, Croft and Sallis14]. Furthermore, childhood trauma is linked to genetic predisposition to neurodevelopmental and psychiatric conditions, as measured by polygenic scores (PGS: risk estimates based on common genetic variants), including autism, attention-deficit/hyperactivity disorder (ADHD), post-traumatic stress disorder (PTSD), major depressive disorder (MDD), and schizophrenia [Reference Warrier, Kwong, Luo, Dalvie, Croft and Sallis14–Reference Woolway, Smart, Lynham, Lloyd, Owen and Jones17]. Other studies have found a significant negative association with the BD PGS, with individuals with this genetic vulnerability reporting less childhood trauma [Reference Aas, Bellivier, Bettella, Henry, Gard and Kahn18]. These patterns provide evidence of possible gene–environment correlations (rGE), whereby one’s genetic predisposition can influence exposure to specific environments [Reference Plomin, DeFries and Loehlin19].
There is also evidence of gene–environment interactions (environmental and genetic effects being dependent on each other; GxE) [Reference Kendler and Eaves20] with childhood trauma in hypomania-related phenotypes. Specific polymorphisms (e.g., Val66Met in the brain-derived neurotrophic factor gene) have been reported to increase vulnerability to childhood trauma’s effect on subclinical psychosis [Reference De Castro-Catala, Van Nierop, Barrantes-Vidal, Cristóbal-Narváez, Sheinbaum and Kwapil21], and the PGS for ADHD and BD have been found to moderate the impact of childhood trauma on BD [Reference Park, Shekhtman and Kelsoe22,Reference Wilcox, Fullerton, Glowinski, Benke, Kamali and Hulvershorn23]. Negative GxE effects have also been found; individuals reporting high trauma levels and with a low BD PGS presented a more unstable form of BD in a clinical sample study [Reference Aas, Bellivier, Bettella, Henry, Gard and Kahn18]. No gene–environment interplay studies have focused on subclinical hypomania, but similarly complex patterns are expected given its genetic overlap with BD [Reference Hosang, Martini, Ronald, Larsson, Lundström, Lichtenstein and Taylor24], its genetic association with the conditions correlated with childhood trauma (ADHD, schizophrenia and MDD) [Reference Hosang, Martin, Karlsson, Lundström, Larsson and Ronald3], and, more broadly, based on evidence that psychiatric symptoms and life impairment are underpinned by a common cross-trait genetic factor [Reference Selzam, Coleman, Caspi, Moffitt and Plomin25].
Study aims
The overall objective of this study was to investigate the association between childhood trauma and subclinical hypomania in early adulthood using a genetically informative approach, addressing three aims. Firstly, the phenotypic association between childhood trauma and subclinical hypomania was examined. Secondly, the degree of genetic and environmental overlap between childhood trauma and subclinical hypomania was estimated using bivariate twin models. Thirdly, the interplay between childhood trauma and PGS for nine relevant psychiatric and neurodevelopmental conditions (e.g., ADHD and BD) was investigated, focusing on rGE and GxE effects on subclinical hypomania. To fully capture this phenotype, we conceptualize subclinical hypomania in two ways: i) as a continuous trait (symptom count) and ii) as a categorical construct (individuals at high-risk for BD based on established classifications).
Methods
Participants
Participants were members of the Twins Early Development Study (TEDS) [Reference Lockhart, Bright, Ahmadzadeh, Breen, Bristow and Boyd26–Reference Rimfeld, Malanchini, Spargo, Spickernell, Selzam and McMillan28], a longitudinal prospective community sample of twins born in England and Wales (1994–1996) identified through the Office of National Statistics. We analyzed data from two data collection waves: ages 21 (N = 8,464; 63% female) and 26 years (N = 7,748; 65% female). Only participants with data available at both timepoints (N = 6,473) were included in analyses. Participants were excluded if they had specific medical conditions and/or extreme perinatal outliers, in line with standard TEDS procedures [Reference Haworth, Davis and Plomin27]. TEDS has ethical approval from King’s College London Ethics Committee (Reference: PNM/09/10–104). Informed consent was obtained from all participants at each wave.
Materials
Childhood trauma
Childhood trauma was assessed retrospectively when participants were 21 years old using eight self-report items derived from the Avon Longitudinal Study of Parents and Children “Life at 22+” questionnaire (Supplementary Table 1) [Reference Houtepen, Heron, Suderman, Tilling and Howe29]. Using this measure, participants reported how frequently they experienced different types of childhood emotional/physical abuse (e.g., “how often did an adult push, grab, or shove you?”), on a scale of “Never” (0) to “Very often” (4). Total scores range from 0 to 32.
Subclinical hypomania
Subclinical hypomania was measured at age 26 using the self-reported Mood Disorder Questionnaire (MDQ) [Reference Wagner, Hirschfeld, Emslie, Findling, Gracious and Reed30]. The MDQ consists of 13 yes/no items examining hypomanic symptoms [1]. The MDQ is one of the best-validated instruments for youth BD[Reference Youngstrom, Genzlinger, Egerton and Van Meter31] and has shown good sensitivity (0.73) and specificity (0.90) for identifying bipolar spectrum disorders [Reference Hirschfeld, Williams, Spitzer, Calabrese, Flynn and Keck32]. We categorized individuals as high-risk for BD using the criteria employed in the Genetic Links to Anxiety and Depression (GLAD) study [Reference Davies, Kalsi, Armour, Jones, McIntosh and Smith33], which is slightly less conservative than the MDQ criteria [Reference Hirschfeld, Williams, Spitzer, Calabrese, Flynn and Keck32], based on the following:
-
1. Number of symptoms
-
a. 7 of the 13 symptoms were reported, or
-
b. 1 elation symptom and at least 3 of any of the other symptoms, or
-
c. 1 symptom of irritability and any other 4 symptoms.
-
-
2. Symptoms clustered in the same period, and
-
3. Reported moderate/severe impairment as a consequence.
Genome-wide polygenic scores
DNA samples were obtained from saliva and buccal cheek swabs. Genotyping was carried out by TEDS researchers on Affymetrix GeneChip 6.0. or HumanOmniExpressExome8v1.2. [Reference Selzam, McAdams, Coleman, Carnell, O’Reilly and Plomin34]. More genotyping information is available elsewhere [35]. PGS were derived from genome-wide association studies (GWAS) and were calculated using LDpred or LDpred-2. PGS summarize trait-associated effect sizes of individual genetic variants (weighted sum of condition-associated alleles). PGS for nine relevant psychiatric and neurodevelopmental conditions were used in this study: BD (overall, BD I and BD II) [Reference Mullins, Forstner, O’Connell, Coombes, Coleman and Qiao36], MDD [Reference Wray, Ripke and Mattheisen37], schizophrenia [Reference Trubetskoy, Pardiñas, Qi, Panagiotaropoulou, Awasthi and Bigdeli38], PTSD [Reference Nievergelt, Maihofer, Klengel, Atkinson, Chen and Choi39], autism [40], anxiety [Reference Purves, Coleman, Meier, Rayner, Davis and Cheesman41], and ADHD [Reference Demontis, Walters, Athanasiadis, Walters, Therrien and Nielsen42]. PGS were selected due to their previously reported association with childhood trauma, subclinical hypomania, BD, and/or other genetically overlapping conditions [Reference Peel, Purves, Baldwin, Breen, Coleman and Pingault15, Reference Aas, Bellivier, Bettella, Henry, Gard and Kahn18, Reference Mullins, Forstner, O’Connell, Coombes, Coleman and Qiao36, Reference Jiang, Zai, Dimick, Kennedy, Young and Birmaher43, Reference Richards, Cardno, Harold, Craddock, Di Florio and Jones44].
Additional measures
Subclinical hypomania at age 16 was measured using the self-report Hypomania Checklist-16 (HCL-16; Supplementary Materials) [Reference Forty, Kelly, Jones, Jones, Barnes and Caesar45]. Hypomania data at both time points were available for 1,901 participants. Participants assessed at age 16 were part of the Longitudinal Experiences and Perceptions Project within TEDS. LEAP has ethical approval from King’s College London Ethics Committee (Reference: HR/DP-20/21–22060).
Data analysis
Analyses were pre-registered on the Open Science Framework (https://osf.io/pvqda/) prior to receiving access to the dataset [Reference Calvo, Ronald, Taylor, Havers and Hosang46]. All analyses were performed using R (Version 2022.12.0 + 353 (2022.12.0 + 353)). Phenotypic variables were transformed using square root transformation techniques to meet the assumption of having a normal distribution for twin modelling. Sex was included as a covariate in all models. The Benjamini–Hochberg procedure (false discovery rate) was used to correct for multiple testing (at p ≤ .05) within each research question.
Phenotypic analyses
The effects of childhood trauma on hypomanic symptoms and high-risk for BD were tested using linear and logistic regressions, respectively, using standardized scores. Analyses were implemented as doubly robust generalized estimating equations (GEE; drgee package) [Reference Zetterqvist and Sjölander47] to account for related individuals in the sample and calculate robust standard errors. Sensitivity analyses were used to further examine these associations while controlling for the influence of hypomania at age 16 years, since there is evidence that adolescents who display hypomania-related symptoms (e.g., impulsivity and irritability) are at increased risk of experiencing both childhood trauma [Reference Hadianfard48] and later hypomania [Reference Hosang, Cardno, Freeman and Ronald2]. Hypomania at age 16 (N = 2,943, 57% female) was assessed as part of the Longitudinal Experiences And Perceptions project (LEAP) [Reference Ronald, Sieradzka, Cardno, Haworth, McGuire and Freeman49].
Twin analyses
The classic twin design allows to decompose phenotypic variance and covariance into genetic and environmental influences using data from monozygotic (MZ) and dizygotic (DZ) twins. This method is based on the principle that MZ twins share 100% of their genetic influences (compared with DZ twins, who share approximately 50%), both MZ and DZ twins share all their common environment (i.e., factors in the same family), and are exposed to non-shared environmental factors unique to the individual, contributing toward differences between both MZ and DZ twin pairs. A detailed description of this method can be found elsewhere [Reference Knopik, Neiderheiser, DeFries and Plomin50]. Univariate structural equation twin models were used to estimate additive genetic (A), non-additive genetic (D), shared environmental (C), and non-shared environmental (E) contributions to childhood trauma, hypomanic symptoms, and high-risk for BD. Liability threshold models were used for high-risk for BD, given the categorical nature of this variable. Bivariate twin models were then fitted to investigate the causes of covariation between childhood trauma and i) hypomanic symptoms (continuous model) and ii) high-risk for BD (joint categorical–continuous model). Specifically, an ACE model was used to estimate the proportion of covariance between childhood trauma and hypomanic symptoms explained by genetic (bivariate heritability) and environmental factors (bivariate shared environment and bivariate non-shared environment), as well as the genetic (ra) and environmental correlations (rc and re) between these phenotypes. These correlations reflect the extent to which the genetic and environmental influences on childhood trauma overlap with those on subclinical hypomania. Based on evidence of non-additive genetic effects, a bivariate ADE model was fitted for childhood trauma and high-risk for BD. Twin analyses were performed in OpenMx (v2.21.11), using the method of maximum likelihood estimation. In line with standard behavioral genetics procedure, the effects of sex and age were regressed out, and analyses were conducted using standardized residuals.
Polygenic score analyses
PGS analyses were performed using GEE (accounting for sample relatedness, as described above). All models were adjusted for the first ten principal components (PCs) of ancestry, genotyping chip and batch, and sex. First, rGE were estimated using univariable linear regressions testing each PGS’s association with childhood trauma using the total sample. A multivariable linear regression model was subsequently fitted, including all 9 PGS as predictors of childhood trauma. In line with previous studies [Reference Hosang, Shakoor, King, Sanches, Vincent and Kennedy51, Reference Mullins, Power, Fisher, Hanscombe, Euesden and Iniesta52], all analyses were repeated separately for the high-risk for BD and control groups to determine whether rGE were specific to either one.
Second, GxE were examined using both multiplicative and additive models, fitted separately for each PGS. Multiplicative GxE models test whether the combined effect of each PGS and childhood trauma differs from the product of their individual effects (i.e., relative risk). Multiplicative interactions were tested using linear (hypomanic symptoms) and logistic regressions (high-risk for BD/control status) [Reference Hosang, Shakoor, King, Sanches, Vincent and Kennedy51–Reference VanderWeele and Knol53]. The main effects of the PGS and childhood trauma and the interaction between the PGS and childhood trauma were included as the predictors, controlling for sex, the first 10 PCs, the interaction term between each PC and the PGS, and the interaction term between each PC and childhood trauma [Reference Hosang, Shakoor, King, Sanches, Vincent and Kennedy51,Reference Keller54]. Additive GxE models test absolute risk. Additive interactions were tested using linear regressions on both the number of hypomanic symptoms and high-risk for BD/control status, with the PGS x childhood trauma interaction as the predictor, controlling for the same covariates used in the multiplicative models [Reference Hosang, Shakoor, King, Sanches, Vincent and Kennedy51–Reference VanderWeele and Knol53].
Results
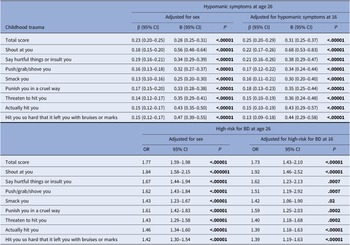
A description of the sample is presented in Table 1. Males reported significantly more hypomanic symptoms (M = 3.40, SD = 3.48) than females (M = 2.94, SD = 3.39; β = 0.07, SE = 0.09, p < .0001). Sex was not significantly associated with being at high-risk for BD (OR = 1.00, 95% Confidence Intervals [CI]: 0.80–1.27, p = .95). The average total score of childhood trauma did not differ significantly by sex (β = 0.02, SE = 0.02, p = .21). Childhood trauma descriptive statistics can be found in Supplementary Table 1.
Table 1. Sample description
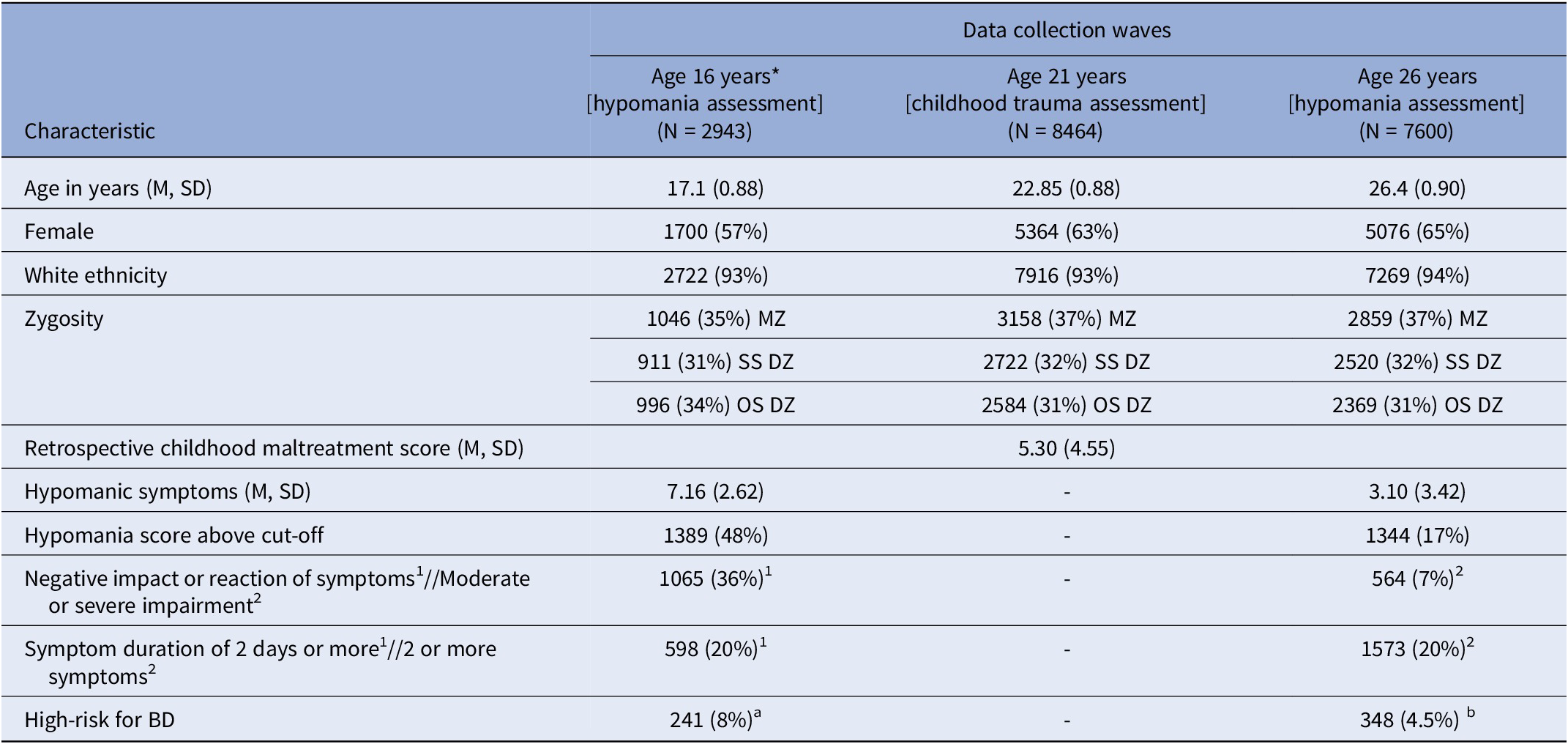
Abbreviations: M (Mean), SD (standard deviation), N (number), MZ (monozygotic), SS DZ (same-sex dizygotic), OS DZ (opposite sex dizygotic), BD (bipolar disorder).
Note: See aage 16 and bage 26 hypomania measures for an explanation of how the high-risk groups were calculated, including information on the cut-off scores. *Data included in sensitivity analyses.
1 Characteristics measured at age 16 years. 2 Characteristics measured at age 26 years.
Phenotypic associations between childhood trauma and subclinical hypomania
Childhood trauma (total score and each item) was significantly associated with hypomanic symptoms and increased odds of being at high-risk for BD at age 26 (Table 2). All associations remained significant in sensitivity analyses, which adjusted for hypomania at age 16.
Table 2. Association between childhood trauma, hypomanic symptoms, and high-risk for bipolar disorder
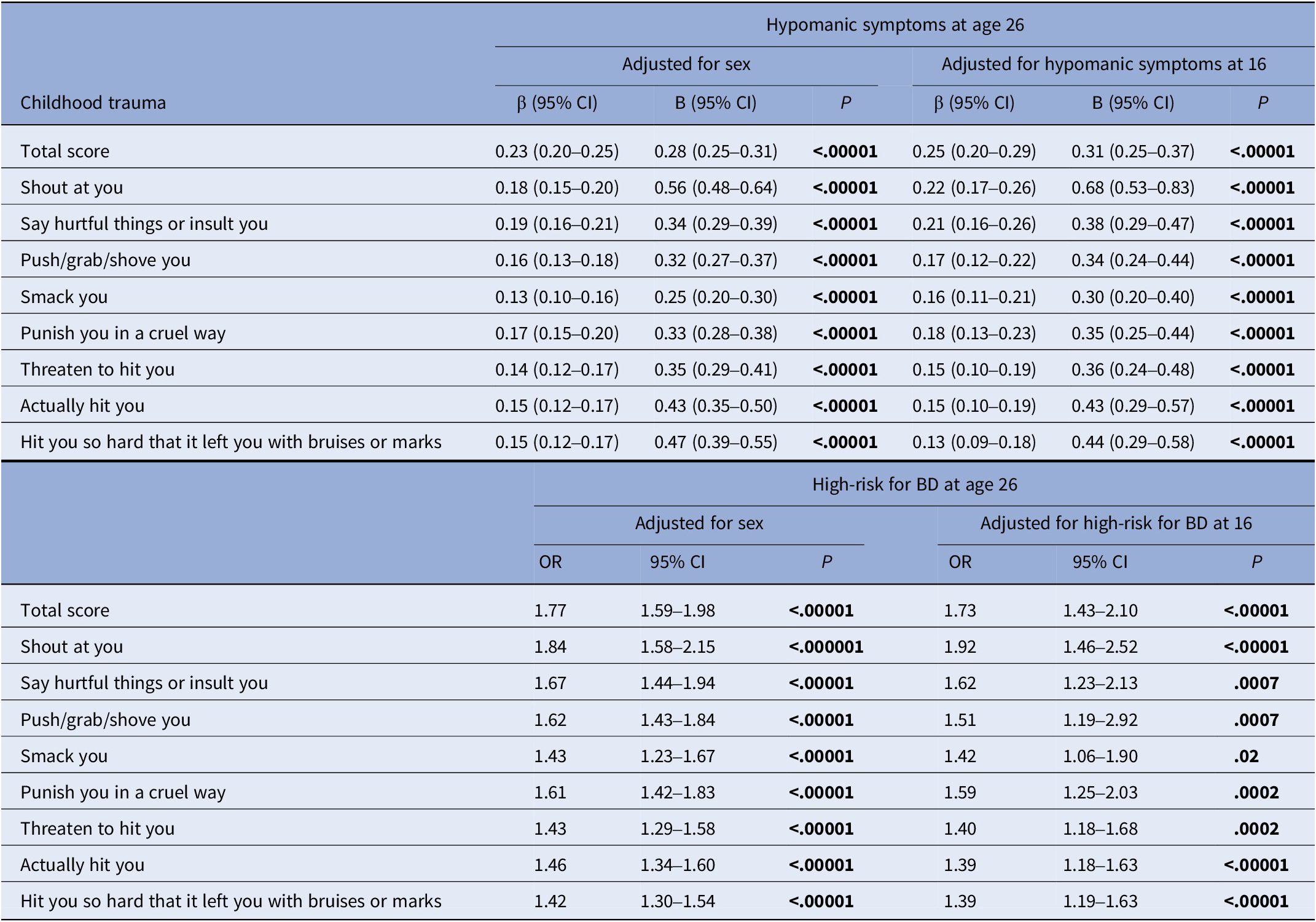
Abbreviations: β (Standardized estimates); CI (Confidence Intervals); B (unstandardized estimates); OR (Odds Ratio).
Note: P-values are reported FDR-adjusted (Benjamini–Hochberg procedure).
The significance of bold values are indicated the values of p ≤ .05.
Genetic and environmental influences on childhood trauma and subclinical hypomania
Twin model assumptions were met (i.e., mean and variance differences were not statistically significantly influenced by twin order or zygosity). Univariate twin correlations for childhood trauma, hypomanic symptoms, and high-risk for BD are presented in Supplementary Table 2. The genetic and environmental estimates from the univariate twin models are shown in Supplementary Table 3. These models did not provide a significantly worse fit when compared with their corresponding saturated models.
Bivariate cross-twin cross-trait (CTCT) correlations for childhood trauma and hypomanic symptoms (Supplementary Table 2) showed that MZ CTCT correlations were larger than DZ CTCT, suggesting that their covariance was influenced by A factors. MZ CTCT correlations were slightly less than the phenotypic association (0.25, 95% CI: 0.22–0.28), indicating E influence. C influences were indicated by the DZ CTCT correlations being greater than half the MZ CTCT correlations. For childhood trauma’s association with high-risk for BD, CTCT correlations suggested A, E, and D effects, because DZ correlations were less than half the MZ correlations.
Childhood trauma and hypomanic symptoms
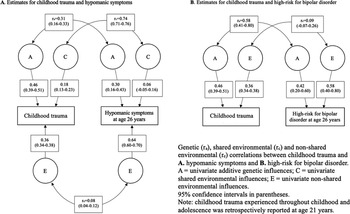
In line with the CTCT correlations for childhood trauma and hypomanic symptoms, results from the bivariate model showed that the ACE model fitted the data better than the nested models (Supplementary Table 4). The association between childhood trauma and hypomanic symptoms was mostly explained by genetic (51%) and shared environmental influences (32%). However, univariate shared environmental estimates for hypomanic symptoms showed that CIs overlapped with zero and thus lacked statistical significance. The correlation estimates showed a moderate degree of genetic overlap (ra = 0.31), a large shared environmental overlap (rc = 0.74), and a small non-shared environmental overlap (re = 0.08) between these phenotypes (Figure 1).

Figure 1. Genetic and environmental univariate estimates and bivariate correlations for childhood trauma and subclinical hypomania (number of symptoms and high-risk for bipolar disorder).
Childhood trauma and high-risk for bipolar disorder
While the CTCT correlations indicated A, D, and E effects, the bivariate AE model was the best fitting. However, all models provided a significantly worse fit than the saturated model (Supplementary Table 4). The association between childhood trauma and high-risk for BD was largely explained by genetic effects (90%), with a moderate genetic overlap (ra = 0.58). A small non-shared environmental correlation was detected (re = 0.09), but CIs overlapped with zero (Figure 1).
Interplay between childhood trauma and polygenic scores
Gene–environment correlations between polygenic scores and childhood trauma
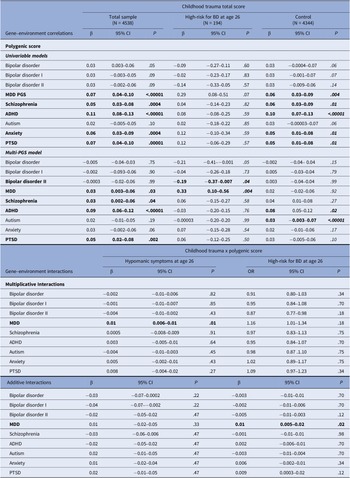
In the overall sample, childhood trauma was significantly positively correlated with five of the nine PGS examined in univariable models: MDD, schizophrenia, ADHD, anxiety, and PTSD. A similar pattern was detected when multi-PGS models were utilized, except for the anxiety PGS. When analyses were repeated for the group at high-risk for BD, there was evidence of a negative rGE between childhood trauma and the BD-II PGS, and a positive rGE with the MDD PGS, but only in the multi-PGS model (Table 3).
Table 3. Gene–environment correlations between polygenic scores and childhood trauma and gene–environment interaction effects between childhood trauma and polygenic scores on hypomanic symptoms and high-risk for bipolar disorder
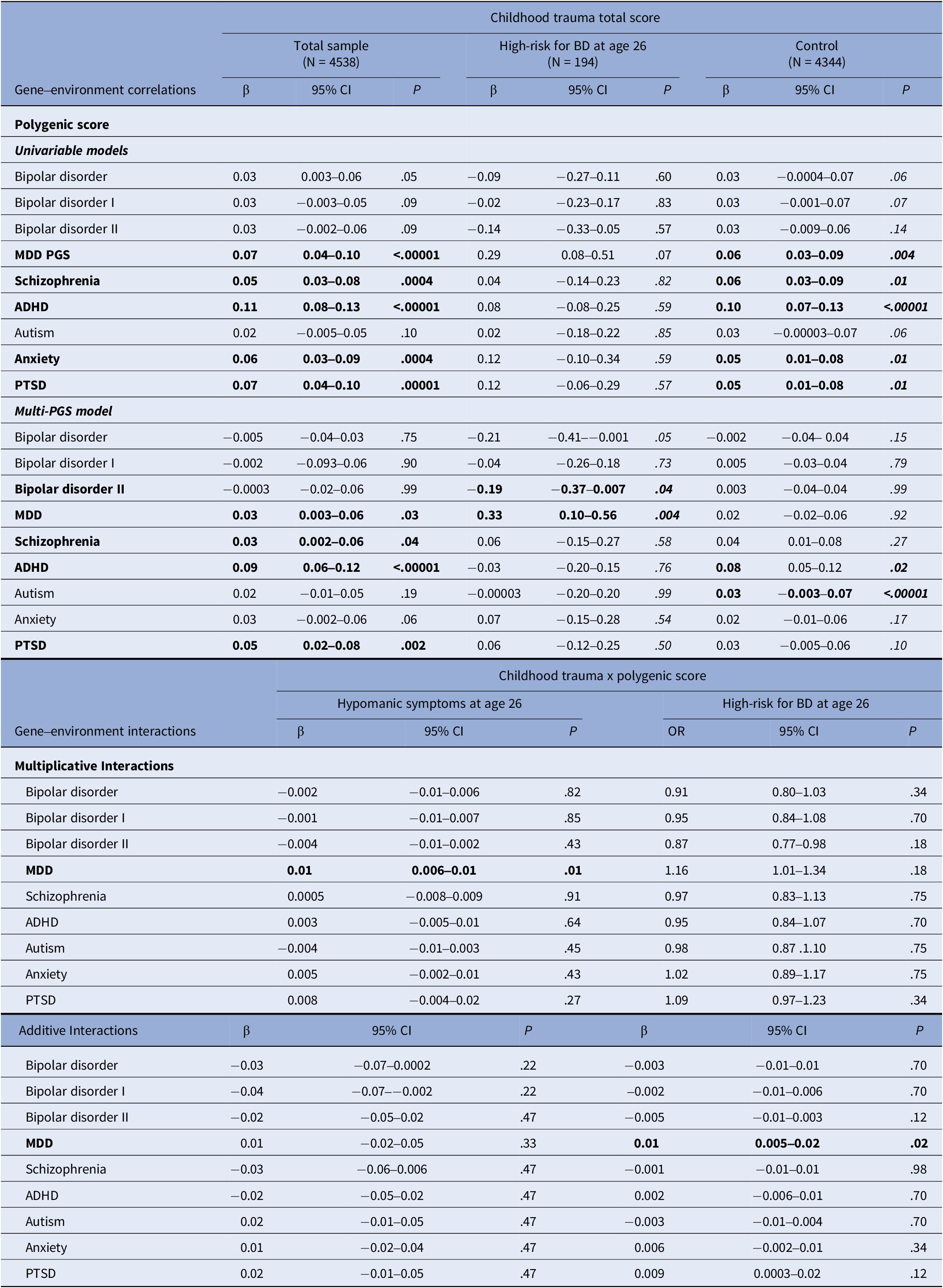
Note: P-values are reported FDR-adjusted (Benjamini–Hochberg procedure); β = Standardized beta. OR = Odds ratio Two multi-PGS models were fitted (one including the overall BD PGS and the other the BD I PGS and BD II PGS) to avoid multicollinearity.
The significance of bold values are indicated the values of p ≤ .05.
Gene–environment interactions between childhood trauma and polygenic scores on subclinical hypomania
The MDD PGS was found to significantly interact with childhood trauma on a multiplicative level in its influence on hypomanic symptoms, and additively on its effect on high-risk for BD (Table 3). No other significant GxE were detected.
Discussion
To our knowledge, this is the first study to test the association between childhood trauma and subclinical hypomania using a genetically sensitive design in a non-clinical sample. Childhood trauma was significantly associated with hypomanic symptoms and being at high-risk for BD at age 26. We found evidence of substantial genetic influence. Additive genetic effects explained 51 and 90% of childhood trauma’s covariance with hypomanic symptoms and high-risk for BD, respectively. Moreover, moderate to strong genetic correlations between childhood trauma with hypomanic symptoms (0.31) and high-risk for BD (0.58) were observed. Next, the interplay between childhood trauma and PGS for psychiatric and neurodevelopmental conditions was examined to elucidate the specific genetic factors and the nature of their relationship with childhood trauma. Childhood trauma was significantly positively correlated with five PGS (i.e., MDD, schizophrenia, ADHD, anxiety, and PTSD), and negatively with the BD II PGS. GxE effects were detected between childhood trauma and the MDD PGS on both hypomanic symptoms (multiplicative) and high-risk for BD (additive).
Childhood trauma and subclinical hypomania
Our findings showing the effect of self-reported childhood trauma on subclinical hypomania in early adulthood extend those of a recent study reporting a link between parent-rated childhood maltreatment and adolescent (hypo)mania [Reference Gajwani, Dinkler, Lundström, Lichtenstein, Gillberg and Minnis9]. Our sensitivity analyses indicated that, despite some influence, these associations were not entirely due to earlier hypomania. The strongest effects were detected for emotional abuse relative to physical maltreatment, mirroring the patterns found for hypomania-related phenotypes, including other subclinical symptoms (e.g., psychotic experiences) and BD [Reference Etain, Henry, Bellivier, Mathieu and Leboyer55–Reference Aas, Henry, Andreassen, Bellivier, Melle and Etain57]. Possible mechanisms may include emotional trauma’s impact on brain development, particularly emotion regulation (e.g., cortical thinning) [Reference Heim, Mayberg, Mletzko, Nemeroff and Pruessner58], which is a key feature of mood disorders [Reference Kurtz, Mohring, Förster, Bauer and Kanske59]. Research in the area of neurocognition may be advantageous for clarifying the nature of these associations.
Genetic and environmental overlap between childhood trauma and subclinical hypomania
A novel finding from the present study is the moderate genetic overlap between childhood trauma and hypomania. These results are in line with those for related forms of psychopathology (e.g., broadly defined emotional disorder and depression) [Reference Sartor60, Reference South, Schafer and Ferraro61]. The influence of shared genetic factors was particularly prominent in the context of high-risk for BD. This suggests that the same genes responsible for individual differences in both measures of subclinical hypomania (particularly more severe cases) may also underlie the development of characteristics that increase the risk of experiencing (i.e., active rGE) and/or evoking (i.e., evocative rGE) traumatic childhood events, such as adverse parental reactions. For instance, children and adolescents who present impulsive or irritable behaviors are more likely to experience conflict with caregivers [Reference Hadianfard48, Reference Barkley62]. These behaviors are more prevalent – and severe – among those at high-risk for BD [Reference Hosang, Cardno, Freeman and Ronald2].
Another innovative finding from this study is that bivariate twin modelling suggested common shared environmental effects on childhood trauma’s association with hypomanic symptoms. This finding may reflect true shared environmental mediation, although it could also be indicative of passive rGE; children of parents with mental health problems (e.g., BD) have greater risk of inheriting a genetic predisposition to psychopathology (e.g., mood disorder traits), but parents affected by mental illness are also more vulnerable to adversity, which additionally increases the risk of offspring childhood trauma [Reference Bastos, Campos, Faria-Schützer, Brito, Da Silva and Dos Santos-Junior63, Reference Reupert and Maybery64]. However, the shared environment had a very small influence on hypomanic symptoms, and CIs overlapped with zero. Further research is necessary to expand our results; adoption studies using registry data could help define the role of passive rGE by disentangling overlapping genetic, shared, and non-shared environmental effects [Reference Plomin, Loehlin and DeFries65].
The influence of shared environmental effects on childhood trauma’s covariance with high-risk for BD may have been masked by the presence of non-additive genetic effects, as these are confounded in twin models [Reference Rettew, Rebollo-Mesa, Hudziak, Willemsen and Boomsma66]. Nevertheless, the lack of effects may indicate etiological differences (related to shared environmental factors) in childhood trauma’s association with subclinical hypomania based on the latter’s severity.
Non-shared environmental factors were minor contributors to childhood trauma’s covariation with both measures of hypomania, but CIs overlapped with zero, and thus lacked statistical significance, in the context of high-risk for BD. Given the limited sample size of our “high-risk for BD” group, it would be important for studies with greater power to replicate these results. While not direct evidence for causality, our finding showing a small non-shared environmental overlap does not rule out causal effects of childhood trauma on hypomania as a quantitative trait [Reference Bornovalova, Huibregtse, Hicks, Keyes, McGue and Iacono67].
Gene–environment interplay between polygenic scores, childhood trauma, and subclinical hypomania
We provide further evidence of rGE between childhood trauma and PGS for several conditions: MDD, schizophrenia, ADHD, anxiety, and PTSD [Reference Peel, Purves, Baldwin, Breen, Coleman and Pingault15, Reference Ratanatharathorn, Koenen, Chibnik, Weisskopf, Rich-Edwards and Roberts16, Reference ter Kuile, Hübel, Cheesman, Coleman, Peel and Levey68]. Genetic liability for these conditions may partly explain some of the active/evocative rGE suggested by the bivariate twin models, particularly MDD, as this PGS showed the most pronounced and consistent effect across models.
A significant correlation in the opposite direction was detected between the BD-II PGS and childhood trauma in the high-risk for BD group. This finding is consistent with those from a study that used a clinical BD sample [Reference Aas, Bellivier, Bettella, Henry, Gard and Kahn18]. The reason for this negative correlation is unclear, but one possible explanation is that individuals at high-risk for BD with a genetic predisposition for BD may evoke/select themselves into less environmental adversity. For instance, the “bright” dimension of hypomania has been defined as socially advantageous, and some of these symptoms (e.g., elation) are more prevalent among high-risk individuals [Reference Hosang, Cardno, Freeman and Ronald2]. Nevertheless, both positive and negative rGE are likely to be underpinned by a more complex multifactorial interplay [Reference Aas, Bellivier, Bettella, Henry, Gard and Kahn18]. Other genes (e.g., PGS for childhood maltreatment) [Reference Dalvie, Maihofer, Coleman, Bradley, Breen and Brick69], environmental (socioeconomic status) [Reference Walsh, McCartney, Smith and Armour70], and protective factors (e.g., resilience) [Reference Park, Lee, Jang, Lee, Yu and Yoon71] linked to childhood trauma warrant further investigation in this context.
The final novel finding is that genetic risk for MDD was linked to increased susceptibility to childhood trauma’s effect on both hypomanic symptoms and high-risk for BD, with evidence of departure from additivity in relation to the latter. This is in line with the implication of biological mechanisms in the development of more severe cases and can be indicative of a particularly vulnerable group that would benefit most from intervention [Reference VanderWeele and Knol53]. Similar GxE effects have been reported for depression [Reference Peyrot, Milaneschi, Abdellaoui, Sullivan, Hottenga and Boomsma72], although these were contested by a more recent meta-analysis [Reference Peyrot, Van Der Auwera, Milaneschi, Dolan, Madden and Sullivan73]. It is important that our results are replicated in subclinical hypomania and BD samples to be conclusive.
Implications
These initial findings increase our understanding of childhood trauma’s role in the etiology of subclinical hypomania, showing considerable genetic effects in the form of i. bivariate heritability, ii. genetic overlap, iii. rGE, and iv. GxE with psychiatric and neurodevelopmental PGS. This highlights the importance that future research testing the influence of childhood trauma account for the impact of complex gene–environment interplay using a range of genetically sensitive designs. While high polygenic risk for MDD was found to increase vulnerability to childhood trauma’s effect on both measures of hypomania, there was evidence of departure from additivity in the context of high-risk for BD. This finding is compatible with the implication of biological processes and underscores this group’s potential as a target for research (e.g., phenotypic definition and examination of developmental pathways to psychiatric conditions, including BD) and clinical efforts (e.g., prevention and intervention) [Reference Ronald, Sieradzka, Cardno, Haworth, McGuire and Freeman49].
More broadly, our findings linking childhood trauma and hypomania to genetic risk for various conditions are consistent with the evidence that psychiatric and adversity phenotypes share a genetic basis [Reference Selzam, Coleman, Caspi, Moffitt and Plomin25]. This reinforces the relevance of examining the influence of different PGS, and not only those measuring a specific trait of interest. Finally, it must be noted that evidence of genetic effects does not imply that those who experience childhood trauma are responsible for their own victimization (e.g., neither as active nor as evocative rGE), or that parental mental illness is inherently linked to offspring childhood trauma. Rather, it suggests that the association between trauma and hypomania may not be causal, underscoring the need to account for genetic characteristics to improve the course and outcomes of psychiatric phenotypes [Reference Andreassen, Hindley, Frei and Smeland74], such as hypomania. Individuals presenting with symptoms of hypomania may be at increased risk of experiencing childhood events as traumatic and may benefit from additional assessment and clinical support targeting the impact of childhood trauma.
Methodological considerations
The main strength of this study is the use of a large genetically informed twin sample, permitting the examination of genetic and environmental influences on the association between childhood trauma and subclinical hypomania using twin and polygenic data. There are also limitations that should be considered when interpreting the findings. Firstly, childhood trauma was assessed retrospectively using a self-report measure. Retrospective self-reports of childhood trauma are robust predictors of psychiatric problems and have shown good reliability and validity among individuals with BD [Reference Hosang, Manoli, Shakoor, Fisher and Parker75], but they can be vulnerable to bias (e.g., recall bias).
Secondly, the present study only focused on recollection of emotional and physical maltreatment, but other overlapping types of trauma (e.g., emotional neglect and sexual abuse) are linked to psychopathology such as BD [Reference Palmier-Claus, Berry, Bucci, Mansell and Varese7, Reference Hosang, Fisher, Hodgson, Maughan and Farmer8] and need to be studied in the context of subclinical hypomania. Thirdly, the power of the PGS included in this study differs in terms of the quality and size of the GWAS they were derived from. For instance, our findings showing a nonsignificant rGE between the autism PGS and childhood trauma are not consistent with previous findings [Reference Peel, Purves, Baldwin, Breen, Coleman and Pingault15] and may be due to the smaller sample size of the GWAS used for this PGS [40], In addition, most GxE effects are small, requiring very large sample sizes to detect reliable effects [Reference VanderWeele and Knol53, Reference Dudbridge76]. Our results should be interpreted with caution and replicated in a larger sample. Lastly, PGS data in TEDS were only available for participants of European ancestry, limiting the generalizability of findings. Diverse GWAS are needed to address this issue and improve power.
Conclusion
In this study, childhood trauma was associated with hypomanic symptoms and being at high-risk for BD at age 26 years. These associations were partially driven by shared genetic factors with childhood trauma, particularly high-risk for BD. There was evidence of rGE with childhood trauma, explained by the PGS for MDD, schizophrenia, ADHD, anxiety, PTSD, and BD II. GxE were detected between childhood and the MDD PGS on both hypomanic symptoms and high-risk for BD. These findings offer support for childhood trauma as a risk factor for subclinical hypomania, while highlighting the influence of gene–environment interplay on these phenotypes and their association. These results do not imply that survivors of childhood trauma are responsible for their experiences via genetic mechanisms, but suggest that the association between trauma and hypomania may not be causal and emphasize the importance of accounting for genetic characteristics to improve hypomania’s course and outcomes.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1192/j.eurpsy.2025.10117.
Data availability statement
TEDS data used within this study are accessible on request via an online proposal form. Please see https://www.teds.ac.uk/researchers/teds-data-access-policy/ for further details. Please note that the TEDS website contains details of all data that are available through a fully searchable data dictionary (https://datadictionary.teds.ac.uk/home.htm).
Acknowledgements
We gratefully acknowledge the ongoing contribution of the participants in the Twins Early Development Study (TEDS) and their families. TEDS is supported by a UK Medical Research Council to Professor Thalia Eley (MR/V012878/1) and previously to Professor Robert Plomin (MR/M021475/1). The authors would like to thank all members of AMPLIFY (A Meaningful Peer-Led Involvement Network for Young People) who provided patient and public involvement (PPI) input throughout the development of this study.
Author contribution
Authors IGC, GH, and AR conceptualized and designed the study. IGC undertook the statistical analysis under the supervision of GH, AR, MT, and LH. IGC and GH wrote the first drafts of the manuscript. AR, MT, LH, EGL, and GH contributed to drafting and editing of the manuscript. AR led the LEAP study. All authors have approved the final manuscript.
Financial support
Irene Gonzalez-Calvo is supported by an Economic Social Research Council (ESRC) London Interdisciplinary Social Science Doctoral Training Partnership (LISS DTP) studentship (ES/P000703/1). The LEAP study was funded by a Medical Research Council grant to Professor Angelica Ronald (G1100559). Erin G. Lawrence is supported by an ESRC LISS DTP studentship (ES/P000703/1).
Competing interests
The authors declare none.


Comments
No Comments have been published for this article.