Parkinson’s disease (PD) is one of the most prevalent neurodegenerative disorders and is primarily characterised by tremors, increased muscle rigidity, bradykinesia, and impaired gait and balance, collectively known as Parkinsonism(Reference Van Den Eeden, Tanner and Bernstein1,Reference Lampropoulos, Malli and Sinani2) . Currently, PD constitutes one of the leading causes of disability globally, imposing substantial economic and social burdens(Reference Van Den Eeden, Tanner and Bernstein1,Reference Tysnes and Storstein3) . According to epidemiological reports, PD affects 1 % of individuals over the age of 60 years, and its incidence varies from one to two cases per 1000 people in unselected groups(Reference Tysnes and Storstein3,Reference Ou, Pan and Tang4) . Notably, PD is uncommon before the age of 50 years, and its prevalence increases to 4 % in older age groups(Reference Dorsey, Elbaz and Nichols5). At autopsy, α-synuclein (α-syn) positive protein clumps (Lewy bodies) and the degeneration of dopaminergic neurons in the substantia nigra pars compacta are the two hallmarks of PD(Reference Baizabal-Carvallo and Jankovic6). Several hypotheses have been proposed to explain the neurodegeneration observed in PD, including oxidative stress, mitochondrial dysfunction, lysosomal dysfunction and inflammation,(Reference Schonhoff, Williams and Wallen7–Reference Robea, Balmus and Ciobica10). Although the precise aetiology of PD remains unclear, emerging genetic data have confirmed that PD is caused by a complex interplay between age, genetic predisposition and environmental exposure(Reference Raza, Anjum and Shakeel11,Reference Grayson12) .
Among all bodily tissues, the brain has one of the highest lipid concentrations. It contains a wide range of lipids, including fatty acids, TAG, phospholipids, sterols and glycolipids(Reference Mori, Imai and Hattori13). Large-scale high-throughput sequencing has identified several PD risk genes associated with lipid metabolism(Reference Neumann, Bras and Deas14). Specifically, the most prevalent genetic variables that raise the risk of PD are missense mutations in GBA1, which encodes the lysosomal hydrolase glucocerebrosidase(Reference Neumann, Bras and Deas14,Reference Sidransky, Nalls and Aasly15) . Additionally, PD risk is associated with mutations in SMPD1, which encodes an acid, sphingomyelinase(Reference Gan-Or, Ozelius and Bar-Shira16). Previous evidence has shown that polymorphisms in GALC and ASAH1, which encode lysosomal enzymes that catabolise sphingolipids, also increase the risk of PD(Reference Chang, Nalls and Hallgrímsdóttir17,Reference Robak, Jansen and van Rooij18) . Other lipid-related genes associated with an increased risk of PD include DGKQ (encoding diacylglycerol kinase theta)(Reference Simón-Sánchez, Schulte and Bras19,Reference Nalls, Pankratz and Lill20) , which is crucial for the formation of synaptic vesicles by mediating the regeneration of phosphatidylinositol from diacylglycerol(Reference Goldschmidt, Tu-Sekine and Volk21,Reference Puchkov and Haucke22) , and SREBF1, which encodes sterol regulatory element binding transcription factor 1(Reference Do, Tung and Dorfman23), essential for biosynthesis and cell membrane integrity(Reference Lee, Phelan and Shin24). Interestingly, new high-resolution histochemical methods have shown that Lewy bodies comprise membrane lipids, mitochondria, lysosomes and α-syn fibrils, among other cellular components(Reference Shahmoradian, Lewis and Genoud25), indicating that the functions of lipids in biomembranes are vital in both physiological and pathological contexts of α-syn(Reference van Dierendonck, Vrieling and Smeehuijzen26). In addition, the identification of ASAH1, GBA1 and GALC as genetic risk factors for PD with substantial Lewy body deposition reinforces the hypothesis that lipids play a role in the aggregation and propagation of α-syn.
TAG, the predominant dietary lipids found in fats and vegetable oils (such as olive oil), are neutral lipids consisting of a glycerol backbone attached to three fatty acyl chains(Reference McLelland, Lopez-Osias and Verzijl27). As the primary energy storage components in a variety of species, including algae, mammals and oleaginous bacteria, TAG are essential for cellular energy balance, lipid homoeostasis, development and maintenance(Reference Xu and Shanklin28). In humans, most (and possibly all) cell types synthesise TAG. Abnormalities in TAG levels have been linked to several diseases, including obesity and CVD. Evidence indicates that obesity and metabolic syndrome are linked to high levels of TAG (hypertriacylglycerolaemia) and that the release of TAG from adipose tissue may contribute to cachexia, a multi-organ wasting disease(Reference Shulman29–Reference Cases, Smith and Zheng31). Recent longitudinal studies have demonstrated a relationship between midlife blood TAG levels and the risk of cognitive decline in older adults(Reference Power, Rawlings and Sharrett32,Reference Kalmijn, Foley and White33) . Tan et al. demonstrated(Reference Huang, Ng and Chia34) that moderate cognitive impairment in patients with PD is linked to elevated blood TAG levels. However, Zhang et al. found an association between reduced serum TAG levels and worse motor performance in patients with PD(Reference Zhang, Chen and Liu35). Therefore, the association between blood TAG levels and PD is complicated by conflicting findings from various studies.
Well-powered genome-wide association studies (GWAS) have identified hundreds of SNP associated with PD, serum lipids, circulating immune cells and circulating inflammatory protein levels, presenting an opportunity to test the genetic relationships between a series of risk factors and PD using Mendelian randomisation (MR) analysis. The causal association between serum TAG levels and the risk of PD remains unknown. Therefore, in this study, we examined the potential causal relationship between serum TAG levels and PD risk using MR analysis. Additionally, a range of lipids, such as sterols, fatty acids and their metabolites, complex lipids (such as glycerophospholipids and sphingolipids), and lipoproteins, have been found to have immunomodulatory and pro-/anti-inflammatory properties(Reference Andersen36,Reference Araújo, Caridade-Silva and Soares-Guedes37) . Chronic neuroinflammation plays a major role in dopaminergic neuron degeneration. Thus, our study examined the causal relationship between plasma lipidomes and PD, related primarily to serum TAG (51:4) levels and attempted to explore the possible role of mediation through circulating immune cells or inflammatory proteins.
Methods
Traits analysed and genome-wide association studies
The GWAS summary meta-analysis for PD, circulating immune cells, inflammatory proteins and the plasma lipidome have been made publicly available. The largest meta-analysis of individuals of European ancestry currently available provides genetic association data for the plasma lipidome(Reference Ottensmann, Tabassum and Ruotsalainen38). Briefly, we employed genotype data at the summary level from the prospective GeneRISK cohort, which examined genome-wide correlations for 179 lipid species among 7174 participants. Genetic information for circulating immune cells was derived from GWAS summary statistics from Orru et al. (2022)(Reference Orrù, Steri and Sidore39), which included a total of 731 immunophenotypes across four categories: absolute cell counts (n 118), median fluorescence intensities reflecting surface antigen levels (n 389), morphological parameters (n 32) and relative cell counts (n 192). GWAS data on circulating inflammatory proteins were obtained from a GWAS project and assessed in eleven cohorts of 14 824 people of European ancestry using the Olink Target Inflammation Panel(Reference Zhao, Stacey and Eriksson40). The genetic details of PD were derived from a cross-population map of genetic correlations(Reference Sakaue, Kanai and Tanigawa41). In short, we employed the findings from an inverse variance meta-analysis limited to individuals of European and East Asian ancestry, taking into account age, sex and the main components representing ancestry (2978 cases; 635 168 controls).
Mendelian randomisation analyses
GWAS summary statistics were used to conduct bidirectional two-sample MR, with α Lipids→PD representing the causal impact of lipids (exposure) on PD (outcome) and α PD→Lipids representing the causal impact of PD (exposure) on lipids (outcome) (Fig. 1). Harmonised SNP significantly associated (p < 5e-8) with the exposure were clumped (p1 = 0·0001, p2 = 0·01, kb = 10 000 and r2 = 0·01) with PLINK v1.9(Reference Chang, Chow and Tellier42) and retained as instrumental variables (IV). Due to the complex long-range linkage disequilibrium structure of the HLA locus, SNP mapping to that region (chr6:25 000 000–37 000 000; GRCh37/hg19) were also excluded from our IV(Reference van der Graaf, Zorro and Claringbould43). For each exposure–outcome pair, further IV were removed based on differences in allele frequency (≥ 0·05) and Steiger filtering (Z ≤ –1·96). Bidirectional MR analyses were carried out with the TwoSampleMR R package (v0.5.7)(Reference Hemani, Zheng and Elsworth44), primarily through the inverse variance-weighted (IVW) method. Additionally, MR methods that were complementary were used. We employed MR-Egger regression, which is generally regarded as conservative in the presence of pleiotropic variants and less likely to produce inflated test statistics leading to false-positive associations(Reference Bowden, Davey Smith and Haycock45–Reference Slob and Burgess47). The weighted median estimator was also used for its benefit in producing valid estimates if at least 50 % of the information in the analysis comes from SNP that are valid IV(Reference Bowden, Davey Smith and Haycock45–Reference Slob and Burgess47). Ultimately, the weighted mode-based estimation approach was employed, which yields the most robust estimates in cases where the majority of invalid instruments nevertheless yield a consistent estimate of the real causal impact.
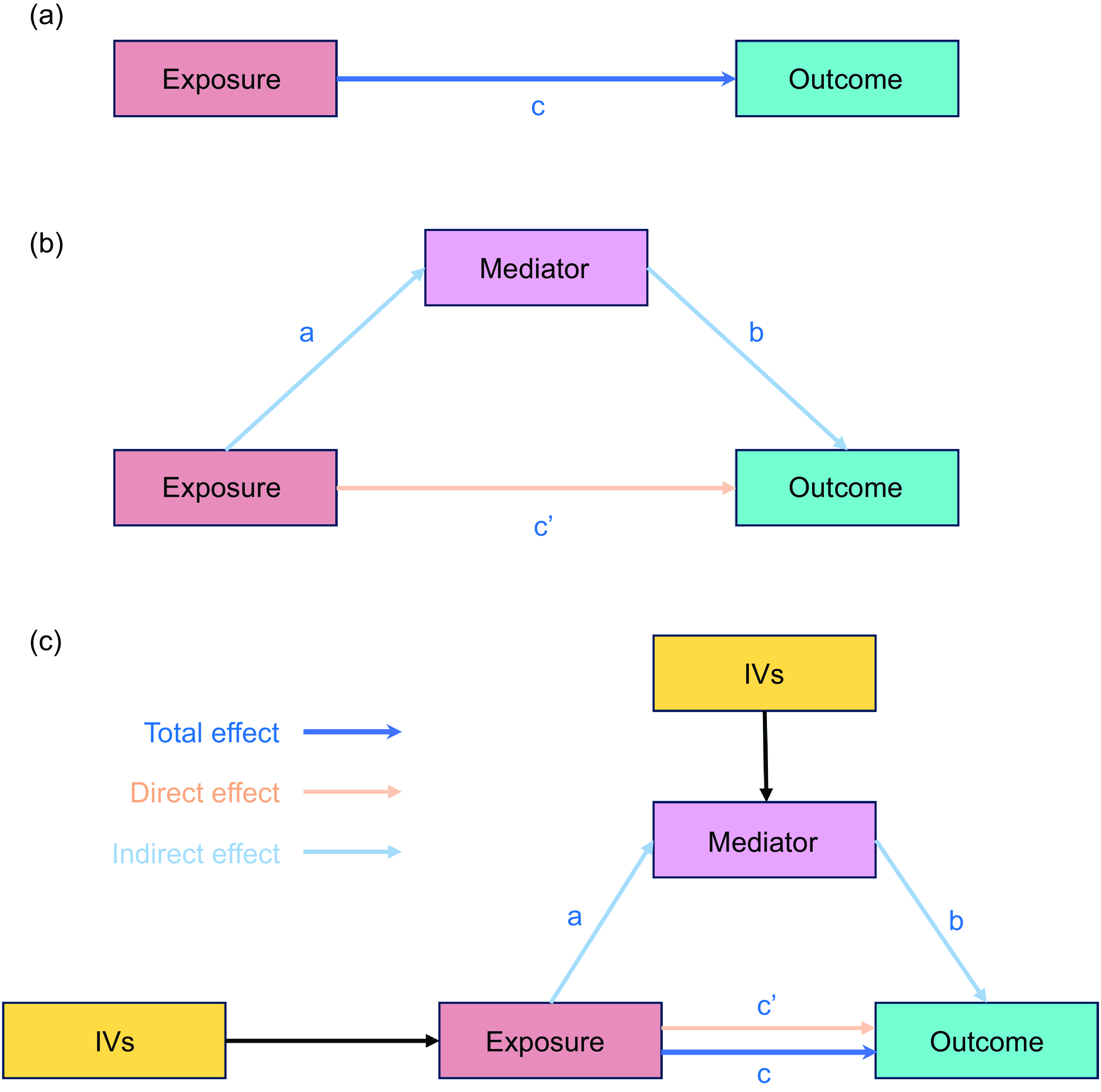
Figure 1. MR analysis diagram. The top half represents the unmediated causal relationship between exposure (X) and outcome (Y) (a). Path c represents the total effect. In the bottom half of the diagram, a third variable (M), the mediator, has been included to explain the relationship between X and Y, in the sense that part or all the effects of X on Y are channelled through M. The coefficient a indicates the effect of X on M, b indicates the effect of M on Y and c’ is the unique effect of X on Y after M has been controlled for. The latter is known to have a direct effect. The indirect effect was defined as product ab (b) and (c). MR, Mendelian randomisation; IVW, inverse variance-weighted.
Mediation analysis
Two-step MR (also known as network MR) is similar to the product of coefficient methods. Two-step MR estimates were calculated to find: (i) the causal effect of exposure on the mediator and (ii) the causal effect of mediator on the outcome (Fig. 1(c)). We used two-step MR for mediation when we found the following (1) evidence to support an effect of the trait on circulating immune cells and inflammatory proteins (step 1); (2) limited evidence to support an effect of circulating immune cells and inflammatory proteins on the trait; and (3) evidence of an effect on circulating immune cells and inflammatory proteins on PD (step 2). An arbitrary false discovery rate of 5 %, calculated according to the Benjamini–Hochberg method, was used as an indicator of supportive evidence for an association(Reference Davey Smith and Hemani48). For the indirect effect of the mediator of the two-step MR for mediation, we multiplied the estimate of the effect of the trait on circulating immune cells and inflammatory protein levels obtained from the univariate MR by the effect of the circulating immune cells and inflammatory protein levels on PD obtained from the univariate MR. Additionally, total effect was defined as the effect of the exposure on the outcome through all potential pathways, or the total effect was the effect of exposure on the outcome without any mediator (Fig. 1(a)).
Multivariate Mendelian randomisation analyses
Multivariate MR (MVMR) regression estimates were compared with the univariate MR estimates. For the univariate MR, we either used the same IV as in the MVMR or employed a subset of IV that were retained after Steiger filtering between both the outcome and the exposure of interest, as well as between the exposure of interest and other exposures. There was weak instrument bias using conditional F-statistics(Reference Sanderson, Spiller and Bowden49) and heterogeneity using Cochran’s Q-statistic(Reference Bowden, Hemani and Davey Smith50).
Selection of instrumental variables and sensitivity analyses
In our analyses, only IV that met the relevance, independence and exclusion restriction assumptions satisfied the three MR assumptions (Fig. 2). The validity of the first MR assumption was confirmed by identifying a significant association between all exposure IV used in the subsequent sensitivity analyses and exposure to MR at P< 5E-8. Additionally, we verified the accuracy of the second MR assumption by carefully choosing independent variables with a linkage disequilibrium score of less than 0·01 after clumping at a 1000 kb range. Subsequently, to assess the robustness of the results, sensitivity studies for associations with significant IVW MR effects were conducted using alternative MR methods such as MR-Egger, simple mode, weighted median and weighted mode. Cochran’s Q-statistics were used to evaluate heterogeneity. Additionally, we ran MR-PRESSO(Reference Verbanck, Chen and Neale51) for relationships with significant IVW MR effects, given a high fraction of raised Q-statistics. To confirm that our findings were not influenced by pleiotropy, which violates the MR assumption that exposure alone influences the outcome(Reference Sanderson, Glymour and Holmes52), we first filtered genome-wide significant exposure SNP and harmonised these SNP with the available GWAS summary statistics, confirming that SNP were present in the summary statistics of all traits. This step was completed prior to clumping to ensure that the identified IV were consistently present across all outcomes and allowed for future comparisons. To ensure that the selected SNP were more strongly connected with the exposure than with any of the other identified characteristics, Steiger filtering was used between the trait and exposure after clustering. SNP that passed all trait filtering were kept as IV and MR studies were performed on them. Considering the diversity of our phenotypes, this method served as an effective pleiotropic filter. We know that no overlap can result in the ‘winner’s curse’ bias, even while sample overlap in two-sample MR may skew results towards observational effects(Reference Burgess, Davies and Thompson53,Reference Mounier and Kutalik54) . Weak instruments tend to exacerbate this bias. We employed MR-APSS(Reference Hu, Zhao and Lin55) (default settings and linkage disequilibrium scores from 1000 Genomes Data(Reference Zheng, Erzurumluoglu and Elsworth56)), which handles both sample overlap and pleiotropy; however, thorough simulations have shown that both problems result in mild bias(Reference Mounier and Kutalik54).
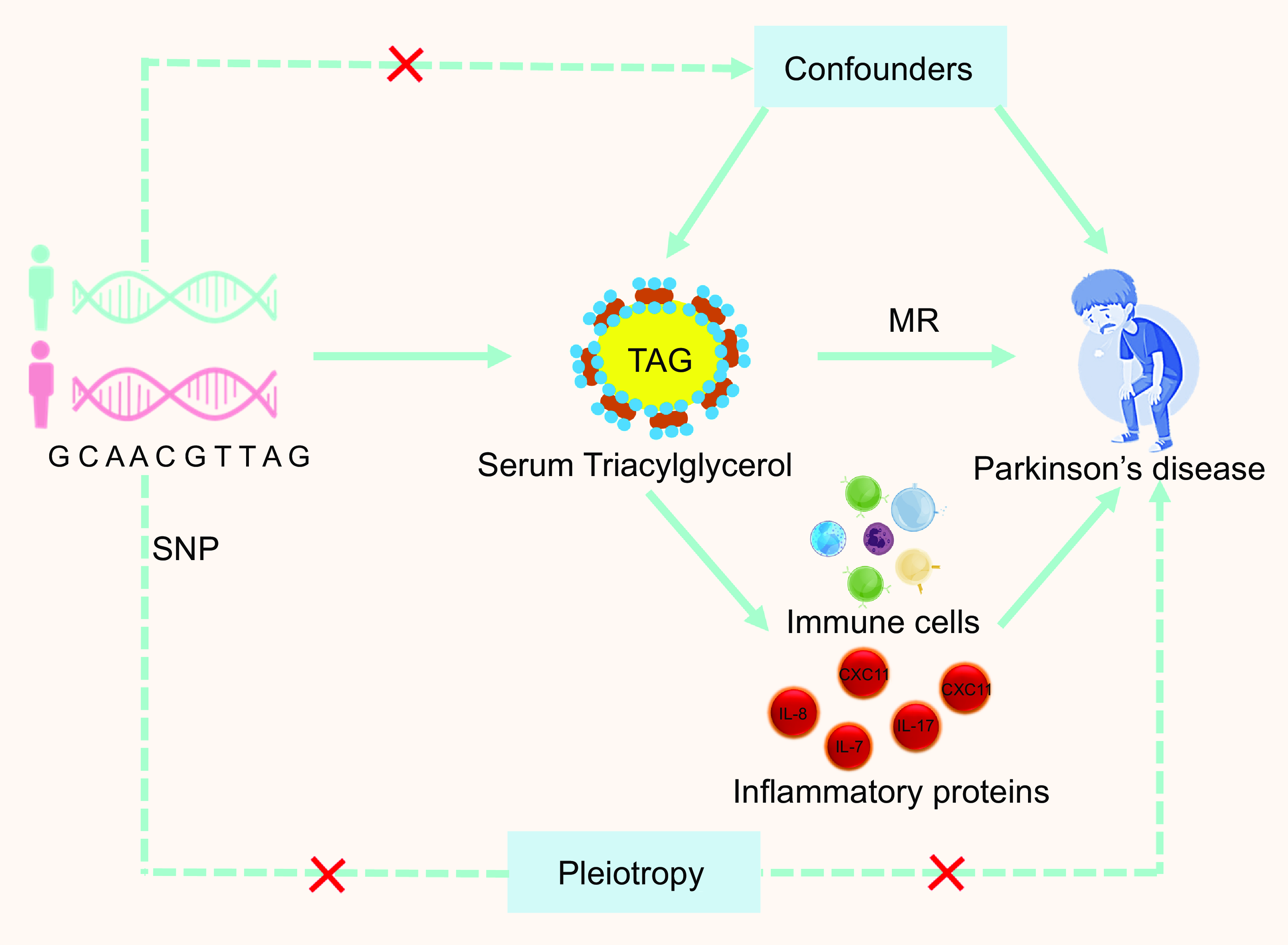
Figure 2. The three key assumptions of Mendelian randomisation (MR) studies. Depicted in this illustration are three assumptions of MR. The relevance assumption can be readily verified as long as the F-statistic for the SNP–exposure association exceeds 10. It is important to ensure that the correlations with established confounders are null, although the independence assumption is difficult to verify because of potential issues with pleiotropy and population substructure. Exclusion restriction assumptions are generally difficult to verify given that SNP may have pleiotropic effects or may be correlated with genes in LD that affect outcomes regardless of exposure. LD, linkage disequilibrium; IV, instrumental variables.
Statistical analysis
Two-sample MR analyses were used to estimate the causal effects of the risk factors related to serum TAG levels in patients with PD. The IVW method was selected as the primary analysis to meta-analyse individual Wald-type ratios of IV under a random-effects model, with OR described per s d increase in the risk factor level. TwoSampleMR (version 0.5.6), MR-cML (version 0.0.0.9) and MR-PRESSO (version 1.0) packages implemented in R (version 3.4) were used for analyses. Forest plots were constructed using the ggplot2 package (version 2.0.1).
Results
Association of serum TAG (51:4) levels with Parkinson’s disease
Initial IVW-MR revealed a significant association between elevated serum TAG levels (51:4) and a lower risk of PD. Despite the fact that other robust MR analysis approaches did not find a significant causal relationship between serum TAG (51:4) levels and the risk of PD (OR = 0·93 for the MR-Egger, P= 0·238; OR = 0·89 for the weighted median, P= 0·17; OR = 0·83 for the simple mode, P= 0·164; and OR = 0·85 for the weighted mode, P= 0·861), the direction of the results from these approaches was still consistent with the results from IVW-MR (online Supplementary Fig. S1A). Additionally, we found a significant association between increased risk of PD and increased serum phosphatidylcholine (17:0_18:1) and TAG (58:7) levels (online Supplementary Fig. S1B and C). We also found a robust relationship between higher serum TAG (51:4) levels and a lower risk of PD after further adjustment for phosphatidylcholine (17:0_18:1) and TAG (58:7) levels using MVMR. Particularly, there was a 21 % reduction in the risk of PD with every sd increase in serum TAG (51:4) levels (OR = 0·79, IVW: P= 0·015, OR = 0·78, MR-Egger: P= 0·030; OR: 0·79, Lasso: P= 0·016) (Fig. 3). Although the weighted median method did not yield a significant causal relationship between serum TAG (51:4) levels and PD risk (OR = 0·76, weighted median, P= 0·079), the results indicate agreement with the other methods.
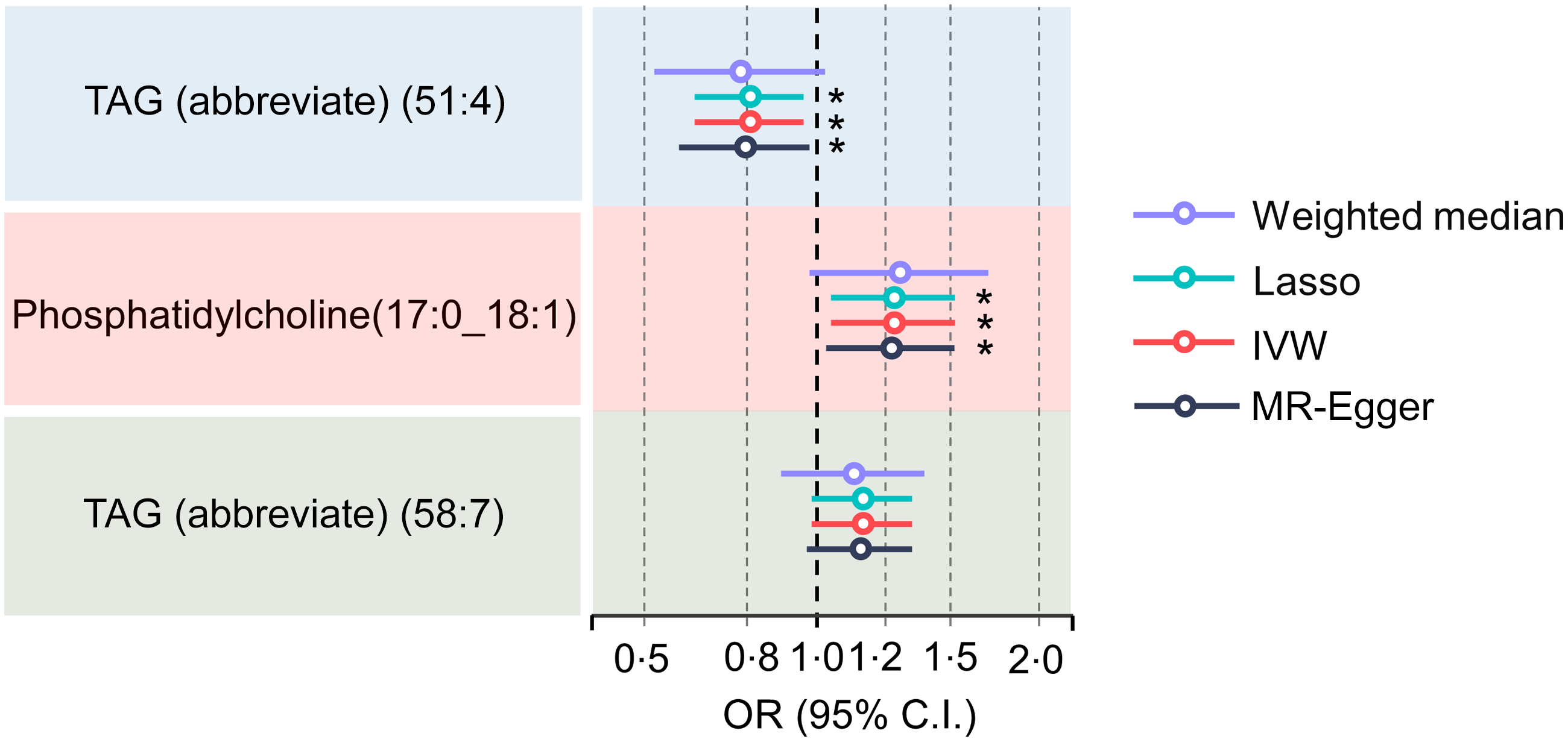
Figure 3. Forest plot for the results from MR analyses testing the effect of serum TAG (51:4) levels on the risk of PD. The results of the five MR analysis approaches are presented OR and 95 % CI. MR, Mendelian randomisation; PD, Parkinson’s disease; P values less than 0·05 are indicated by *, 0·01 and 0·001 by **, *** and *, respectively.
Association of serum TAG (51:4) levels with circulating immune cell counts and circulating inflammatory protein levels
Additionally, we discovered that there may be a causal relationship between serum TAG (51:4) levels and the counts of various subtypes of circulating immune cells. Lower T lymphocyte counts from fourteen different subtypes were linked to elevated blood TAG (51:4) levels (Fig. 4). Among them, the relative count of immature CD28- CD25++ CD8 + T cells (immature CD28- CD25++ CD8 + T cells %) decreased most significantly (with 1 sd genetically instrumented higher serum TAG (51:4) level leading to a 21 per cent (95 % CI −0·38, −0·03) lower immature CD28- CD25++ CD8 + T cells %, P= 0·021). Additionally, our findings indicated that increased serum TAG (51:4) levels were associated with reductions in the count of five subtypes of B lymphocyte (CD20 on IgD + CD38- unswitched memory B cells, IgD- CD38- B cell %, IgD- CD38- B cell % lymphocytes, IgD- CD38- B cell absolute counts and switched memory B cell % lymphocytes), two subtypes of dendritic cells (FSC-A on myeloid dendritic cells and CD86+ plasmacytoid dendritic cells %), four subtypes of natural killer (FSC-A on HLA DR+ natural killer, HLA DR on HLA DR+ natural killer, HLA DR+ natural killer absolute count and SSC-A on HLA DR+ natural killer) and the HLA DR++ monocyte %leucocyte (Fig. 4(a)).
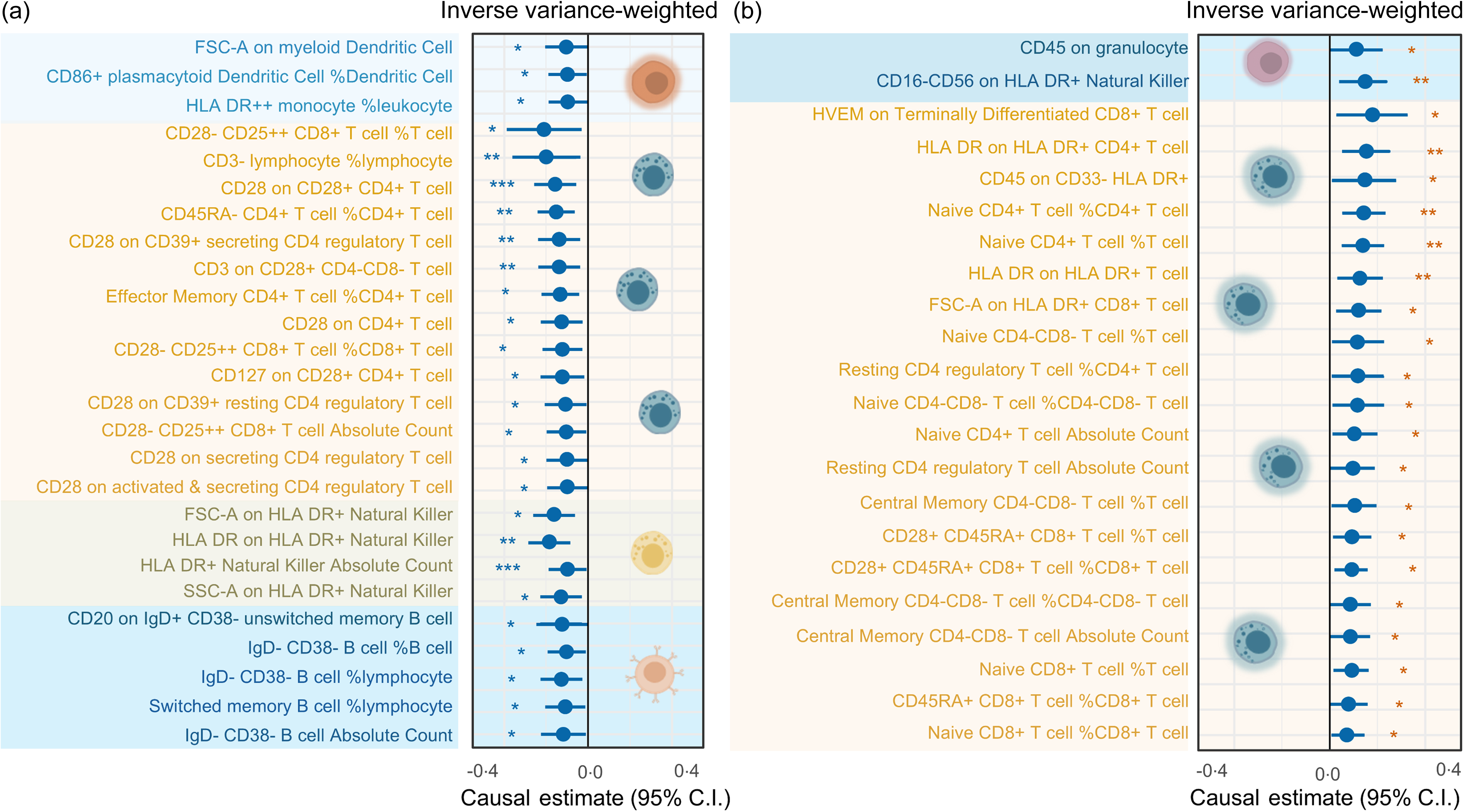
Figure 4. Forest plot from MR analyses testing the effect of serum TAG (51:4) levels on various circulating immune cell counts. The results of the five MR analysis approaches are presented as causal estimates and 95 % CI. MR, Mendelian randomisation; IVW, inverse variance-weighted. P values less than 0·05 are indicated by *, 0·01 and 0·001 by **, *** and *, respectively.
The IVW methods also demonstrated a potential causal relationship between increased serum TAG (51:4) levels and an increase in the counts of 20 T lymphocyte cell subtypes. Among them, the most significant increase was found in the Herpes Virus Entry Mediator (HVEM) on terminally differentiated CD8 + T cell count (with 1 sd genetically instrumented higher serum TAG (51:4) levels leading to an 18 % (95 % CI 0·03, 0·33) higher HVEM on terminally differentiated CD8 + T cells, P= 0·020) (Fig. 3(b)). In addition to T lymphocytes, our MR results also showed a potential causal relationship between increased serum TAG (51:4) levels and an increase in the count of CD16-CD56 on HLA-DR + natural killer and CD45 on granulocyte cells (Fig. 4(b)).
We also examined the association between serum TAG (51:4) levels and circulating inflammatory proteins. Notably, we found a positive causal relationship between increased serum TAG (51:4) levels and levels of eight circulating inflammatory proteins (with 1 sd genetically instrumented higher serum TAG (51:4) levels leading to a 6 % (95 % CI 1·01, 1·11) higher caspase 8 levels, P= 0·013; 6 % (95 % CI 1·01, 1·11) higher C-C motif chemokine 20 levels, P= 0·024; 8 % (95 % CI 1·02, 1·14) higher fibroblast growth factor 19 levels, P= 0·006; 6 % (95 % CI 1·1, 1·11) higher macrophage inflammatory protein 1a levels, P= 0·017; and 5 % (95 % CI 1·01, 1·10) TNF ligand superfamily member 12, P= 0·049) (Fig. 5).
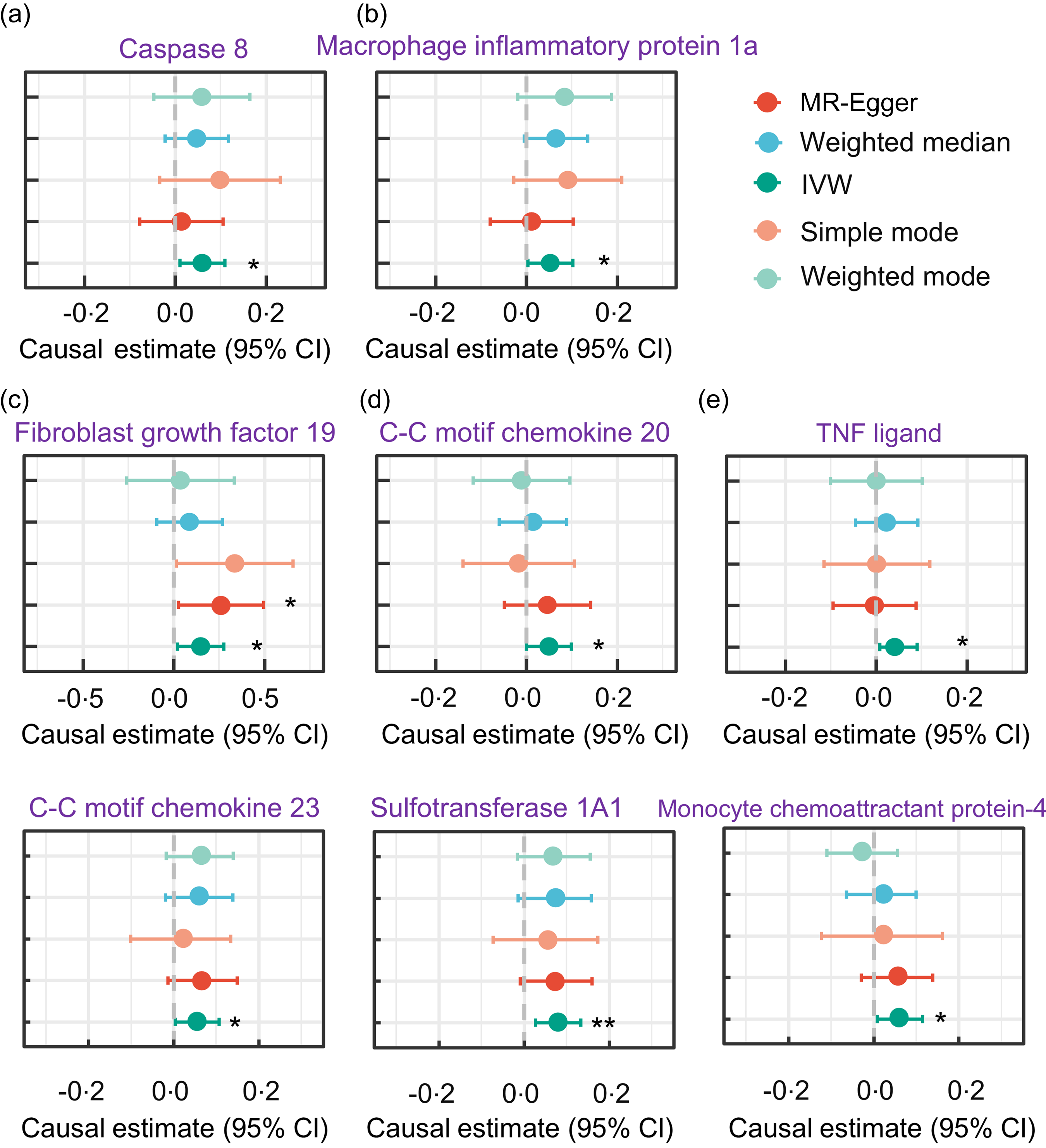
Figure 5. Forest plot MR analyses testing the effect of serum TAG (51:4) levels on various circulating inflammatory proteins levels. The results of the five MR analysis methods were presented as causal estimates and 95 % CI. MR, Mendelian randomisation. * indicates a P-value less than 0·05, ** denotes a P-value less than 0·01 and *** denotes a P-value less than 0·001.
Association of circulating immune cell counts with Parkinson’s disease
Our MR analysis provided robust evidence supporting a causal relationship between the number of circulating immune cells and increased risk of PD. Specifically, increased IgD-CD38-B lymphocyte counts were associated with a reduction in the risk of PD (IVW OR, 0·91 (95 % CI 0·85, 0·99), P= 0·006). This suggests that the risk of PD is decreased by 9 % with 1 % genetically instrumented higher circulating IgD-CD38-B cell % lymphocyte count. Additionally, we found that increased resting CD4 regulatory T cell %CD4 + T cell counts were associated with a reduction in the risk of PD (with a 1 % genetically instrumented higher resting CD4 regulatory T cell %CD4 + T cell leading to a 4 % (95 % CI 0·93, 0·99) lower risk of PD, P= 0·038) (Fig. 6).
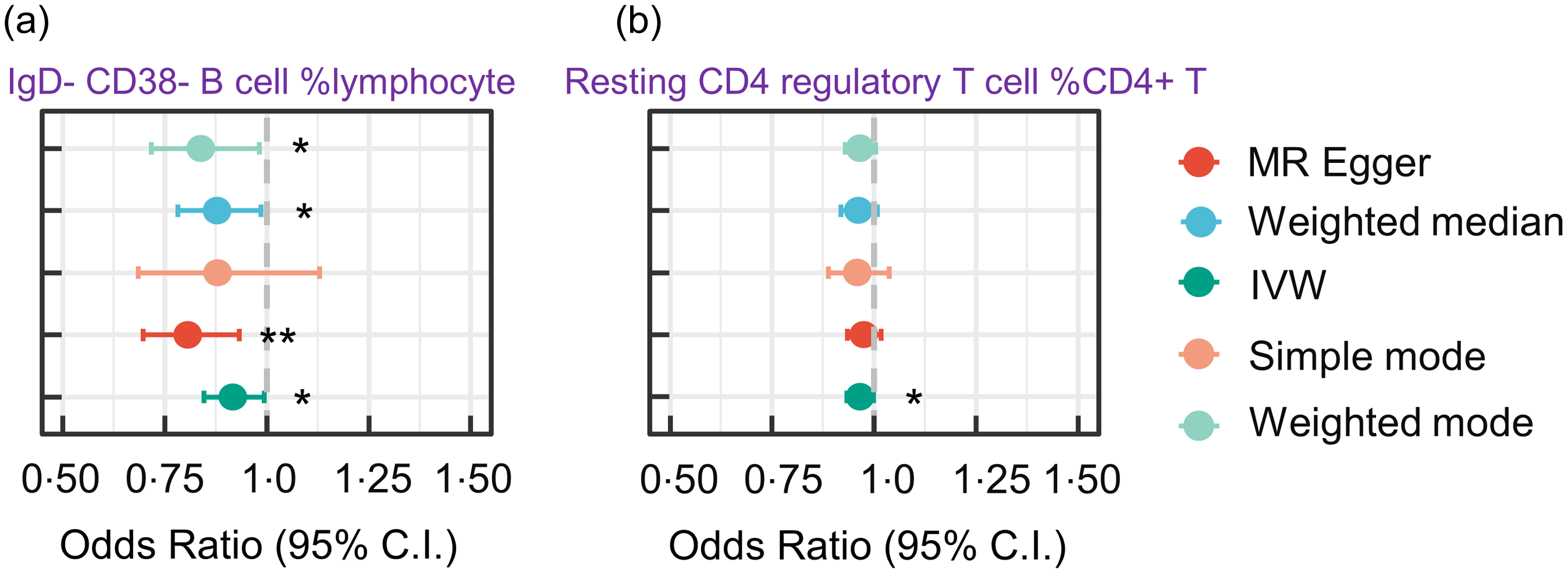
Figure 6. Forest plot for MR analyses testing the effect of circulating immune cells on the risk of PD. The results of the five MR analysis methods are presented as OR and 95 % CI. * indicates a P-value less than 0·05, ** denotes a P-value less than 0·01 and *** denotes a P-value less than 0·001. MR, Mendelian randomisation; PD, Parkinson’s disease; IVW, inverse variance-weighted.
Proportion of the association between serum TAG (51:4) levels and Parkinson’s disease mediated by circulating immune cell counts
The effect of serum TAG (51:4) levels was partially mediated by the IgD-CD38-B cell for all-cause PD (proportion of circulating IgD-CD38-B cell mediated: 6·9 %; CI 2·1 %, 16 %; P= 0·024). Additionally, we found that the mediating effect of resting CD4 regulatory T cells in association with serum TAG (51:4) levels and PD was not significant (proportion of circulating resting CD4 regulatory T cell mediated: 3·7 %; CI −6·5 %, 14 %; P= 0·336) (Table 1). Interestingly, none of the ninety-one circulating inflammatory proteins, except circulating immune cells, mediated the relationship between serum TAG (51:4) levels and PD risk.
Table 1. The estimated proportions of the association between serum TAG (51:4) levels and the risk of PD accounted for by circulating immune cells

PD, Parkinson’s disease; IVW, inverse variance weighted.
Sensitivity analysis
Online Supplementary Tables S1–S8 summarise the findings of our sensitivity analyses, which included pleiotropy and heterogeneity tests. Notably, we found no evidence of horizontal pleiotropy for circulating inflammatory proteins, immune cells or serum TAG (51:4) levels (P> 0·05). Additionally, all analytical findings showed concordance between the IVW and MR-Egger results, with no indication of heterogeneity (P> 0·05).
Discussion
According to genetic variant analysis, lipid and lipid transport, autophagic-lysosomal pathways, and inflammatory pathways are now known to carry a substantial and influential genetic risk for age-dependent neurological illnesses(Reference Chang, Nalls and Hallgrímsdóttir17,Reference Shi, Belbin and Medway57,Reference Klemann, Martens and Sharma58) . However, the mechanisms through which these molecular interactions contribute to the pathology of many age-related neurodegenerative illnesses, including dementia, Alzheimer’s disease and PD, remain poorly understood. We used GWAS summary data to evaluate for a causal relationship between plasma lipidomes and PD, related primarily to serum TAG (51:4) levels and PD. We also attempted to clarify a possible role for mediation through circulating immune cells but not circulating inflammatory proteins.
Several observational studies(Reference Coulombe, Kerdiles and Tremblay59–Reference Kubo, Nemani and Chalkley61) have examined the relationship between lipid metabolism and PD onset. However, the specific types of lipid metabolism disturbances associated with the risk of PD remain unclear. In the present study, which included 179 lipid species in 7174 adult Finnish subjects, we employed MR analysis to investigate the potential association between plasma lipidomes and PD risk. The MR findings demonstrated a causative relationship between higher serum TAG (51:4) levels and a reduced risk of PD. Consistent with our results, a meta-analysis showed that higher levels of total serum TAG and cholesterol were associated with reduced PD risk or were higher in control group compared with the PD group(Reference Fu, Wang and He62). Similarly, several clinical observational studies have reported lower serum TAG levels in patients with PD compared with healthy individuals(Reference Scigliano, Musicco and Soliveri63–Reference Meng, Zheng and Zhang65). Further, several clinical studies have also demonstrated that higher serum levels of TAG confer protective benefits against PD(Reference Sääksjärvi, Knekt and Männistö66,Reference Vikdahl, Bäckman and Johansson67) . Additionally, the findings of animal experiments revealed that mice overexpressing α-syn A53T and other PD models have considerably lower intracellular TAG levels(Reference Meng, Zheng and Zhang65,Reference Guerreiro, Coelho and Sousa-Lima68) . However, Zhang et al. discovered a correlation between worse motor performance and higher serum TAG levels in patients with PD(Reference Zhang, Chen and Liu35). We inferred that this may be due to confounding effects in observational studies, which is efficiently eliminated by MR analysis.
Evidence suggests that a variety of lipid species, including sterols, fatty acids and their metabolites, complex lipids (such as glycerophospholipids and sphingolipids) and lipoproteins, have immunomodulatory, and pro- and anti-inflammatory properties(Reference Andersen36). Thus, lipid metabolism is crucial in controlling inflammation in acute and chronic illnesses, including Alzheimer’s disease and PD. Since lipids possess pro- and anti-inflammatory properties and lipoprotein profiles and composition affect immunomodulatory pathways, we explored the role that phenotypes associated with 731 different immune cells and ninety-one distinct circulating inflammatory protein phenotypes play as mediators in the relationship between the plasma lipidome and PD. Interestingly, we found no evidence supporting the mediating role of circulating inflammatory proteins; instead, we identified a potential mediating role for circulating immune cells. More precisely, our results showed that higher percentages of resting CD4 regulatory T cells (%CD4 + T cells) and IgD-CD38-B cells (% lymphocytes) were associated with a lower risk of PD (IVW OR, 0·91 (95 % CI 0·85, 0·99), P= 0·033 and 0·96 (95 % CI 0·93, 0·99), P= 0·038, respectively). Consistent with our findings, Stienstra et al. discovered that lowering adipose TAG lipase-mediated lipolysis led to a reduction in PG-E2 and IL-6 production, which in turn attenuated the inflammatory response in macrophages following ex vivo and in vitro activation(Reference van Dierendonck, Vrieling and Smeehuijzen26). Similarly, Fülöp T and colleagues discovered that changes in plasma membrane characteristics are linked to a suppressive effect on peripheral T-cell CD28-dependent activation caused by selective increase in intravascular lipolysis and plasma linoleic acid content(Reference Larbi, Grenier and Frisch69). Additionally, mediating roles of IgD + CD38dim%B and CD25 ++ CD8br in the causal link between TAG (51:4) and onset of hypotension have been validated in recent MR research(Reference Lin, Han and Hong70). Overall, our results suggest that circulating immune cells play a major mediating role in the relationship between serum TAG (51:4) levels and PD risk.
As components of the adaptive immune system, B lymphocytes perform a range of functions and intricately interact with other pathways of the innate and adaptive immune systems. Recent studies have indicated that immune cells, including B lymphocytes, may engage in intricate interactions with the central nervous system through the meningeal lymphatic system(Reference Louveau, Herz and Alme71,Reference Zou, Pu and Feng72) . Other studies have identified channels in the skull bone marrow that permit the egress of calvarial immune cells, including B lymphocytes(Reference Herisson, Frodermann and Courties73,Reference Kolabas, Kuemmerle and Perneczky74) . These findings imply that B lymphocytes potentially contribute significantly to the inflammatory process in PD, both centrally and peripherally, indicating that they may be a pertinent target for disease-modifying treatments. Notably, it is now well accepted that B cell counts are reduced in PD(Reference Gruden, Sewell and Yanamandra75–Reference Li, Tropea and Baratta78), which is consistent with our results. Importantly, postmortem research has shown that IgG binds to approximately 30 % of neurons in the substantia nigra(Reference Orr, Rowe and Mizuno79). We found a causal relationship between increased serum TAG (51:4) levels and a reduction in the percentage of IgD-CD38-B lymphocytes. More significantly, a causal association was present between a lower percentage of IgD-CD38-B lymphocytes and reduced risk of PD. These findings indicate that IgD-CD38-B cell lymphocytes mediate the effects of serum TAG (51:4) levels on PD.
In addition to B lymphocytes, several animal models have demonstrated the important role of Treg cells in preventing neurodegeneration in PD. A decrease in IFN-g-producing type 1 helper T cells was correlated with an increase in CD41CD251 cells in lymphoid tissues in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model(Reference Huang, Liu and Wang80). Similarly, a more robust microglial response and greater loss of dopaminergic neurons in the MPTP model are linked to considerably lower numbers of CD41CD251 cells observed in aquaporin-4 knockout mice when compared with wild-type mice(Reference Chi, Fan and He81). In the same model, systemically increasing the number of Treg cells by injecting Treg-expanding bee venom phospholipase A2 or adoptive transfer of CD3-activated Treg cells prevented the degeneration of dopaminergic neurons in the substantia nigra and reduced microglial activation and CD41 cell infiltration(Reference Chung, Kim and Lee82). Furthermore, growing evidence suggests that Treg cell malfunction may play a role in the pathophysiology of PD. PTEN-induced kinase 1 (PINK1) mutations are associated with familial early-onset parkinsonism, and Treg cell formation and function are impaired in PINK1 knockout mice(Reference Ellis, Zhi and Akundi83). Although Treg cells have been reported to have a greater suppressive capacity in patients with PD(Reference Duffy, Keating and Perera84), other studies have revealed that this condition reduces serum Treg cell counts(Reference Chen, Qi and Xu85) and affects their ability to suppress effector T cell function in vitro (Reference Saunders, Estes and Kosloski86). We found that there was a causal relationship between higher serum TAG (51:4) levels and an increase in the proportion of resting CD4 regulatory T cells %CD4 + T cells. More importantly, a causal relationship was observed between a lower risk of PD and a greater ratio of resting CD4 regulatory T cells to CD4 + T cells, which was revealed as another mediator in the relationship between plasma lipidome exposure and the risk of PD. Consequently, we speculated that the effect of serum TAG (51:4) levels was partially mediated by resting CD4 regulatory T cells and CD4 + T cells.
Human cross-sectional and cohort studies have highlighted that high total fat intake can increase the risk of allergy and allergic diseases(Reference Lim, Reginald and Say87). High total fat intake, especially saturated fat, often leads to increased TAG levels in the blood, which may exacerbate neuroinflammation and neurodegeneration in PD(Reference Lim, Lim and Reginald88–Reference Shokri-Mashhadi, Ghiasvand and Feizi90). Notably, the effect of dysregulated lipid metabolism on the risk of PD, specifically with regard to TAG, remains unclear. Our results are important, in part, as they offer a mechanistic explanation for the association between blood TAG (51:4) levels and development of PD, which has been observed and demonstrated in other studies. Several studies have indicated a strong relationship between oxidative stress and chronic inflammation caused by endogenous danger signalling molecules, as well as the onset and progression of PD(Reference Jayaram and Krishnamurthy91–Reference Zimmermann and Brockmann93). Notably, nutritional studies have shown that TAG derived from red sorghum have potent antioxidant, anti-inflammatory and radical scavenging properties(Reference Li, Xu and Zheng94). Furthermore, Deng et al. verified that dietary consumption of medium- and long-chain TAG considerably enhanced lipid metabolism and decreased inflammation in obese rats fed a high-fat diet(Reference Du, Chen and Zhu95). Additionally, Campos et al. found esterification of arachidonic acid into TAGs in Fe-overloaded neurons, and an increase in TAG levels and appearance of lipid droplets suggested that dopaminergic neurons respond to oxidative injury by storing peroxidable fatty acids in the TAG pool, thus avoiding the persistence of PUFA in the phospholipid fraction(Reference Sánchez Campos, Rodríguez Diez and Oresti96). These results imply that the anti-inflammatory and antioxidant properties of TAG (51:4) may be the underlying mechanisms by which it protects against PD.
In addition to modulating immune responses, TAG play a vital role in improving lipid metabolism, particularly within glial cells, which may be crucial for maintaining neuronal health and homoeostasis(Reference Li, Zeng and Huang97). Enhanced lipid metabolism helps mitigate disruptions that could otherwise lead to neurodegeneration(Reference Yang, Wang and Zhang98). Studies have suggested that higher TAG levels are often associated with neuroprotection in the context of PD, potentially reducing the risk of developing the condition and promoting better outcomes in affected individuals(Reference Fang, Zhan and Hammar99). Additionally, TAG are integral to energy metabolism in the brain and essential for sustaining neuronal function. An adequate energy supply is critical for maintaining neuronal integrity, and disruptions in energy metabolism can lead to neurodegenerative changes(Reference Li, Zeng and Huang97). By supporting energy metabolism, TAG contribute to overall neuronal health and may prevent the progression of neurodegenerative diseases, such as PD(Reference Gómez-Soler, Cordobilla and Morató100). Interestingly, autophagy activation, a critical cellular degradation pathway, is another mechanism by which TAG exhibit protective effects(Reference Xie, Li and Kang101). Enhanced autophagic activity linked to TAG may promote clearance of misfolded proteins and damaged organelles, which are characteristic of neurodegenerative diseases, such as PD(Reference Zhang, Peng and Yang102). This process could be particularly effective in reducing protein aggregation, such as that of α-syn, which has been implicated in the pathology of PD. Additionally, TAG stored in lipid droplets may serve as buffers against toxins and oxidative stress that contribute to PD. For instance, synucleins, which are proteins linked to PD, are known to bind TAG and protect them from degradation(Reference Cole, Murphy and Grider103), thereby potentially shielding cells against PD-related damage. In summary, TAG, a type of lipid, may offer protection against PD through several mechanisms, although the exact process is not fully understood.
Limitations
Understanding the estimates obtained by MR can be challenging, as MR depends on assumptions that might not be testable, such as exclusion criteria, especially in cases involving unmeasured or unknown potential confounding factors. For example, MR estimates may be confounded by horizontal pleiotropy, a phenomenon in which genetic variants are independently associated with traits other than those studied. Sensitivity analyses that are more resilient to these types of pleiotropy can be employed to assess the susceptibility of MR estimates to horizontal pleiotropy. In this study, the MR-Egger and weighted median approaches were utilised for this purpose. Additionally, allele frequencies and illness risk range between populations of various genetic ancestries might introduce genetic confounders into an MR study and potentially lead to false causal estimations. The GWAS approaches used in this investigation to identify associations between circulating immune cell counts and PD considered population stratification and cryptic relatedness should minimise the chances of these confounding factors.
The present study found evidence supporting a potential causal relationship between the reduction in PD risk and higher serum TAG (51:4) levels and clarified the possible role of circulating immune cells (including IgD-CD38-B cell lymphocytes and resting CD4 regulatory T cell %CD4 + T cells), but not circulating inflammatory proteins. The molecular mechanisms underlying this association may involve pathways associated with inflammation; however, they can also operate independently of classical regulatory mechanisms.
Suggestions for future work
Some studies have found that therapeutic strategies aimed at lipid metabolism in PD can significantly alleviate disease progression by reducing the aggregation of α-syn(Reference Baekelandt, Lobbestael and Xicoy104). For instance, targeting specific enzymes involved in lipid metabolism, such as LIPE, may decrease α-syn inclusions and improve neuronal health(Reference Adom, Hahn and McCaffery105). Enhanced fatty acid metabolism can also facilitate energy production and reduce oxidative stress, which are detrimental to dopaminergic neurons. Immune modulation is another promising approach for therapeutic intervention in PD. It is generally accepted that chronic neuroinflammation is a key contributor to dopaminergic neuron degeneration, and targeting inflammatory pathways may protect neuronal integrity(Reference Tansey, Wallings and Houser106). Findings based on the present study, thus, combining lipid metabolism modulation with immune modulation may be a potent therapeutic strategy. For example, interventions that reduce neuroinflammation by targeting specific enzymes that regulate lipid metabolism in neurons may synergistically improve patient outcomes. A multifaceted approach may facilitate the reduction of toxic protein aggregates, while simultaneously supporting overall brain health.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114524002137
Acknowledgements
The authors are grateful to Dr Marco and Dr Huang Shu-na for their comments on the manuscript and assistance with statistical analyses. The authors also acknowledge the teams of Yukinori Okada, James E. Peters, Valeria Orrù and Matti Pirinen for providing data support for our study by opening up the circulating inflammatory proteins phenotype-related GWAS summaries, the immune cells phenotype-related GWAS summaries, Parkinson’s disease phenotype-related GWAS summaries and the plasma lipidome phenotype-related GWAS summaries.
This work was supported by the National Natural Science Foundation of China (Project No. 82171327).
Y. J. J. and H. L. Z. contributed to conceptualisation. Y. M. C. and J. Y. S. contributed to the literature search. Y. J. J. and J. Y. S. contributed to data analysis. Y. J. J., J. Y. S. and J. Y. S. contributed to original draft writing. D. Z. K., L. Y. H. and D. L. W. wrote, reviewed and edited the manuscript. All authors have read and approved the final version of the manuscript.
The authors declare none.









