Suboptimal diet quality accounts for a greater population burden of morbidity and mortality from chronic diseases than tobacco, alcohol and physical activity combined(Reference Lim, Voz and Flaxman1). Consumption of diets including high proportions of fruits and vegetables are associated with reduced risks of developing CVD, type 2 diabetes and cancer(Reference Boeing, Bechthold and Bub2). However, the majority of US adults consume less than the recommended amounts(3). This is especially true for individuals facing food insecurity, the limited or uncertain ability to acquire adequate food due to insufficient money and other resources(Reference Coleman-Jensen, Rabbitt and Gregory4,Reference Leung, Epel and Ritchie5) .
Individuals experiencing food insecurity may employ compensatory strategies such as skipping meals, reducing portion sizes and reducing variety in their diets, which can increase the risk of development or exacerbation of diet-sensitive chronic disease(Reference Seligman and Schillinger6,Reference Laraia7) . A combination of physiological and behavioural responses to food insecurity and the associated stress has been offered as an additional explanation for the observed relationships between food insecurity, suboptimal diets and chronic disease(Reference Laraia7–Reference Laraia, Leak and Tester9). This is especially salient for low-income black populations in the Southeast US who experience disproportionate chronic disease burden(Reference Carnethon, Pu and Howard10). Even when controlling for socio-economic status, significant racial differences in chronic disease outcomes are evident(Reference Phelan and Link11). Structural, institutional, interpersonal and internalised racism lead to health inequalities through social, economic and political exclusion resulting in less access to resources and greater physiological embodiment of stress, both of which lead to poorer health outcomes(Reference Simons, Lei and Klopack12).
Given the important roles of diet quality and food insecurity in chronic disease(3,Reference Laraia7) , there has been a proliferation of interest in interventions incorporating Food is Medicine™ initiatives into healthcare systems to facilitate access to healthy foods for marginalised patients(Reference Veldheer, Scartozzi and Knehans13–Reference Downer, Berkowitz and Berkowitz15). One such approach is a produce prescription (PRx) model, in which healthcare providers refer their patients to free or discounted healthy produce(Reference Mozaffarian, Mande and Micha14,Reference Bhat, Coyle and Trieu16) .
PRx programmes use a partnership model of care that involves a referring healthcare provider and produce retailers(Reference Swartz17). Financial incentive models, including PRx programmes, are informed by the principles of operant conditioning, whereby behaviours eliciting rewards are repeated(Reference Skinner18,Reference Burns, Donovan and Ackermann19) . Incentives, in this case produce, may act as facilitators for healthy cooking and eating practices by increasing access and convenience of acquiring fresh produce, enabling participants to practice skills outside of class sessions and build self-efficacy. In this way, incentives act as catalysts for behaviour change and repeated engagement may become intrinsically motivating, facilitating sustained behaviour change(Reference Purnell, Gernes and Stein20). Some PRx programmes incorporate group-based nutrition education and cooking sessions(Reference Burrington, Hohensee and Tallman21–Reference Watt, Appel and Lopez26). Nutrition education increases knowledge and awareness while hands-on cooking sessions provide skills and increase self-efficacy to engage in the behaviour(Reference Michie, van Stralen and West27–Reference Prochaska and Velicer29). These behaviours are then reinforced through educational sessions involving peer and provider support and through practice at home as facilitated by the provision of free or discounted produce(Reference Swartz17,Reference Purnell, Gernes and Stein20,Reference Michie, van Stralen and West27,Reference Bellg30) .
There is consistent evidence that PRx programmes increase food security and fruit and vegetable consumption(Reference Bhat, Coyle and Trieu16). However, few studies have reported on health outcome measures. A recent meta-analysis estimated that PRx programmes are associated with decreases in BMI of 0·6 kg/m2 (95 % CI 0·2, 1·1) and HbA1c of 0·8 % (95 % CI 0·1, 1·6) with no significant changes observed for blood pressure or lipid concentrations(Reference Bhat, Coyle and Trieu16). Among studies reporting health outcomes from participation in PRx programmes, only one used longitudinal data(Reference Emmert-Aronson, Grill and Trivedi22) and none to our knowledge has reported on multiple years of programme implementation. Additionally, no studies to our knowledge have assessed the relationship between programme attendance and health outcomes within the context of a PRx programme. No studies evaluating health outcomes have been conducted in the Southeastern US or with predominantly black participants to our knowledge. Given the health disparities in this population(Reference Mazimba and Peterson31), there is a great need for research to include more black participants and other underrepresented groups. To address these needs, we assessed the relationship between programme attendance and changes in CVD risk factors in the Georgia Food for Health (GF4H) programme, a PRx programme implemented in inner-city Atlanta, Georgia with a majority black participant population.
Methods
GF4H is a multi-partner collaboration that aims to improve food access and provide experiential nutrition and cooking education. The 6-month GF4H programme provided vouchers worth $1 per household member per day, redeemable weekly for fresh produce at retail locations throughout Atlanta. Additionally, participants received monthly group-based nutrition education and hands-on cooking classes for the first 6 weeks of the programme.
Local context and partnership roles
Located in inner-city Atlanta, Georgia, Grady Health Systems is a safety-net hospital that served as the healthcare partner and implementation site for the programme. Grady Health Systems serves marginalised populations in Fulton and Dekalb counties who have limited or no health insurance. Data collected from the Grady Health Systems Primary Care Center suggest that the majority of patients experience poverty (90 % report annual family incomes < $20 000), multiple chronic health conditions (two-thirds have ≥ 4 chronic diseases) and demonstrate low patient activation (60 % report low knowledge and confidence to take action in self-management of health). Open Hand Atlanta is a community-based organisation that served as the cooking education partner and provided funding for produce. Wholesome Wave Georgia is a community-based organisation that provided administrative support and funding for produce. The Common Market Southeast, the East Point Farmers Market and the MARTA markets, a local food distributor and community farmers markets, respectively, provided produce and prescription redemption sites for the programme. Emory University is a research institution and served as the research and evaluation partner.
Recruitment
Participants were referred by healthcare providers from five clinics within Grady Health Systems including three primary care clinics, a diabetes clinic and an infectious disease clinic. Eligibility requirements included a positive screen for food insecurity in the previous 12 months using a validated 2-item food insecurity screener(Reference Hager, Quigg and Black32,Reference Gundersen, Engelhard and Crumbaugh33) . Participants were 18 years or older, patients of the Grady Health Systems Primary Care Centers and expressed commitment to the 6-month programme(Reference Hager, Quigg and Black32). Recruitment strategies varied somewhat by year and clinic. In 2017, clients were referred directly by their healthcare providers during clinic visits and followed up by registered dietitians for enrolment into the programme. In 2018 and 2019, participants from four of the five clinics were recruited from a pool of patients who were attending group nutrition education sessions offered at the clinics by registered dietitians. At the fifth clinic, participants were referred directly during clinic visits by their healthcare providers and followed up by registered dietitians for enrolment.
Intervention
Over the first 6 weeks, six hands-on cooking classes were taught by a Registered Dietitian from Open Hand Atlanta using Cooking Matters™, an evidence-based curriculum(34). Classes included resource management tips, with the goal of teaching participants to prepare healthy meals on a limited budget. At each weekly cooking skills class, seasonal produce was provided according to participant household size. Concurrently, participants attended monthly Eat Well, Live Well wellness courses for the duration of the 6-month GF4H programme. The education content of the Eat Well, Live Well nutrition sessions covered shopping and cooking healthfully on a budget, exercise demonstrations and gardening sessions. At each monthly Eat Well, Live Well nutrition session, vouchers were distributed worth $1 per family member per day. These were redeemable at local retail locations such as MARTA markets and farmers markets located in train stations in participants’ communities. To address common barriers to participation, the GF4H programme offered assistance with transportation, allowed participants to bring children to group sessions and offered opportunities to make up missed group sessions with one-on-one meetings with providers as needed. See Table 1 for a description of each component of the programme.
Table 1 Georgia Food for Health programme components

Graduation
Participants were considered graduates if they attended 4 out of 6 of both the Cooking Matters classes and Eat Well, Live Well sessions. In 2017, forty-three participants were enrolled in the programme across two cohorts and thirty-four of those participants graduated (79 %). In 2018, the programme expanded, adding additional cohorts with 115 participants enrolled. Of those, ninety-one graduated (79 %). In 2019, 173 participants were enrolled and 157 graduated (91 %).
Measures
Surveys were administered at baseline, at the final Cooking Matters session 6 weeks later, and at the end of the programme 6 months following baseline. Surveys were self-administered by participants with evaluators present to assist with questions, verbally administer surveys as needed and check for survey completion.
Socio-demographic information collected at baseline included sex (female, male), age in years (18–29, 30–39, 40–29, 50–59 and 60+), ethnicity (Hispanic or Latino: Yes/No), race (Asian/Asian American, American Indian/Alaskan Native, Black/African American or Caribbean American, Hawaiian/Pacific Islander, White/Caucasian and Other/Multi-racial), highest level of education attained (less than high school degree, high school or GED certificate, two-year college or technical school degree, some college/technical school, but have not graduated, four-year college or technical school degree and more than four-year college degree), employment status (working full-time, working part-time, retired, not employed/homemaker, student, on disability and other), health insurance status (uninsured, insured by Medicaid, Medicare or other public insurance, insured through employer, insured through private insurance and other), annual household income (less than $25 000, $25 000–$34 999, $35 000 or greater) and household size including non-relatives living in the home.
The 2-item Hunger Vital Signs tool(Reference Hager, Quigg and Black32) was used to determine eligibility for the programme. At enrolment, 6 weeks of participation and the end of the programme, participants completed the 6-item United States Department of Agriculture Household Food Security Survey Module(Reference Blumberg, Bialostosky and Hamilton35) with a 30-d recall to assess recent food security status and change over time. The 6-item module was chosen over the longer 18-item United States Department of Agriculture module for programme evaluation to avoid unduly increasing participant burden while still providing granularity of food security status beyond that of the 2-item tool used in recruitment(Reference Blumberg, Bialostosky and Hamilton35). Food security was categorised using the scoring guide with categories including: high or marginal food security (0–1 affirmative responses to screening questions), low food security (2–4 affirmative responses) and very low food security (5–6 affirmative responses)(Reference Blumberg, Bialostosky and Hamilton35).
At each monthly Eat Well, Live Well visit, clinical staff collected height, weight, blood pressure and waist circumference prior to programme education sessions. Height was collected using ScaleTronix stadiometers, weight using ScaleTronix scales, blood pressure using Omron Blood Pressure Monitor Model BP742N and waist circumference using retractable measuring tape. BMI was derived from monthly height and weight variables as weight in pounds divided by height in inches squared and multiplied by 703(36).
Redeemed vouchers were collected by the individual markets at the time of redemption and returned to study staff. Household per-capita redemption was calculated as the dollar amount of vouchers redeemed divided by household size.
Ethics
This project was deemed exempt from review by Emory University’s institutional review board, as it was considered a quality improvement project for an existing and ongoing intervention and was approved by Grady Health Systems’ Office of Research Administration. Though informed consent was not required, participants were informed of data collection procedures and informed that all data collection was voluntary, and they could choose not to participate in these procedures without affecting their ability to continue in the programme.
Analytic sample
Participants who were enrolled but did not complete the programme (n 49) were excluded from the analysis as follow-up data were not available for those who did not complete the programme due to alignment of programme sessions and data collection. The mean number of visits attended among those lost to follow-up was 1·1, meaning only baseline data were available for those lost to follow-up, limiting our ability to conduct an intent-to-treat analysis. The overall graduation rate across all 3 years was 83 %, resulting in a final analytical sample of 282. We conducted an attrition analysis comparing socio-demographic, household characteristic and food security information provided at baseline for those retained and those lost to follow-up using frequencies and chi-square tests to identify significant differences between the groups.
Statistical methods
We used descriptive analyses, including means and frequencies to characterise study participants and paired t tests to test the significance of change in values for continuous outcomes. We used a longitudinal, repeated measures, single-arm approach to estimate the association between the number of monthly programme visits attended and changes in BMI, weight, waist circumference, systolic blood pressure and diastolic blood pressure. In this study, we restricted the analysis to programme graduates, restricting the range of monthly visits attended to 4–6, so while the model uses all available data from visits 1–6 in estimation, the coefficients reflect the association between a one-unit increase in visits attended beyond visit 4 and outcome. We controlled for potential confounding factors by including fixed effects for programme site, year, participant sex, and age, race and ethnicity, Supplemental Nutrition Assistance Program participation, and household size and random effects for intercepts and slopes for participants and site of participation, which accounts for individual and site-level variation in outcomes at baseline and over time. Fixed effect covariates were selected using forward selection procedures and comparing Akaike information criterion and Bayesian information criterion values as indicators of model fit. We used restricted maximum likelihood to estimate the model parameters and we presented estimates with 95 % CI.
Some socio-demographic data were missing for fifty-five of the programme graduates (19·5 %). Specifically, race and ethnicity were missing for 8 (2·8 %), highest level of education attained was missing for 7 (2·5 %), health insurance status was missing for 38 (13·5 %), employment status, income or receipt of public benefits was missing for 6 (2·1 %, respectively) and household size was missing for 9 (3·2 %). Some covariate data were additionally missing: sex was missing for 4 graduates (1·4 %) and age was missing for 9 (3·2 %). Additionally, blood pressure was missing for 1 observation for 7 graduates (2·5 %), BMI was missing for 3 (1·1 %) and waist circumference was missing for 5 (1·8 %). We used multivariate imputation by chained equations method to estimate observed outcomes in the scenario of no missing data (see online Supplemental Materials)(Reference White, Royston and Wood37). All analyses were conducted in STATA version 17.0(38). Statistical significance was determined at P < 0·05.
Results
Participant characteristics
A flow chart displaying the number of participants enrolled, lost to follow-up and graduating is presented in Fig. 1. Demographic characteristics of programme graduates are presented in Table 2. Most participants were black (93·1 %), female (71·6 %) and aged 40 years or older (91·9 %). A majority of participants (65·3 %) received public health insurance and 86·6 % had a household income of less than $25 000 annually. Most were retired (24·3 %) and/or receiving disability benefits (40·2 %). At baseline, 60·4 % of participants were characterised as having low or very low food security and 59·4 % received Supplemental Nutrition Assistance Program benefits.

Fig. 1 Flow chart of participants enrolled, lost to follow-up and final analytical sample of programme graduates
Table 2 Demographic characteristics of Georgia Food for Health programme graduates, 2017–2019 (n 282)

SNAP, Supplemental Nutritional Assistance Programme
Programme graduates are defined as those who completed at least 4 of the 6 monthly programme visits.
Food security status was assessed using the 6-item United States Department of Agriculture Household Food Security Survey Module and categorised as high or marginal (0–1 affirmative responses to screening questions), low (2–4 affirmative responses) or very low (5–6 affirmative responses).
Results of an attrition analysis show those retained in the programme (n 282) were more likely to be over the age of 50 years (P = 0·002) and less likely to have been referred from the infectious disease clinic (P = 0·023) compared to those lost to follow-up (n 49). No differences in retention were observed based on sex, race and ethnicity, highest level of education attained, employment, household income, household size, receipt of public benefits, health insurance or food security status at baseline.
Clinical outcomes
At baseline, programme graduates had a mean BMI of 36·5 (95 % CI 35·5, 37·6) kg/m2, a mean weight of 227 (95 % CI 220, 233) lbs., a mean waist circumference of 45·3 (95 % CI 44·5, 46·1) inches, mean systolic blood pressure of 140·4 (95 % CI 138·1, 142·6) mmHg and mean diastolic blood pressure of 82·8 (95 % CI 80·4, 83·2) mmHg. In unadjusted models, we observed significant reductions in mean BMI, weight, waist circumference, systolic and diastolic blood pressure from the first programme visit attended to the last programme visit attended (Table 3).
Table 3 Unadjusted mean changes in clinical indicators between first and last visit attended, among Georgia Food for Health programme graduates, 2017–2019

After controlling for programme site, year of implementation, participant sex, race and ethnicity, Supplemental Nutrition Assistance Program status and household size, each additional programme visit beyond four visits was associated with a 0·6 (95 % CI –0·1, –0·0) kg/m2 reduction in BMI, a 0·4 (95 % CI –0·7, 0·0) lb. reduction in weight, a 0·4 (95 % CI –0·5, –0·3) inch reduction in waist circumference, a 1·0 (95 % CI –1·5, –0·6) mmHg reduction in systolic blood pressure and a 0·4 (95 % CI –0·7, –0·2) mmHg reduction in diastolic blood pressure (Table 4). Estimates using imputed data were consistent with those from the original dataset. However, coefficients for the association between programme participation and blood pressure were slightly lower in magnitude when using the imputed data (online Supplemental Table 1).
Table 4 Estimated association of an increase from 4 to 5 sessions and 5 to 6 sessions attended with change in clinical measures among Georgia Food for Health programme graduates, 2017–2019
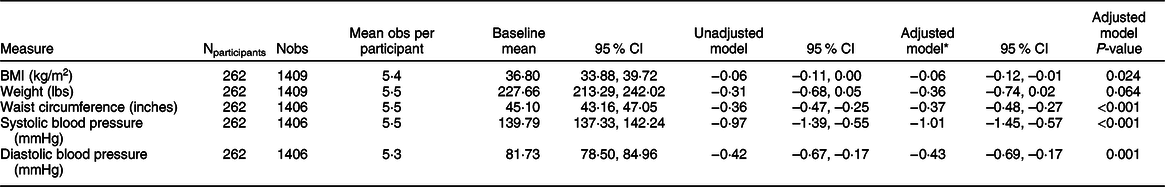
All estimates produced from linear mixed models including random intercepts and slopes for participants and site of participation.
* Adjusted models include fixed effects: year, sex, and age, race and ethnicity, Supplemental Nutrition Assistance Program participation status and household size
Discussion
Among graduates of the GF4H programme, the number of programme visits attended was associated with modest but statistically significant reductions in BMI, weight, waist circumference and blood pressure measures. Most published studies on evaluations of similar programmes report increases in fruit and vegetable consumption and improvements in food security but have not reported on health outcomes(Reference Bhat, Coyle and Trieu16). A meta-analysis pooling results of three studies reporting BMI, four studies reporting blood pressure and five studies reporting HbA1c estimated that PRx programmes were associated with modest decreases in BMI by 0·6 kg/m2 (95 % CI –2·8, –0·3) and HbA1c by 0·8 % (95 % CI –1·6, –0·1) across studies(Reference Bhat, Coyle and Trieu16). In this meta-analysis, no significant changes in blood pressure or lipid concentrations were observed. Our results are generally comparable in magnitude to these few published evaluations of PRx programmes. However, heterogeneity in programme duration and implementation, participant characteristics and study design limit the ability to make direct comparisons between programmes. Other programmes range in duration from 13 weeks(Reference Bryce, Guajardo and Ilarraza39,Reference Cavanagh, Jurkowski and Bozlak40) to 10 months(Reference Hager, Du and Li41) and involve a variety of programme components such as mindfulness meditation and physical activity(Reference Emmert-Aronson, Grill and Trivedi22).
Nutrition education components vary substantially across publications, with one programme providing healthy eating information handouts(Reference Berkowitz, O’Neill and Sayer42), others involving one-on-one nutrition counselling sessions(Reference Cavanagh, Jurkowski and Bozlak40,Reference Omar, Heidemann and Blum-Alexander43) and another providing hour-long group-based sessions over a meal(Reference Emmert-Aronson, Grill and Trivedi22). Although many programmes incorporate recipes and cooking demonstrations(Reference Bryce, Guajardo and Ilarraza39,Reference Omar, Heidemann and Blum-Alexander43) , there are no published studies of PRx programmes that include hands-on cooking education. One study evaluating Cooking Matters, the evidence-based programme used in GF4H, demonstrated effectiveness in improving confidence with food resource management and food resource management practices such as comparison shopping and planning meals ahead of time(Reference Pooler, Morgan and Wong44). In another study evaluating Cooking Matters in conjunction with Diabetes Self-Management Education and weekly meal provision (4 servings per week), improvements in diabetes management, diet, food security and health-related quality of life were observed(Reference Williams, Shrodes and Radabaugh45). No studies to our knowledge have evaluated health outcomes among participants of Cooking Matters, with the exception of Williams et al., who reported no overall change in HbA1c in their study although participants experiencing food insecurity showed greater improvements in HbA1c than their food secure counterparts. While the monthly registered dietitian-led sessions, Eat Well, Live Well, included in the GF4H programme were not based on an existing evidence-based programme, the content included frequency and duration of sessions, and expertise of educators aligned with recognised best practices in nutrition education for low-income audiences(Reference Baker, Auld and Ammerman46).
PRx programmes are designed to improve chronic disease risk factors by increasing food security and diet quality(Reference Downer, Berkowitz and Berkowitz15). The combination of increased access to high-quality food and nutrition education supports participants’ engagement in healthy shopping and eating practices throughout the programme(Reference Downer, Berkowitz and Berkowitz15,Reference Swartz17,Reference Hager and Mozaffarian47) . By practicing these behaviours, participants gain confidence in their skills and ability to acquire and cook healthy food on a budget, improving ability to maintain these behaviours after the programme has ended(Reference Pooler, Morgan and Wong44,Reference Adedokun, Plonski and Aull48) . Sustained improvements in diet quality reduce the risk of chronic disease risk factor progression and exacerbation of existing conditions(3). While evidence is converging to support the effectiveness of PRx programmes in improving food security, diet and self-efficacy related outcomes, results from studies reporting on health outcomes remain mixed. This study of the GF4H programme examining the association between programme attendance and health outcomes adds to the evidence of effectiveness of PRx on improving health risk factors and improves the literature base by including a majority black participant population, which is much-needed given health disparities evident among this group. Still, further studies are needed to examine the long-term benefits of these programmes and to better understand the impacts of individual programme components.
Limitations
This study has several limitations. Follow-up data were not available for those who were lost to follow-up, limiting our findings to those who completed the programme. The mean number of visits attended for those lost to follow-up was 1·1 (95 % CI 1·0, 1·2), limiting our ability to investigate changes in the interim points for those who did not graduate. However, graduation rates across the 3 years of the programme were relatively high at 83 %, comparable to those observed in published evaluations of similar programmes(Reference Emmert-Aronson, Grill and Trivedi22,Reference Bryce, Guajardo and Ilarraza39,Reference Berkowitz, O’Neill and Sayer42) . For some clinics, participants were recruited from a pool of patients who had completed four introductory group nutrition classes, so those enrolled may have differed from the general patient population in that they may have been more motivated to participate based on previous positive experiences with the introductory programme or greater interest in diet-related programming. These participants may have also had more schedule flexibility to participate in the 6-month programme involving both group education sessions and weekly market visits for produce voucher redemption. It is also possible that those who graduated the programme remained engaged due to their perceived benefits of participation, indicating potential for reverse causality. However, the findings from this study remain useful for understanding the potential among motivated patients for chronic disease risk factor improvement after participation in a PRx programme.
We do not have information on why participants dropped out of the programme or were lost to follow-up. As Stotz and colleagues note, PRx participants often have competing barriers to programme engagement and may require additional services such as transportation to facilitate engagement(Reference Stotz, Budd Nugent and Ridberg49). Implementation of a process for routinely collecting and recording information on factors contributing to disengagement would be helpful for understanding the barriers to participation and generating ideas on how to address them to better retain participants.
Another limitation is the lack of a comparison group in evaluation. It is possible that changes observed in this study were related to factors outside of the intervention such as participation in other nutrition programming or factors tangential to effects of the intervention related to potential increases in engagement in care or improvements in medication adherence related to increases in food security. Additional investigations involving control groups and randomised study design are needed to strengthen our understanding of the potential of PRx programmes for achieving health outcome improvements.
Additionally, some missing data were present due to skipped questions in surveys or, in some cases, participants missing data collection days. While the proportion of missing data was low, analysis of a dataset created using multiple imputation was performed and compared to the results of complete-case analysis. Estimates of clinical change over the course of the programme were similar and help to confirm the validity of the findings presented here.
This study is also limited by the lack of ability to assess comparative effectiveness of the components of the programme on health outcomes. Although this study examined the relationship between programme attendance and CVD risk factors, it did not isolate the effects of nutrition education, cooking education and the provision of free produce. Future research to address this gap could involve the use of randomisation of programme components to allow for comparison. Additionally, structural equation modelling techniques such as pathway analysis could be useful for understanding the specific contributions of each component on different outcomes and help understand the role of mediating factors.
Strengths
The major strengths of this evaluation include the use of 3 years of programme data from multiple sites of implementation and longitudinal data with objective biometric measures. This programme was implemented in an urban, safety net health system context, with low-income participants. These populations face the highest barriers to engaging with an in-person programme. However, we observed high graduation rates (83·0 % graduated across all years) and graduation improved with each year of programme implementation (from 79·1 % in 2017 and 2018 to 90·8 % in 2019). Improvements in programme graduation are potentially related to continuity of staff and increased competence with operating procedures over time including increased communication between programme partners, resulting in greater clarity of goals and a more cohesive and flexible programme structure for participants(Reference Cook, Ward and Newman50).
Conclusions
Overall, our findings support the hypothesis that increased access to fresh produce and education in nutrition, cooking and food resource management techniques is associated with modest improvements in chronic disease risk factors over the course of a 6-month intervention in a low-income, urban population. Each additional programme visit attended beyond the graduation threshold was associated with modest but significant improvements in CVD risk factors, suggesting that increased engagement in cooking and nutrition education within the context of a PRx programme improves health outcomes. These findings can also help with participant and programme staff goal setting and inform realistic outcomes from participation in similar programmes.
Acknowledgements
We would like to first thank the participants of the Georgia Food for Health (GF4H) programme for their commitment to the programme. We are also deeply grateful for all the GF4H partner organisations including Grady Health Systems, Open Hand Atlanta, Wholesome Wave Georgia, The Georgia Common Market, The Fresh MARTA Markets, East Point Farmers Market and Emory University, who worked tirelessly to successfully deliver this programme.
Financial support
The Georgia Center for Diabetes Translational Research Pilot Award funded the evaluation of this programme and had no role in the design, analysis or writing of this article.
Conflict of interest
M.A.C.: While this work was conducted as part of her doctoral studies, M.A.C. is now employed by Open Hand Atlanta as their Research and Evaluation Manager. She has additionally served as a consultant on evaluation projects for Grady Health Systems and was an intern with Wholesome Wave Georgia previously. K.T. is the Director of Medical Nutrition Therapy for Grady Health Systems. T.R. is the Director of Cooking Matters for Open Hand Atlanta. S.M. is the Director of Operations for Wholesome Wave Georgia. K.M. is the Senior Manager of Community Benefit and Population Health for Grady Health Systems. Cecilia Tran was employed as a Registered Dietitian for Grady Health Systems at the time this work was conducted. C.B. was employed as a Registered Dietitian for Grady Health Systems at the time this work was conducted. Stacie Schmidt is an internist for Grady Health Systems in addition to her role as an associate professor for Emory University. A.D.S.: None. A.W.G. is a former board member for Wholesome Wave Georgia and serves as an evaluation partner for Open Hand Atlanta.
Authorship
M.A.C.: (1) Substantial contribution to the study including: (a) conceptualisation/design, (b) methodology, (c) investigation, (d) data curation, (e) data analysis and (f) interpretation. (2) Participation in the writing of the manuscript including: (a) drafting the initial manuscript and (b) review and editing of the manuscript. (3) Gave final approval of the version to be published. (4) Agrees to be accountable for all aspects of the work. K.T.: (1) Substantial contribution to the study including: (a) conceptualisation/design, (b) investigation, (c) data curation and (d) interpretation. (2) Participation in the writing of the manuscript including: (a) review and editing of the manuscript. (3) Gave final approval of the version to be published. (4) Agrees to be accountable for all aspects of the work. T.R.: (1) Substantial contribution to the study including: (a) conceptualisation/design, (b) methodology, (c) data curation and (d) interpretation. (2) Participation in the writing of the manuscript including: (a) review and editing of the manuscript. (3) Gave final approval of the version to be published. (4) Agrees to be accountable for all aspects of the work. S.M.: (1) Substantial contribution to the study including: (a) conceptualisation/design, (b) methodology and (c) investigation. (2) Participation in the writing of the manuscript including: (a) review and editing of the manuscript. (3) Gave final approval of the version to be published. (4) Agrees to be accountable for all aspects of the work. K.M. (1) Substantial contribution to the study including: (a) conceptualisation/design, (b) methodology, (c) investigation and (d) interpretation/discussion points. (2) Participation in the writing of the manuscript including: (a) review and editing of the manuscript. (3) Gave final approval of the version to be published. (4) Agrees to be accountable for all aspects of the work. C.T.: (1) Substantial contribution to the study including: (a) investigation and (b) data curation. (2) Participation in the writing of the manuscript including: (a) review and editing of the manuscript. (3) Gave final approval of the version to be published. (4) Agrees to be accountable for all aspects of the work. C.B.: (1) Substantial contribution to the study including: (a) investigation and (b) data curation. (2) Participation in the writing of the manuscript including: (a) review and editing of the manuscript. (3) Gave final approval of the version to be published. (4) Agrees to be accountable for all aspects of the work. S.S.: (1) Substantial contribution to the study including: (a) conceptualisation/design, (b) methodology, (c) investigation, (d) data curation and (e) interpretation (clinical relevance). (2) Participation in the writing of the manuscript including: (a) review and editing of the manuscript. (3) Gave final approval of the version to be published. (4) Agrees to be accountable for all aspects of the work. A.D.S. (1) Substantial contribution to the study including: (a) methodology, (b) data curation, (c) data analysis and (d) interpretation. (2) Participation in the writing of the manuscript including: (a) review and editing of the manuscript. (3) Gave final approval of the version to be published. (4) Agrees to be accountable for all aspects of the work. A.W.G.: (1) Substantial contribution to the study including: (a) conceptualisation/design, (b) methodology, (c) investigation, (d) data curation, (e) data analysis, (f) interpretation and (g) funding acquisition. (2) Participation in the writing of the manuscript including: (a) review and editing of the manuscript. (3) Gave final approval of the version to be published. (4) Agrees to be accountable for all aspects of the work.
Ethics of human subject participation
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving research study participants were reviewed and approved by the Emory University Institutional Review Board, which designated this study as exempt, as it was considered a quality improvement project for an existing and ongoing intervention. The study was additionally approved by the Grady Health Systems Office of Research Administration. Verbal informed consent was obtained from all participants upon enrolment and formally recorded.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S1368980023001611







