The effect of heat stress on productive performance has been extensively studied in poultry, especially in high-producing hens( Reference Star, Kemp and van den Anker 1 – Reference Tang 4 ). High environmental temperature negatively influences the performance of laying commercial hens( Reference Star, Kemp and van den Anker 1 , Reference Mashaly, Hendricks and Kalama 2 ) and broiler breeders( Reference McDaniel, Bramwell and Wilson 3 , Reference Tang 4 ) by reducing feed intake, egg production and eggshell quality. In addition to altered productive performance, heat stress can also disturb the redox balance and induce oxidative stress, with the production of reactive oxygen species (ROS), in broiler breeders( Reference Xie, Tang and Lu 5 ) and commercial hens( Reference Lin, De Vos and Decuypere 6 ). Although substantial attention has been paid to the roles of antioxidant trace minerals (Se, Zn) in minimising the harmful effect of heat stress in broilers( Reference Mahmoud and Edens 7 ) and commercial laying hens( Reference Sahin and Kucuk 8 ), the role of Mn in stress reduction has not been well studied. Mn is a crucial component of the metalloenzyme Mn superoxide dismutase (MnSOD)( Reference Luo, Su and Huang 9 ), which plays a key role in the detoxification of superoxide free radicals. A series of studies in our laboratory have demonstrated that dietary Mn may increase heart MnSOD activity and reduce lipid peroxidation in broilers( Reference Luo, Su and Huang 10 , Reference Lu, Luo and Liu 11 ) and commercial laying hens( Reference Luo, Guo and liu 12 ), as well as up-regulate heart MnSOD expression( Reference Li, Luo and Liu 13 – Reference Bai, Lu and Wang 16 ) in broilers under thermoneutral conditions. In addition, moderately chelated organic Mn is the most effective in augmenting these activities. Our recent results have shown that high environmental temperature significantly impaired egg production performance and eggshell quality and induced lipid peroxidation and tissue damage, whereas dietary supplementation of either organic or inorganic Mn improved eggshell strength and thermotolerance and reduced protein oxidation, and that organic Mn with its moderate chelation strength could alleviate the negative effect of high temperature on egg production performance of broiler breeders at 32–45 weeks of age( Reference Zhu, Xie and Li 17 ). However, it is not clear whether dietary supplementation with Mn, especially organic Mn with moderate chelation strength, can reduce oxidative damage and increase heat stress resistance with the enhancement of antioxidant ability in broiler breeders. Hyperthermia increases the synthesis of heat shock proteins (HSP) in broilers to aid in thermotolerance( Reference Wang and Edens 18 , Reference Soleimani, Zulkifli and Hair-Bejo 19 ). However, hyperthermia induces HSP70 expressions via oxidative stress or the increased production of ROS( Reference Flanagan, Moseley and Buettner 20 , Reference Burdon, Gill and Rice-Evans 21 ). In this scenario, HSP70 accumulation might be used as a biomarker for potential heat stress damage( Reference Tedeschi, Kennington and Berry 22 ). Increased activities of superoxide dismutase (SOD) and catalase enzymes in cells blocked for induction of HSP expression result in a less-severe heat-stressed response( Reference Omar and Pappolla 23 ). It is assumed that dietary Mn supplementation might inhibit the expressions of HSP/heat shock factors (HSF) by enhancing the antioxidant response of broiler breeders under high environmental temperature. Therefore, the objective of this study was to investigate the effect of dietary supplementation with Mn, especially organic Mn with moderate chelation strength, on tissue Mn content, antioxidant status and expression of HSP/HSF in tissues of laying broiler breeders reared at normal and high environmental temperatures.
Methods
Experimental design and treatments
A completely randomised design involving 2 environmental temperatures×3 dietary Mn treatments was used in this experiment. The two environmental temperatures were a normal temperature of 21±1°C (NT) and a high temperature of 32±1°C (HT). The three dietary Mn treatments were a maize–soyabean meal basal diet without Mn supplementation (CON), and the basal diet supplemented with 120 mg of Mn/kg of diet on a fed-basis, which was either inorganic Mn sulphate (iMn) or organic Mn proteinate (oMn) with a moderate chelation strength. Thus, there were a total of six different treatments (NT-CON, NT-iMn, NT-oMn, HT-CON, HT-iMn and HT-oMn).
Birds and diets
This study was approved by the Animal Welfare Committee of Institutes of Animal Sciences, Chinese Academy of Agricultural Sciences. The managements of broiler breeders and dietary treatments for this experiment were the same as described previously( Reference Zhu, Xie and Li 17 ). In brief, 144 18-week-old female broiler breeders (Arbor Acres, Huadu Broiler Company) were randomly allotted to one of six treatments, with six replicates (four birds per replicate) for each treatment based upon body weight. Four birds in each replicate were kept in two neighbouring galvanised steel cages (length 50 cm×width 50 cm×height 50 cm) with 2 birds/cage. All broiler breeders were handled in accordance with the Arbor Acres breeder management guidelines for lighting and feeding and allowed ad libitum access to tap water containing no detectable Mn during the adaptation period from 18 to 29 weeks of age. After the adaptation, all broiler breeders were fed the same maize–soyabean meal basal diet with no Mn addition (Table 1, containing 32·5 g Ca/kg and 14·3 mg Mn/kg by analysis) to deplete Mn stores from 30 to 31 weeks of age. After Mn depletion, the room temperature for the NT-CON, NT-iMn and NT-oMn groups was maintained at 21±1°C, whereas the room temperature for the HT-CON, HT-iMn and HT-oMn groups was increased step-wise from 21 to 32°C over 2 d for these birds to acclimatise to the experimental chronic heat challenge, and then maintained at 32±1°C for the rest of the experiment. Relative moisture in the two rooms was kept at 40±5 % during the experimental period of 14 weeks (32–45 weeks of age). Our previous results( Reference Zhu, Xie and Li 17 ) on rectal temperature indicated that all of the laying broiler breeders under HT were in a heat stress status throughout the experimental period. The daily feed intake of birds in each replicate was recorded and reported previously( Reference Zhu, Xie and Li 17 ). All broiler breeders from both NT and HT were feed restricted with the same amount of treatment diets each day from 32 to 35 weeks of age. However, the feed intake of broiler breeders was still significantly lower under HT (125·5 g/bird per d) than under NT (136·1 g/bird per d) during this period. From 36 to 45 weeks of age, in order to eliminate the potential effect of reduced feed intake under HT, the birds under NT were pair-fed the same amount of feed consumed by birds under HT on the previous day, and the feed intake of breeders under HT and NT were 130·8 and 130·9 g/bird per d, respectively.
Table 1 Composition and nutrient levels of the basal diet (as-fed basis)
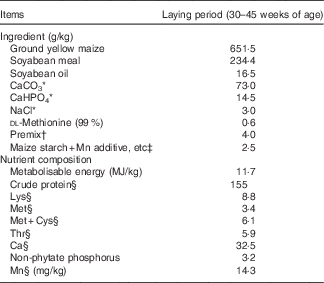
* Reagent grade.
† The premix contained the following vitamins and minerals (per kg of diet): retinol, 4·5 mg; cholecalciferol, 0·10 mg; α-tocopherol acetate, 36·0 mg; menadione, 3·9 mg; thiamin, 4·5 mg; riboflavin, 10·5 mg; pyridoxine, 4·5 mg; cyanocobalamin, 0·024 mg; pantothenate, 18 mg; niacin, 39 mg; folic acid, 1·5 mg; biotin, 0·18 mg; choline, 1000 mg; Cu, 10 mg; Fe, 50 mg; Zn, 100 mg; I (KI), 2·0 mg; I, 2·0 mg; Se, 0·30 mg.
‡ Mn additive, lysine-HCl or dl-methionine were added to diets by replacing an equal weight of maize starch.
§ Analysed values based on triplicate determinations.
The Mn-unsupplemented maize–soyabean meal basal diet for the Mn-depleting and experimental stages (Table 1) was formulated to meet or exceed the National Research Council( 24 ) requirements for laying broiler breeders, except for Mn, which was added to the basal diet according to the experimental design. The Mn sulphate (MnSO4·H2O) was reagent grade (purity>99 %; Beijing Chemical Company), whereas Mn proteinate was provided by Hebei Amino Acid Company (purity>90 %). A single batch of basal diet was mixed and then divided into three aliquots according to the experimental treatments. Lysine and methionine levels in the control diet or diet supplemented with inorganic Mn were balanced by adding synthetic lysine-HCl and dl-methionine based upon supplemental amounts of lysine and methionine from Mn proteinate source. The analysed Mn contents in diets, which were very close to calculated values, are presented in Table 2.
Table 2 Analysed manganese content in experimental dietsFootnote * (Mean values and standard deviations based on triplicate determinations)
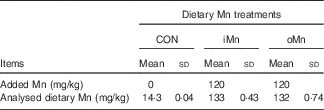
CON, Mn-unsupplemented basal diet; iMn, basal diet+120 mg Mn/kg as MnSO4·H2O; oMn, basal diet+120 mg Mn/kg as the Mn proteinate with a moderate chelation strength of 61·9 Q f (10·2 % Mn).
* The Mn concentrations are on an as-fed basis.
Sample collections and preparations
Samples of the Mn sources, diets and tap water were collected for analyses of Mn, Ca and dietary crude protein (CP) contents. At the end of the experiment, two birds from each replicate were selected on the basis of body weight and slaughtered humanely by carbon dioxide asphyxiation( Reference Sahin and Kucuk 8 ). Heart, liver and breast muscle samples were collected immediately. A set of tissue subsamples were snap-frozen in liquid N2 and then stored at −80°C for the mRNA and protein analyses, whereas another set of subsamples were kept on ice and stored at −20°C for subsequent measurements of Mn contents, malondialdehyde (MDA) levels and SOD activities. Tibiotarsal bones were boiled for approximately 10 min in deionised water; all soft tissue was removed, dried for 12 h at 105°C, and then ashed in a muffle furnace at 550°C for 16 h to obtain bone ash samples. For each tissue, all samples from the two birds in each replicate were pooled into one sample of equal weight before analyses.
Measurements of manganese, calcium, crude protein and amino acid contents and Q f value of manganese proteinate
The Mn and Ca contents in diets and Mn contents in Mn sources, tap water, tissues and bone ash were measured using an inductively coupled plasma emission spectroscope (model IRIS Intrepid II, Thermal Jarrell Ash) after wet digestions with HNO3 and HClO4 as described by Luo et al. ( Reference Luo, Su and Huang 9 ). Validation of the mineral analysis was conducted using bovine liver powder (GBW (E) 080193, National Institute of Standards and Technology) as a standard reference material. Contents of CP and Ca in feed ingredients and diets were determined using AOAC methods (1990)( 25 ). Amino acids in Mn proteinate were analysed using an amino acid analyser (model L-8500A, Hitachi Ltd), and the Q f value of Mn proteinate was determined using polarography as described by Li et al. ( Reference Li, Luo and Liu 13 , Reference Li, Lu and Hao 15 ). The control basal diet was also sampled for analyses of amino acid contents.
Determinations of malondialdehyde levels and superoxide dismutase activities in tissues
Heart, liver and breast muscle samples were homogenised in 10 % (w/v) physiological saline on ice for 60 s and then sonicated with an ultrasonic wave cell grinder (JY92-11; NingBo Scientz Biotechnology Company) for 1 min (on 1 s, interval 2 s). The homogenates were centrifuged at 1000 g for 15 min at 4°C and supernatants were collected to determine total protein contents and MDA levels and SOD activities. Total protein concentration was determined using a BCA Protein Assay kit (cat no. 23225; Pierce). The levels of MDA in the supernatant were determined using a commercial assay kit (cat no. A003-1; Nanjing Jiancheng Bioengineering Institute) and expressed as MDA content per mg protein. The total superoxide dismutase (TSOD) and MnSOD activities were measured following the nitrite method described by Li et al. ( Reference Li, Luo and Liu 13 ), and copper zinc superoxide dismutase (CuZnSOD) activities were calculated by subtracting MnSOD activity from TSOD activity. The MnSOD and CuZnSOD activities in the tissues were expressed as nitrite units per mg protein.
RNA extractions, reverse transcriptions and real-time PCR
Total RNA was isolated from heart, liver and breast muscle samples using Trizol reagent (cat no. 15596018; Life Technologies) according to the manufacturer’s instructions. The concentration of each isolated RNA sample was determined using a NanoDrop Spectrophotomter (ND-2000; Gene Company Ltd) and the integrity of the RNA was checked using denatured RNA electrophoresis.
A total of 1 μg of RNA was used to obtain complementary DNA (cDNA) by reverse transcription using the QuantiTect Reverse Transcription Kit (cat no. 205311; Qiagen). The samples were treated with RNase-Free DNase and reverse-transcribed according to the manufacturer’s instructions. Expression of genes coding for HSP70, HSP90, HSF1, HSF3 and MnSOD in the heart, liver and breast muscles was quantified by real-time PCR using Power SYBR Green Master Mix (cat no. 4367659; Life Technologies) with an ABI 7500 Real-Time PCR Detection System (Life Technologies). The target genes and primers (Invitrogen) of each target gene are given in Table 3. All samples were arranged in the same plate to ensure the analysis was run under the same reaction conditions. The protocol of PCR was as follows: denaturation at 95°C for 2 min followed by forty cycles at 95°C for 60 s, 60°C for 30 s and 72°C for 30 s. For each reaction plate of the same target gene, the cDNA pool of all samples was used as the reference control sample. The geometric mean of internal references, β-actin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), was used to normalise the expression of the targeted genes(
Reference Xie, Tang and Lu
5
). The
![]() $$2^{{{\minus}\Delta \Delta C_{t} }} $$
was used to calculate the mRNA level of each target gene, where the average mean of C
t
from the NT-CON group was used as the calibrator.
$$2^{{{\minus}\Delta \Delta C_{t} }} $$
was used to calculate the mRNA level of each target gene, where the average mean of C
t
from the NT-CON group was used as the calibrator.
Table 3 Primer sequences for real-time PCR amplifications
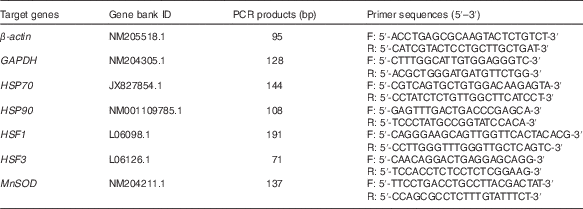
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HSP70 and HSP90, heat shock proteins 70 and 90; HSF1 and HSF3, heat shock factors 1 and 3; MnSOD, manganese superoxide dismutase; F, forward; R, reverse.
Tissue preparations and Western blotting
Frozen tissue samples (40 mg) were minced and homogenised in 0·7 ml of ice-cold RIPA lysis buffer (cat no. P0013B; Beyotime Institute of Biotechnology) supplemented with protease inhibitor (cat no. 4693159001; Roche). The homogenates were centrifuged at 12 000 g for 5 min at 4°C and then the cytosolic fractions were collected. Total protein concentration was determined using a BCA Protein Assay kit. Protein extract (30 µg) from each sample was then loaded onto 10 % SDS–PAGE gels and the separated proteins were transferred onto nitrocellulose membranes (cat no. IPVH00010; Merck-Millipore). After the transfer, membranes were blocked for 1 h at room temperature in blocking buffer with 5 % skimmed milk and then incubated overnight at 4°C with the following primary antibodies purchased from Abcam: HSP70 (1:2000, ab69412), HSP90 (1:2000, ab64182), MnSOD (1:3000, ab13533) and GAPDH (1:5000, ab22555). After four washes for 10 min each with Tris-buffered saline containing Tween, membranes were incubated with goat anti-rabbit horseradish peroxidase-conjugated secondary antibodies (1:5000, cat no. CW0103A; ComWin Biotech) for 1 h at room temperature. After four washes for 10 min each, bands were visualised by enhanced chemiluminescence using a SuperSignal West Pico Trial Kit (cat no. 34077; Pierce). The signals were recorded with an Image Quant LAS 4000 scanner (GE Healthcare Life Sciences) and analysed with TotalLab Quant software (TotalLab). GAPDH protein was used to normalise the expressions of the targeted proteins, and the average expression of proteins in the NT-CON was used as a calibrator.
Statistical analyses
All data were analysed by two-way ANOVA using the general linear model procedure of the SAS 9.2 (SAS Institute Inc.), and the model included the main effects of temperature, dietary Mn and their interactions. Each replicate served as the experimental unit. Differences among means were tested by the least significant difference (LSD) method, and statistical significance was set at P≤0·05 with a trend at 0·05<P≤0·10.
Results
Manganese contents of manganese sources, and amino acid contents and Qf value of manganese proteinate
The Mn contents of Mn sulphate (MnSO4·H2O) and Mn proteinate were 32·2 and 10·2 % on an as-fed basis, respectively. The Mn proteinate contained amino acids (% of the product) with aspartic acid (6·77), serine (2·05), glutamic acid (4·49), threonine (0·57), glycine (12·36), arginine (1·56), alanine (3·76), proline (7·80), valine (1·04), phenylalanine (1·28), isoleucine (0·46), leucine (1·23), lysine (6·75) and methionine (0·34) on an as-fed basis. The chelation strength (Q f value) of the Mn proteinate was analysed to be 61·9, which was categorised as a moderate chelation strength based on the classification of Li et al. ( Reference Li, Luo and Liu 13 ).
Manganese contents in tissues
No interactions (P>0·10) between temperature and dietary Mn were observed in Mn content in the liver, heart, breast muscle and bone (Table 4). Temperature affected (P<0·003) Mn content in the liver and heart, but not (P>0·19) in breast muscle and bone (Table 4). The Mn content in the measured tissues was affected (P<0·0001) by dietary Mn. Compared with NT, HT decreased (P<0·003) the Mn content in the liver and heart. Broiler breeders fed either an iMn or an oMn diet had 1·5–2·0-fold higher (P<0·0001) Mn content in the liver, heart, breast muscle and bone than did those fed the CON diet, with no differences (P>0·48) between the two Mn sources except for bone Mn content. Broiler breeders in the oMn group had higher (P<0·03) bone Mn content than did those in the iMn group.
Table 4 Effects of environmental temperature and dietary manganese on tissue manganese content (μg/g) of broiler breedersFootnote * (Mean values with their standard errors)
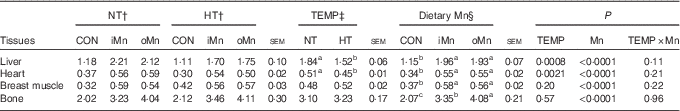
NT, normal temperature; HT, high temperature; TEMP, environmental temperature; CON, Mn-unsupplemented basal diet; iMn, basal diet+120 mg Mn/kg as MnSO4·H2O; oMn, basal diet+120 mg Mn/kg as the Mn proteinate with a moderate chelation strength of 61·9 Q f (10·2 % Mn).
a,b,c Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* The Mn contents in liver, heart and breast muscle were on a fresh basis, and the bone Mn content was on an ash basis.
†‡§ The values represent the means of 6, 18 and 12 replicate cages (n 6, 18 and 12), respectively.
Tissue malondialdehyde levels and superoxide dismutase activities
MDA levels and SOD activities in the liver, heart and breast muscle of broiler breeders are listed in Table 5. No interactions (P>0·20) between temperature and dietary Mn were observed in any of the above-mentioned indices. Both temperature and dietary Mn had no effect (P>0·14) on MDA levels in the liver and heart. The breast muscle MDA level tended (P=0·06) to be influenced by temperature, but not by dietary Mn (P>0·28). Dietary Mn influenced the activities of MnSOD (P<0·0001) and CuZnSOD (P<0·01) in the heart, but not (P>0·29) in the liver and breast muscle. Temperature affected (P=0·02) MnSOD activity in breast muscle and tended (P=0·07) to influence the CuZnSOD activity in the liver and heart. The temperature did not affect (P>0·26) the activities of MnSOD in the liver and the heart, nor that of CuZnSOD in the breast muscle. Compared with NT, HT increased (P<0·05) MnSOD activity and tended (P=0·06) to increase the MDA level in breast muscle, but tended (P=0·07) to decrease the CuZnSOD activities in the liver and the heart. Broiler breeders fed diets supplemented with Mn of either source had 9·4 and 12·0 % higher (P<0·001) activities of MnSOD and CuZnSOD in the heart, respectively, compared with those in CON, with no differences (P>0·78) between the two Mn sources.
Table 5 Effects of environmental temperature and dietary manganese on tissue superoxide dismutase activities and malondialdehyde (MDA) content of broiler breeders (Mean values with their standard errors)
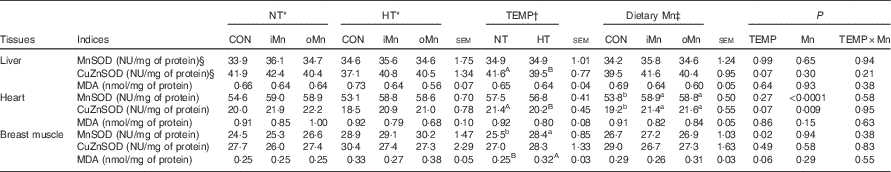
NT, normal temperature; HT, high temperature; TEMP, environmental temperature; CON, Mn-unsupplemented basal diet; iMn, basal diet+120 mg Mn/kg as MnSO4·H2O; oMn, basal diet+120 mg Mn/kg as the Mn proteinate with a moderate chelation strength of 61·9 Q f (10·2 % Mn); CuZnSOD, copper zinc superoxide dismutase; MnSOD, manganese superoxide dismutase.
a,b Mean values within a row with unlike superscript lowercase letters were significantly different (P<0·05); A,B mean values within a row with unlike superscript uppercase letters were significantly different for a trend (0·05<P<0·10).
*†‡ The values represented the means of 6, 18 and 12 replicate cages (n 6, 18 and 12), respectively.
§ One nitrite unit (NU) was defined as the amount of enzyme needed to obtain 50 % inhibition of nitrite formation.
mRNA expression levels of heat shock proteins, heat shock factors and manganese superoxide dismutase in tissues
The mRNA expressions of HSP70, HSP90, HSF1, HSF3 and MnSOD genes in the liver, heart and breast muscle of broiler breeders are listed in Tables 6–8. No interactions (P>0·15) between temperature and dietary Mn were observed in any of the above indices. Neither temperature nor dietary Mn influenced (P>0·57) HSP90 mRNA expression levels in the liver and heart (Tables 6 and 7). The mRNA expression levels of HSP90 (P<0·007) in breast muscle, HSP70 (P<0·001) in the liver and heart, and HSF1 (P<0·006), HSF3 (P<0·04) and MnSOD (P<0·04) in the liver, heart and breast muscle were affected by temperature, but not (P>0·16) by dietary Mn (Tables 6–8). Both temperature and dietary Mn influenced (P<0·05) HSP70 mRNA expression levels in breast muscle (Table 8). Compared with NT, HT increased the mRNA expression levels of HSP70, HSF1, HSF3 and MnSOD in the liver, heart and breast muscle (P<0·05) and that of HSP90 only in breast muscle (P<0·005). Broiler breeders fed either iMn (P<0·05) or oMn (P<0·01) had lower breast muscle HSP70 mRNA expressions than did those in CON, with no difference (P>0·62) between the two Mn sources.
Table 6 Effects of environmental temperature and dietary manganese on mRNA expression of heat shock proteins (HSP), heat shock factors (HSF) and manganese superoxide dismutase (MnSOD) in the liver of broiler breedersFootnote * (Mean values with their standard errors)

NT, normal temperature; HT, high temperature; TEMP, environmental temperature; CON, Mn-unsupplemented basal diet; iMn, basal diet+120 mg Mn/kg as MnSO4·H2O; oMn, basal diet+120 mg Mn/kg as the Mn proteinate with a moderate chelation strength of 61·9 Q f (10·2 % Mn).
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* The geometric mean of internal references, β-actin and glyceraldehyde-3-phosphate dehydrogenase, was used to normalise the expression of targets genes, and the average expression of genes mRNA in the NT-CON was used as a calibrator.
†‡§ The values represent the means of 6, 18 and 12 replicate cages (n 6, 18 and 12), respectively.
Table 7 Effects of environmental temperature and dietary manganese on mRNA expression of heat shock proteins (HSP), heat shock factors (HSF) and manganese superoxide dismutase (MnSOD) in the heart of broiler breedersFootnote * (Mean values with their standard errors)
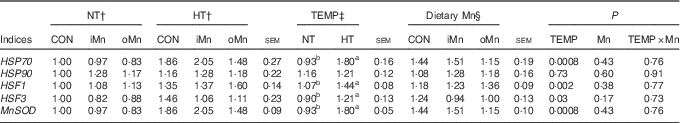
NT, normal temperature; HT, high temperature; TEMP, environmental temperature; CON, Mn-unsupplemented basal diet; iMn, basal diet+120 mg Mn/kg as MnSO4·H2O; oMn, basal diet+120 mg Mn/kg as the Mn proteinate with a moderate chelation strength of 61·9 Q f (10·2 % Mn).
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* The geometric mean of internal references, β-actin and glyceraldehyde-3-phosphate dehydrogenase, was used to normalise the expression of target genes, and the average expression of each gene’s mRNA in the NT-CON was used as a calibrator.
†‡§ The values represent the means of 6, 18 and 12 replicate cages (n 6, 18 and 12), respectively.
Table 8 Effects of environmental temperature and dietary manganese on mRNA expression of heat shock proteins (HSP), heat shock factors (HSF) and manganese superoxide dismutase (MnSOD) in breast muscle of broiler breedersFootnote * (Mean values with their standard errors)

NT, normal temperature; HT, high temperature; TEMP, environmental temperature; CON, Mn-unsupplemented basal diet; iMn, basal diet+120 mg Mn/kg as MnSO4·H2O; oMn, basal diet+120 mg Mn/kg as the Mn proteinate with a moderate chelation strength of 61·9 Q f (10·2 % Mn).
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* The geometric mean of internal references, β-actin and glyceraldehyde-3-phosphate dehydrogenase, was used to normalise the expression of targets genes, and the average expression of genes mRNA in the NT-CON was used as a calibrator.
†‡§ The values represent the means of 6, 18 and 12 replicate cages (n 6, 18 and 12), respectively.
Protein expression levels of heat shock proteins 70 and 90 and manganese superoxide dismutase in tissues
The results and representative immunoblots of HSP70, HSP90 and MnSOD protein expression in the liver, heart and breast muscle are shown in Table 9 and Fig. 1((a)–(c)), respectively. No interactions (P>0·21) between temperature and dietary Mn were observed in any of the above indices. The protein expression levels of HSP70, HSP90 and MnSOD in the liver and heart, and of HSP90 in breast muscle, were not affected (P>0·36) by dietary Mn. Temperature affected (P<0·004) the protein expression levels of HSP70 in the liver and heart, as well as HSP90 in breast muscle, but not (P>0·12) in the liver and heart. The breast muscle HSP70 and MnSOD protein expression levels were influenced (P<0·02) by both temperature and dietary Mn. Compared with NT, HSP70 protein expression levels in the three tissues increased (P<0·0004) under HT, whereas the protein expression levels of HSP90 and MnSOD increased only in breast muscle (P<0·0004) by 1-fold under HT. Broiler breeders fed either iMn (P<0·05) or oMn (P<0·01) had higher MnSOD but lower HSP70 protein expression levels in breast muscle than did those in CON.
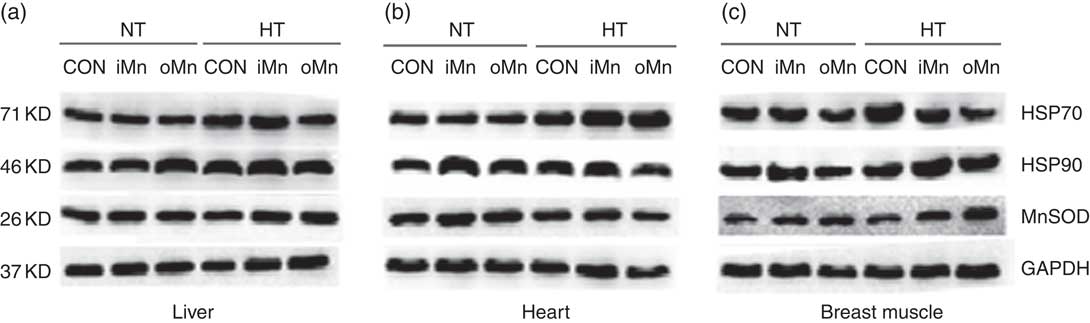
Fig. 1 Representative immunoblots demonstrating heat shock proteins (HSP70, HSP90) and manganese superoxide dismutase (MnSOD) protein expression in the liver, heart and breast muscle of laying broiler breeders subjected to varying temperature and manganese supplementation (a–c). NT, normal temperature; HT, high temperature; CON, manganese-unsupplemented basal diet; iMn, basal diet+120 mg manganese/kg as MnSO4·H2O; oMn, basal diet+120 mg manganese/kg as the manganese proteinate with a moderate chelation strength of 61·9 Q f (10·2 % manganese); GAPDH, glyceraldehydes-3-phosphate dehydrogenase.
Table 9 Effects of environmental temperature and dietary manganese on protein expression of heat shock proteins (HSP70, HSP90) and manganese superoxide dismutase (MnSOD) in tissues of broiler breedersFootnote * (Mean values with their standard errors)

NT, normal temperature; HT, high temperature; TEMP, environmental temperature; CON, Mn-unsupplemented basal diet; iMn, basal diet+120 mg Mn/kg as MnSO4·H2O; oMn, basal diet+120 mg Mn/kg as the Mn proteinate with a moderate chelation strength of 61·9 Q f (10·2 % Mn).
a,b Mean values within a row with unlike superscript letters were significantly different (P<0·05).
* The glyceraldehyde-3-phosphate dehydrogenase protein was used to normalise the expressions of the target proteins, and the average expression level of protein in the NT-CON was used as a calibrator.
†‡§ The values represent the means of 6, 18 and 12 replicate cages (n 6, 18 and 12), respectively.
Discussion
The negative effect of heat stress on mineral balance in broilers has been previously reported( Reference Belay, Wiernusz and Teeter 26 , Reference Sahin, Sahin and Onderci 27 ). In the present study, significantly decreased Mn content in the liver and heart was observed in heat-stressed broiler breeders. The decreased Mn content may have been due to reduced Mn absorption coupled with increased Mn excretion under HT( Reference Smith, Sherman and Miller 28 ). Thus, a higher dietary Mn level may be required for broiler breeders exposed to HT compared with NT. Dietary supplementation of Mn from either Mn source increased tissue Mn content under both NT and HT in this study, which was consistent with previous studies in broilers( Reference Luo, Guo and liu 12 – Reference Li, Lu and Hao 15 ) and laying hens( Reference Lu, Luo and Liu 11 ). Specifically, the organic Mn with moderate chelation strength demonstrated greater bioavailability than did the inorganic Mn in bone. A series of studies also suggest that organic Mn has higher absorption and bioavailability compared with inorganic Mn for broilers( Reference Li, Luo and Liu 13 – Reference Bai, Lu and Wang 16 , Reference Smith, Sherman and Miller 28 , Reference Henry, Ammerman and Littell 29 ) and lambs( Reference Henry, Ammerman and Littell 30 ), and organic Mn sources with moderate chelation strengths exhibit the greatest availability( Reference Li, Luo and Liu 13 – Reference Li, Lu and Hao 15 ).
Heat stress is believed to produce ROS and induce oxidative damage in broilers( Reference Lin, Decuypere and Buyse 31 , Reference Sahin, Onderci and Sahin 32 ) and laying hens( Reference Lin, De Vos and Decuypere 6 ). In the current study, HT showed a clear trend towards elevating breast muscle MDA content, and the increased plasma MDA level was also observed in another study by us( Reference Zhu, Xie and Li 17 ), suggesting that lipid peroxidation occurs under the HT condition. However, at the same time, HT led to an increase in MnSOD activity in breast muscle. Both SOD (MnSOD and CuZnSOD) may allow superoxide radicals to be scavenged and protect cells against toxic oxygen radicals. Therefore, the damage to the breast muscle membrane resulting from free radicals under HT may have induced the corresponding increase in its MnSOD activity because of its function in self-protection during heat stress. The above results suggest that heat stress may disturb the redox balance and induce oxidative damage in the breast muscle of laying broiler breeders.
Evidence suggests that HSP, especially HSP70, are involved in the development of thermotolerance in broilers( Reference Wang and Edens 18 , Reference Soleimani, Zulkifli and Hair-Bejo 19 ) and in human and mouse cells( Reference Li and Mak 33 – Reference Liu, Yuan and Cao 35 ). In the present study, the up-regulations of both HSP70 mRNA and protein in the heart, liver and breast muscle of broiler breeders exposed to HT might favour an anti-heat stress response. Some reports have confirmed that the expressions of HSP are regulated mainly at the level of transcription by HSF( Reference Akerfelt, Morimoto and Sistonen 36 , Reference Wu 37 ). HSF1- and HSF3-mediated mechanisms of cellular adaptation to heat shock have been examined in detail in chicken embryo fibroblasts, and HSF3 is considered an avian-specific factor( Reference Nakai, Kawazoe and Tanabe 38 ). As expected, the synchronised changes in transcriptional activation between HSF1, HSF3 and HSP70 mRNA and protein were observed in the liver, heart and breast muscle under HT. Generally, HSF1, HSF3 can bind the heat shock element in the promoter of HSP70 to up-regulate HSP70 in response to heat stress( Reference Tanabe, Nakai and Kawazoe 39 ). The up-regulation of HSP70 under HT might be dependent upon the presence of ROS and/or oxidative stress induced by heat stress. Previous research indicates that ROS-mediated activation of p38 mitogen-activated protein kinase (p38MAPK) can increase the HSF1 DNA-binding activity to increase HSP70 expression in cells( Reference Banerjee Mustafi, Chakraborty and Dey 40 ). As previously reported, expression of HSF is mainly altered by certain stressors, and exhibits a stress-specific pattern( Reference Mathew, Mathur and Jolly 41 ). This could very well explain why dietary Mn supplementation did not affect the expression of HSF mRNA in the present study.
In addition, the increased expressions of HSP90 mRNA and protein were induced only in breast muscle of birds under chronic heat challenge, but not in the liver and heart, suggesting that the abundance and inducibility of HSP90 might be tissue dependent( Reference Vamvakopoulos 42 ). The muscle was the most sensitive and responsive tissue of heat-stressed broiler breeders based on the expression of HSP90. However, the expressions of HSF1 and 3 mRNA in the liver and heart were also increased under HT. In fact, the HSP90 expressions were regulated not only by HSF( Reference Akerfelt, Morimoto and Sistonen 36 ) but also by other non-HSF transcription factors, such as signal transducer and activator of transcription (STAT) family( Reference Stephanou and Latchman 43 ) and nuclear factor IL-6( Reference Stephanou, Amin and Isenberg 44 ). It is reported that the STAT-3 signalling pathway has inhibitory effects on HSF-mediated activation of HSP90( Reference Stephanou, Isenberg and Akira 45 ). Therefore, it would be possible to have no response of HSP90 expression in the liver and heart under HT. In addition to HSP, antioxidant enzymes are induced by stressors and provide an organism with multiple protective options( Reference Banerjee Mustafi, Chakraborty and Dey 40 , Reference Yamashita, Hoshida and Nishida 46 ). The increases in MnSOD mRNA and protein expressions as well as MnSOD activity were observed in the breast muscle of broiler breeders subjected to HT, suggesting that these up-regulated responses in the breast muscle might be essential for reducing lipid peroxidation damage and protecting mitochondria stability and integrity. The enhancement of MnSOD expression under heat exposure could be caused by the presence of elevated ROS levels via activation of p38MAPK and protein kinase B( Reference Banerjee Mustafi, Chakraborty and Dey 40 ).
Mn is a crucial cofactor of MnSOD, which is the most dominant dismutase involved in scavenging free radicals. Severe Mn deficiency (1 mg/kg) in purified diets decreased MnSOD activities in the heart and other organs (liver, kidney and brain) of rats, mice and chickens( Reference de Rosa, Keen and Leach 47 ). However, previous studies from our laboratory have shown that moderate Mn deficiency (about 16 mg/kg) in a practical maize–soyabean meal diet depressed MnSOD activity only in the heart, and not in the liver and pancreas, and then further led to Mn-responsive abnormalities in the heart mitochondrial ultrastructure, indicating that the heart of broilers is more sensitive to Mn deficiency than other tissues( Reference Luo, Su and Huang 9 , Reference Luo, Su and Huang 10 ). In the present study, we also found that supplemental Mn significantly increased MnSOD activity as well as CuZnSOD activity in the heart regardless of Mn source, indicating that Mn-supplemented broiler breeders might be better prepared to deal with oxidative stress with enhanced antioxidant ability. Similar results have been observed in commercial laying hens( Reference Luo, Guo and liu 12 ) in our previous study under thermoneutrality conditions. Our other studies further demonstrated that heart MnSOD mRNA level in broilers is more sensitive to dietary Mn level than is MnSOD activity( Reference Li, Luo and Liu 13 – Reference Li, Lu and Hao 15 ). However, in this study, no differences in expression of heart MnSOD mRNA or protein were observed in broiler breeders receiving dietary Mn treatments. This discrepancy between broiler chicks and laying broiler breeders might mainly be due to differences in metabolic and physiological statuses at different developmental stages. It appears that Mn accumulation in broiler breeders in the pre-period was sufficient to meet their Mn requirement for heart MnSOD mRNA and protein expressions. Breast muscle MnSOD protein level was increased by dietary Mn supplementation regardless of Mn source, suggesting that the protection of breast muscle against ROS might depend, in part, upon an endogenous pool of antioxidant enzymes stored as proteins during heat stress. These findings also suggest that MnSOD expression in breast muscle and heart may be up-regulated by dietary Mn at different levels of translation and post-translational modification, respectively.
Heat shock, as a promoter of oxidative stress, creates a redox imbalance by increasing the generation of ROS( Reference Lin, Decuypere and Buyse 31 ). Subsequent cellular damage caused by accumulation of ROS has been suggested as a key factor for activation of HSP genes( Reference Mahmoud and Edens 7 , Reference Sahin and Kucuk 8 ). When cells are subjected to heat shock with an increase in lipid peroxidation, HSP70 accumulates and might serve as a tissue biomarker for potential stress damage( Reference Tedeschi, Kennington and Berry 22 ). Thus, constitutive and inducible HSP70 expression levels might be regarded as a response to damage resulting from a strong stress to the organism( Reference Flanagan, Moseley and Buettner 20 , Reference Burdon, Gill and Rice-Evans 21 ). In addition, the increased activities of SOD and catalase likely scavenge free radicals that inhibit the expression of HSP proteins and improve cell survival( Reference Omar and Pappolla 23 ). In the present study, compared with birds fed the control diet, broiler breeders fed diets supplemented with Mn from either source, had lower expression levels of HSP70 mRNA and protein in breast muscle regardless of environmental temperatures. It appears that dietary Mn supplementation may inhibit HSP70 expressions by enhancing the activities of SOD and subsequent removal of ROS. Similarly, it has been reported that reduced induction of HSP70 protein occurs in Se-fed chickens because of the higher glutathione peroxidase activity in the liver( Reference Mahmoud and Edens 7 ).
In conclusion, high environmental temperature decreases Mn retention and increases HSP70, HSF1 and HSF3 expressions in tissues of laying broiler breeders. Breast muscle is more susceptible to heat stress due to the induction of lipid peroxidation, as well as up-regulation of HSP90 expression. Dietary supplementation with Mn from either source may thus enhance the heart antioxidant capacity and inhibit the expressions of HSP70 in muscle. Organic Mn proteinate with a moderate chelation strength might be more available to bone than inorganic Mn sulphate in laying broiler breeders regardless of environmental temperatures.
Acknowledgements
The present study was supported by the Key International Cooperation Program of the National Natural Science Foundation of China (project no. 31110103916), the Agricultural Science and Technology Innovation Program (ASTIP-IAS08) and the China Agriculture Research System (project no. CARS-42).
X.-G. L. was responsible for all issues related to this paper. Y.-W. Z. and L. L. were responsible for the planning of the study, sample analyses, collections and statistical analyses of all data, as well as manuscript writing. W.-X. L. and L.-Y. Z. were involved in the sample collections, analysis and statistical analyses. X. L., H.-C. L., J. O. and C. J. were involved in the experimental design and data interpretations. All authors contributed to the writing of the manuscript and agreed with the final content.
The authors declare that there are no conflicts of interest.












