Introduction
The importance of the gut microbiota to human physiological and immunological development, particularly during the early years of life, has led to efforts to characterize the composition and dynamics of the gut microbiota of humans from birth through infancy and into childhood (Arrieta et al. Reference Arrieta, Stiemsma, Amenyogbe, Brown and Finlay2014; Backhed et al. Reference Backhed, Roswall, Peng, Feng, Jia, Kovatcheva-Datchary, Li, Xia, Xie, Zhong, Khan, Zhang, Li, Xiao, Al-Aama, Zhang, Lee, Kotowska, Colding, Tremaroli, Yin, Bergman, Xu, Madsen, Kristiansen, Dahlgren and Wang2015a; Rodriguez et al. Reference Rodriguez, Murphy, Stanton, Ross, Kober, Juge, Avershina, Rudi, Narbad, Jenmalm, Marchesi and Collado2015). This knowledge is required to identify different factors that influence gut microbiota composition and functionality through time and how changes in the gut microbiota impact on host health at various key stages of life (Subramanian et al. Reference Subramanian, Huq, Yatsunenko, Haque, Mahfuz, Alam, Benezra, DeStefano, Meier, Muegge, Barratt, VanArendonk, Zhang, Province, Petri, Ahmed and Gordon2014; Frese and Mills, Reference Frese and Mills2015).
Upon birth, the human gut is rapidly colonized by a diversity of microbes. Numerous studies have shown that the diversity of the neonate gut microbiota is affected by a range of factors including mode of delivery (vaginal or Caesarean section), whether the infant is breast or formula fed, and antibiotic use (Dominguez-Bello et al. Reference Dominguez-Bello, Costello, Contreras, Magris, Hidalgo, Fierer and Knight2010; Bokulich et al. Reference Bokulich, Chung, Battaglia, Henderson, Jay, Li, Lieber, Wu, Perez-Perez, Chen, Schweizer, Zheng, Contreras, Dominguez-Bello and Blaser2016; Hill et al. Reference Hill, Lynch, Murphy, Ulaszewska, Jeffery, O'Shea, Watkins, Dempsey, Mattivi, Tuohy, Ross, Ryan, Toole and Stanton2017). Following birth, the bacterial population of the gut undergoes gradual succession and matures with key signature species and ecological networks observed at particular time-points (Backhed et al. Reference Backhed, Roswall, Peng, Feng, Jia, Kovatcheva-Datchary, Li, Xia, Xie, Zhong, Khan, Zhang, Li, Xiao, Al-Aama, Zhang, Lee, Kotowska, Colding, Tremaroli, Yin, Bergman, Xu, Madsen, Kristiansen, Dahlgren and Wang2015a). Between ~2 and 5 years of age, the bacterial population of the human gut begins to resemble that of an adult with respect to both diversity and richness (Koenig et al. Reference Koenig, Spor, Scalfone, Fricker, Stombaugh, Knight, Angenent and Ley2011; Yatsunenko et al. Reference Yatsunenko, Rey, Manary, Trehan, Dominguez-Bello, Contreras, Magris, Hidalgo, Baldassano, Anokhin, Heath, Warner, Reeder, Kuczynski, Caporaso, Lozupone, Lauber, Clemente, Knights, Knight and Gordon2012; Bokulich et al. Reference Bokulich, Chung, Battaglia, Henderson, Jay, Li, Lieber, Wu, Perez-Perez, Chen, Schweizer, Zheng, Contreras, Dominguez-Bello and Blaser2016).
In addition to the bacterial fraction of the infant gut microbiota, researchers are now investigating patterns of colonization and diversity for other members of the gut microbiota, e.g. archaea, viruses and fungi, in order to determine the roles that such microorganisms play as drivers and/or moderators of intestinal health during early life (Lim et al. Reference Lim, Zhou, Zhao, Bauer, Droit, Ndao, Warner, Tarr, Wang and Holtz2015; Ward et al. Reference Ward, Knights and Gale2017). However, little is known about the prevalence and diversity of other potentially important microbes, such as protists, and what role, if any, they may play in infant health and disease. This dearth of knowledge extends to the microbial eukaryote Blastocystis, which is a common component of the human adult gut and is estimated to colonize over 1 billion people worldwide (Scanlan and Stensvold, Reference Scanlan and Stensvold2013).
Blastocystis is a member of the Stramenopiles (or Heterokonta) branch of Eukarya (Silberman et al. Reference Silberman, Sogin, Leipe and Clark1996). This diverse assemblage of organisms encompasses both uni- and multi-cellular organisms such as diatoms, algae and oomycetes (Patterson, Reference Patterson1999). Currently, seventeen different Blastocystis subtypes (STs) or species have been described (Alfellani et al. Reference Alfellani, Taner-Mulla, Jacob, Imeede, Yoshikawa, Stensvold and Clark2013a) and, of these, nine have been recovered from human samples. Although a major focus in Blastocystis research is understanding the potential role of this microorganism in infection and intestinal disease, recent data have shown that it is a common component of the healthy adult gut microbiota (Scanlan et al. Reference Scanlan, Stensvold, Rajilic-Stojanovic, Heilig, De Vos, O'Toole and Cotter2014; Beghini et al. Reference Beghini, Pasolli, Truong, Putignani, Caccio and Segata2017). Given that asymptomatic carriage is common, this suggests that Blastocystis’ potential for pathogenicity is limited to certain genotypes and/or specific host–genotype and host–genotype–environment interactions (Scanlan and Stensvold, Reference Scanlan and Stensvold2013).
Blastocystis prevalence rates vary significantly between different geographical regions (Alfellani et al. Reference Alfellani, Stensvold, Vidal-Lapiedra, Onuoha, Fagbenro-Beyioku and Clark2013), with the highest prevalence in a healthy European cohort published to date reported for a subset of the adult Irish population (Scanlan et al. Reference Scanlan, Stensvold, Rajilic-Stojanovic, Heilig, De Vos, O'Toole and Cotter2014). Fifty-five per cent of adults in this study were positive for Blastocystis and, although within-host diversity was low, with the most individuals host to a single Blastocystis ST, 22% were host to two or more different STs (Scanlan et al. Reference Scanlan, Stensvold and Cotter2015). Although the factors responsible for the high prevalence rates of Blastocystis observed in the Irish population compared to other European countries are, as yet, unknown, variation in Blastocystis prevalence has been linked to a number of factors including levels of sanitation and exposure to contaminated water (Leelayoova et al. Reference Leelayoova, Siripattanapipong, Thathaisong, Naaglor, Taamasri, Piyaraj and Mungthin2008; Speich et al. Reference Speich, Croll, Furst, Utzinger and Keiser2016). Following on from this study we wished to provide a more complete picture of the epidemiology of Blastocystis in the Irish population and also shed some light on how and when humans become colonized with Blastocystis. Accordingly, we investigated the prevalence and genetic diversity of Blastocystis in a cohort of healthy infants from a subset of the Irish population that had been sampled at a number of time-points up to 24 months of age.
Material and methods
Overview of study and study participants
The aim of our study was to provide longitudinal data on the prevalence and diversity of the intestinal protist Blastocystis in a healthy infant cohort from a Westernized European country (Ireland). The samples analysed were part of the INFANTMET study cohort (Hill et al. Reference Hill, Lynch, Murphy, Ulaszewska, Jeffery, O'Shea, Watkins, Dempsey, Mattivi, Tuohy, Ross, Ryan, Toole and Stanton2017) for which ethical approval was provided by the Cork University Hospital Research Ethics Committee (ethical approval reference: ECM (w) 07/02/2012). Fecal DNA samples were obtained from infants (n = 59) that were born either at full term (n = 55) or preterm (n = 4) and either via spontaneous vaginal delivery (n = 30) or Caesarean section (n = 29); see Table 1 for more details. Samples taken from week 1, week 8, 12 months and 24 months were analysed for all infants. Samples from 1 additional time-point (week 4) were analysed for three individuals that were positive for Blastocystis.
Table 1. Overview of study participants and results
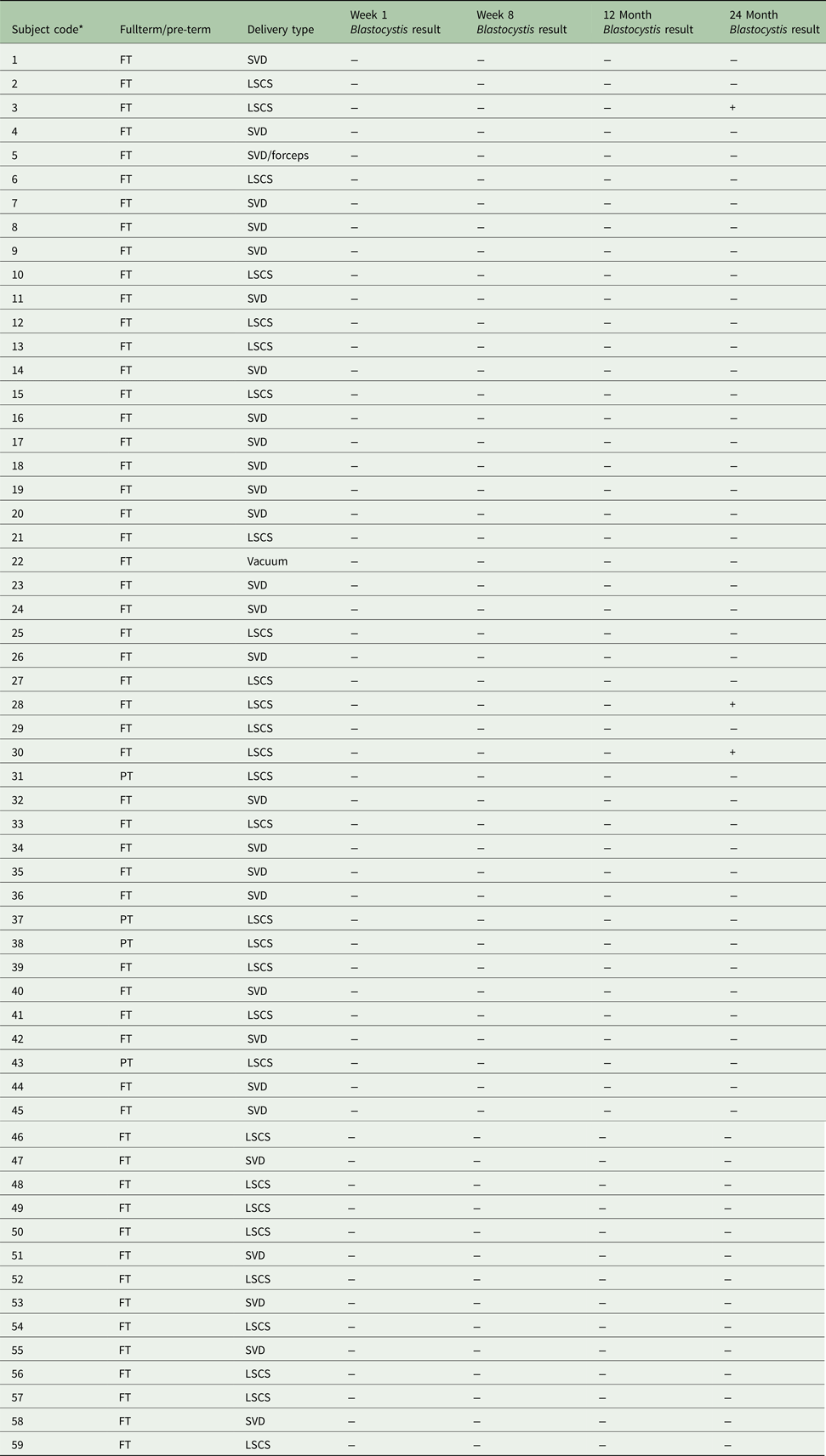
* Positive Blastocystis samples are highlighted in bold.
FT, fullterm; PT, pre-term; SVD, spontaneous vaginal delivery; LSCS, lower segment Caesarean section.
Blastocystis PCR and sequence analysis
Genomic DNA was extracted from fecal samples as outlined previously (Hill et al. Reference Hill, Lynch, Murphy, Ulaszewska, Jeffery, O'Shea, Watkins, Dempsey, Mattivi, Tuohy, Ross, Ryan, Toole and Stanton2017). The primer set RD5 and BhRDr were used to amplify and sequence ~600 bp of the SSU rRNA gene for all samples according to a standard protocol (Scicluna et al. Reference Scicluna, Tawari and Clark2006; Scanlan et al. Reference Scanlan, Stensvold, Rajilic-Stojanovic, Heilig, De Vos, O'Toole and Cotter2014). Positive PCR products were cleaned using the Qiagen QIAquick PCR clean up kit and sequenced (Source Bioscience, Ireland). Sequence data were trimmed and submitted to the online site http://pubmlst.org/Blastocystis/ to assign Blastocystis subtype and allele ID. Sequences were then aligned and analysed in MEGA4 (Tamura et al. Reference Tamura, Dudley, Nei and Kumar2007). Within-host Blastocystis diversity (so-called mixed infections) was also investigated using a recently developed ST-specific primer set as described elsewhere (Scanlan et al. Reference Scanlan, Stensvold and Cotter2015).
Results and discussion
Blastocystis was detected in three of the 59 or 5% of the infant population tested, with all positives being 24-month samples. No positive PCR signals were detected for any of the samples taken from any infants at week 1, week 8 and 12 months including all samples from the three infants that were positive for Blastocystis at 24 months. Unfortunately, data relating to Blastocystis colonization of the mothers of the infants sampled here are not available. However, based on our previously published prevalence data (Scanlan et al. Reference Scanlan, Stensvold, Rajilic-Stojanovic, Heilig, De Vos, O'Toole and Cotter2014, Reference Scanlan, Stensvold and Cotter2015) from a contemporaneous adult cohort living in the same region of Ireland, it is conceivable that >50% of them were positive. Based on this assumption, the absence of Blastocystis in all infants at the early time-points) indicates that Blastocystis was not acquired by any of these infants at birth and, in those individuals that were positive for Blastocystis at 24 months, it is likely that Blastocystis was acquired via horizontal transmission at some stage between years 1 and 2.
Each of the three positive PCR products could be assigned to one of three STs (ST2_allele_9, ST3_allele_31 and ST4_allele_42, respectively) using the online site http://pubmlst.org/Blastocystis/ (Jolley and Maiden, Reference Jolley and Maiden2010; Stensvold et al. Reference Stensvold, Alfellani and Clark2012). There was no evidence for multiple STs present within an individual host. Even though the number of positive hosts is low, the diversity of STs detected in this infant population is typical of those STs present in the healthy adult population (Scanlan et al. Reference Scanlan, Stensvold, Rajilic-Stojanovic, Heilig, De Vos, O'Toole and Cotter2014).
Collectively, our data show that the prevalence of Blastocystis in this infant population is relatively low compared with an earlier study of the adult Irish population and that Blastocystis is likely to be acquired via horizontal rather than vertical transmission. Overall, these results are consistent with studies of Blastocystis prevalence rates in adults and infants in India. The first of these studies surveyed microbial eukaryotic diversity in mothers and their infants (n = 4) and found that whilst Blastocystis was detected by PCR and sequencing of DNAs pooled from the mother's samples, no Blastocystis signal was detected in the infant dataset (Pandey et al. Reference Pandey, Siddharth, Verma, Bavdekar, Patole and Shouche2012). A follow-up study reported a similar trend with Blastocystis prevalent in the adult population (n = 100, prevalence = 27%), and absent in the infant population, i.e. none of the 120 samples that had been obtained from thirty infants at various time-points between 7 days and 12 months old gave a positive result (Pandey et al. Reference Pandey, Verma, Marathe, Shetty, Bavdekar, Patole, Stensvold and Shouche2015). Similarly, a study of Blastocystis prevalence rates in families living in the US state of Colorado (Scanlan et al. Reference Scanlan, Knight, Song, Ackermann and Cotter2016) found that though the overall prevalence rates for Blastocystis was low in this dataset, only one of 19 infants (5%) were positive for Blastocystis. This figure was lower than the adult population with nine out of 101 adults Blastocystis positive (9%). Interestingly, infants in the US dataset were aged between 0.5 months and 2 years and the positive sample was obtained from a 24-month old.
One of the possible explanations for the difference in Blastocystis prevalence rates between adult and infant populations may relate to differences in the diversity and composition of the gut bacteria in adults compared with children (Yatsunenko et al. Reference Yatsunenko, Rey, Manary, Trehan, Dominguez-Bello, Contreras, Magris, Hidalgo, Baldassano, Anokhin, Heath, Warner, Reeder, Kuczynski, Caporaso, Lozupone, Lauber, Clemente, Knights, Knight and Gordon2012). Recent data have shown that the presence of Blastocystis in the adult gut microbiota is correlated with increased bacterial diversity and the presence of specific bacterial species (Andersen et al. Reference Andersen, Bonde, Nielsen and Stensvold2015; Audebert et al. Reference Audebert, Even, Cian, Loywick, Merlin, Viscogliosi and Chabe2016). Given that the infant gut is much less diverse and differs in composition to the adult gut, it is possible that the conditions for successful colonization of Blastocystis (upon exposure) are only present once the infant's gut has matured and reached a more diverse community that develops as the child ages. To test this hypothesis a comparative analysis of the microbiota of large numbers of positive and negative Blastocystis samples is required. Unfortunately, this type of analysis is not possible here given the imbalance (very low numbers) of positives relative to negatives in our sample-set. Nonetheless, this proposed scenario is analogous to a recent observation that hydrogen-consuming microbes such as the Desulfovibrio spp. and Methanobrevibacter smithii are abundant in mothers yet virtually absent in their infants (n = 98) (with the exception of two 12-month infants that were colonized by M. smithii) (Backhed et al. Reference Backhed, Roswall, Peng, Feng, Jia, Kovatcheva-Datchary, Li, Xia, Xie, Zhong, Khan, Zhang, Li, Xiao, Al-Aama, Zhang, Lee, Kotowska, Colding, Tremaroli, Yin, Bergman, Xu, Madsen, Kristiansen, Dahlgren and Wang2015b). Here, the authors suggested that the presence of these microbes in the adult gut and their absence in the infant's gut was possibly due to increased fermentative capacity observed in the adult microbiota that creates a niche for microbes that can dispose of hydrogen as methane or other by-products.
Whilst emerging data highlight potential links between Blastocystis and other members of the gut microbiota as potential determinants of successful Blastocystis colonization, it is clearly necessary to consider exposure rates to this microorganism as another key factor that may explain differences in Blastocystis prevalence rates, particularly between different geographical regions. For example, a recent study of children in Nigeria showed that the proportion of 24-month-old infants that were positive for Blastocystis (n = 7, 40% prevalence) was much higher than the prevalence rates reported here and in the other referenced studies (Pandey et al. Reference Pandey, Verma, Marathe, Shetty, Bavdekar, Patole, Stensvold and Shouche2015; Scanlan et al. Reference Scanlan, Knight, Song, Ackermann and Cotter2016). Although the number of infants sampled in the Nigerian study is low, these data highlight the importance of exposure which is likely to vary considerably between different geographical regions due to living and sanitation conditions, access to clean water and exposure to animals. Accordingly, we can expect to see variation between datasets based on geography for both infant and adult populations. Nonetheless, even if exposure rates can explain some of the variation in prevalence rates, age appears to be emerging as an important factor given that this longitudinal study of Nigerian infants and children also showed that Blastocystis prevalence rates increased significantly with increasing age; children aged four and over (n = 192) had prevalence rates of >80% (Poulsen et al. Reference Poulsen, Efunshile, Nelson and Stensvold2016).
Conclusions
The continued provision of prevalence data on Blastocystis is contributing to our greater understanding of the ecology and epidemiology of this gut microbe. The almost complete absence of Blastocystis in healthy infant groups relative to its higher prevalence in adult populations reported here and elsewhere indicates that Blastocystis is not adapted to the naïve infant gut. Therefore, the successful colonization of Blastocystis in humans may require additional factors relating to the specific composition and diversity (maturity) of the gut microbiota.
Acknowledgements
We are grateful to Professor Paul O' Toole for providing access to samples for this study. We would also like to extend our thanks to all the families who agreed to take part in the INFANTMET study
Financial support
This research was funded by an Intra-European Marie Curie Research Fellowship award to Pauline Scanlan.



