Introduction
The aqueous geochemistry of subglacial environments has been studied since the late 1970s, often in the context of tracing subglacial hydrology through the chemical outputs of proglacial streams (e.g. Collins, Reference Collins1977; Raiswell, Reference Raiswell1984). The first several decades of work focuses primarily on alpine systems, with synthesis work finding that glaciers have significantly higher dissolved loads and distinct chemistry compared to riverine systems of equivalent discharge (Anderson and others, Reference Anderson, Drever and Humphrey1997). The high pressures and transient freeze–thaw effects present in the subglacial ice–rock interface are highly effective at crushing and grinding earth materials, producing abundant fresh minerals with high specific surface area and exposing reactive trace minerals in many subglacial environments. It is these fresh mineral surfaces, whose fractured edges have high cation exchange capacity, and the relative abundance of trace minerals that drive both the higher dissolved loads of alpine glacier systems and their distinct chemistry (Anderson, Reference Anderson2005). In addition to the carbonic acid from the dissolution of carbon dioxide in water (carbonation), oxygen-rich ice and glacial meltwater are important in that they can create a highly oxidizing otherwise closed system in the subglacial environment (Tranter and others, Reference Tranter2002). Such oxidization can form sulfuric acid from sulfides and carbonic acid from organic matter (Wadham and others, Reference Wadham2010), which in turn drive chemical weathering of the subglacial substrate. Microbial communities also play an important role in driving chemical reactions under ice (e.g. Sharp and others, Reference Sharp1999; Christner and others, Reference Christner2014; Dubnick and others, Reference Dubnick2017; Lamarche-Gagnon and others, Reference Lamarche-Gagnon2020).
In recent decades, exploration has focused more strongly on the world's large ice sheets of Greenland and Antarctica. These continental ice masses differ from mountain glaciers first in scale. In much of southern and western Greenland, there is an order of 50 km distance between where surface meltwater reaches the bed through moulins and crevasses and where waters emerge in the proglacial environment (Chandler and others, Reference Chandler2021). Combined with low ice surface gradients that allow for distributed flow (Hoffman and others, Reference Hoffman2016), these distances cause substantially longer flow pathways under the Greenland Ice Sheet than most alpine glacial flow systems. Interior Antarctica represents a setting where surface meltwater does not reach the glacier bed and subglacial meltwater is only generated from geothermal and strain heating (Bell and others, Reference Bell, Banwell, Trusel and Kingslake2018). Consequently, subglacial lakes and connecting porous media hold most of Antarctica's liquid water (Wright and Siegert, Reference Wright and Siegert2012; Livingstone and others, Reference Livingstone2022), with the potential for residence times as long as >10 ka (Bell and others, Reference Bell2002). These are vastly longer than the residence times of 1–100 h in alpine systems (e.g. Willis and others, Reference Willis, Sharp and Richards1990; Bingham and others, Reference Bingham, Nienow, Sharp and Boon2005; Miles and others, Reference Miles2019) and of weeks in portions of the Greenland Ice Sheet controlled by the seasonal input of surface melt (Meierbachtol and others, Reference Meierbachtol, Harper, Humphrey and Wright2016).
The differences between these environments make for three fundamentally different systems of subglacial hydrology: (1) seasonal melt dominated systems with short water residence times (i.e. alpine glaciers); (2) seasonal melt dominated systems with long water residence times (i.e. the Greenland Ice Sheet) and (3) systems without seasonal melt and therefore the longest water residence times (i.e. interior Antarctica). Large and geographically diverse datasets have now been collected at all three system types. This allows for comparison between these three types and analysis of the control of glacial hydrology on subglacial geochemistry.
Synthesis
To synthesize data collected from the chemical outputs of these three glacial-hydrologic systems, we have included a large selection of sample averages from alpine glaciers (Raiswell and Thomas, Reference Raiswell and Thomas1984; Thomas and Raiswell, Reference Thomas and Raiswell1984; Drever and Hurcomb, Reference Drever and Hurcomb1986; Fairchild and others, Reference Fairchild, Bradby, Sharp and Tison1994; Singh and others, Reference Singh, Pandey and Panda1998, Reference Singh2012; Wadham and others, Reference Wadham, Hodson, Tranter and Dowdeswell1998, Reference Wadham, Tranter and Dowdeswell2000; Hodson and others, Reference Hodson, Tranter and Vatne2000; Pandey and others, Reference Pandey, Singh and Hasnain2001; Singh and Hasnain, Reference Singh and Hasnain2002; Hagedorn and Hasholt, Reference Hagedorn and Hasholt2004; Hosein and others, Reference Hosein, Arn, Steinmann, Adatte and Föllmi2004; Yde and others, Reference Yde, Knudsen and Nielsen2005, Reference Yde, Riger-Kusk, Christiansen, Knudsen and Humlum2008, Reference Yde2012; Brown and others, Reference Brown2006; Wynn and others, Reference Wynn, Hodson and Heaton2006; Hindshaw and others, Reference Hindshaw2011; Feng and others, Reference Feng, Li, Jin, Dong and Wang2012; Zeng and others, Reference Zeng, Gremaud, Zeng, Liu and Goldscheider2012; Stachnik and others, Reference Stachnik, Yde, Kondracka, Ignatiuk and Grzesik2016; Graly and others, Reference Graly, Drever and Humphrey2017b, Reference Graly, Humphrey and Licht2018), the Greenland Ice Sheet (Ryu and Jacobson, Reference Ryu and Jacobson2012; Bhatia and others, Reference Bhatia2013; Graly and others, Reference Graly, Humphrey, Landowski and Harper2014, Reference Graly, Harrington and Humphrey2017a; Yde and others, Reference Yde, Knudsen, Hasholt and Mikkelsen2014; Hawkings and others, Reference Hawkings2015, Reference Hawkings2016; Urra and others, Reference Urra2019) and the Antarctic Ice Sheet (Wand and others, Reference Wand, Schwarz, Brüggemann and Bräuer1997; Mikucki and others, Reference Mikucki, Foreman, Sattler, Lyons and Priscu2004; Skidmore and others, Reference Skidmore, Tranter, Tulaczyk and Lanoil2010; Sun and others, Reference Sun2015; Michaud and others, Reference Michaud2016) – see Supplementary appendix for underlying data. This includes both stream chemistry from land terminal outlets of glaciers and ice sheets and samples of subglacial water taken directly from hot water boreholes. Antarctic waters only rarely emerge on the land surface, with notable examples found at Blood Falls in the McMurdo Dry Valleys and Lewis Cliffs Ice Tongue in the central Transantarctic Mountains. However, in Antarctica, the borehole samples include access to subglacial lakes and porewaters of subglacial sediments. In Greenland, the boreholes recharge from a distributed drainage system in the surrounding subglacial environment.
For a broad synoptic view, we plotted major cation and anion data from each glacial terminus or borehole site on a single Piper plot (Fig. 1). With few exceptions, data from alpine glaciers, the Greenland Ice Sheet, and the Antarctic Ice Sheet plot on distinct regions of the diagram. Most waters from alpine glaciers have relatively high Ca2+ + Mg2+ and relatively high HCO3− (Wadham and others, Reference Wadham2010). The diagram also clearly distinguishes alpine glacier settings where carbonate minerals make up a substantial proportion of the bedrock (i.e. those with limestones and similar sedimentary rocks, see Graly and others, Reference Graly, Drever and Humphrey2017b) from those where silicate minerals dominate the bedrock composition, with silicate bedded glaciers relatively depleted in Ca2+ and HCO3−. Glaciers draining basalt bedrock (i.e. those in Disko Island and Iceland) may also have a distinct composition, though only two are represented in the dataset. Most Greenland Ice Sheet waters have relatively high Na+ + K+ and relatively high HCO3−. The terminal outlet samples from Greenland tend to be enriched in Ca2+ + Mg2+ compared to boreholes and lateral outlets. Most Antarctic Ice Sheet waters have relatively high Na+ + K+ and relatively high SO42− + Cl−.
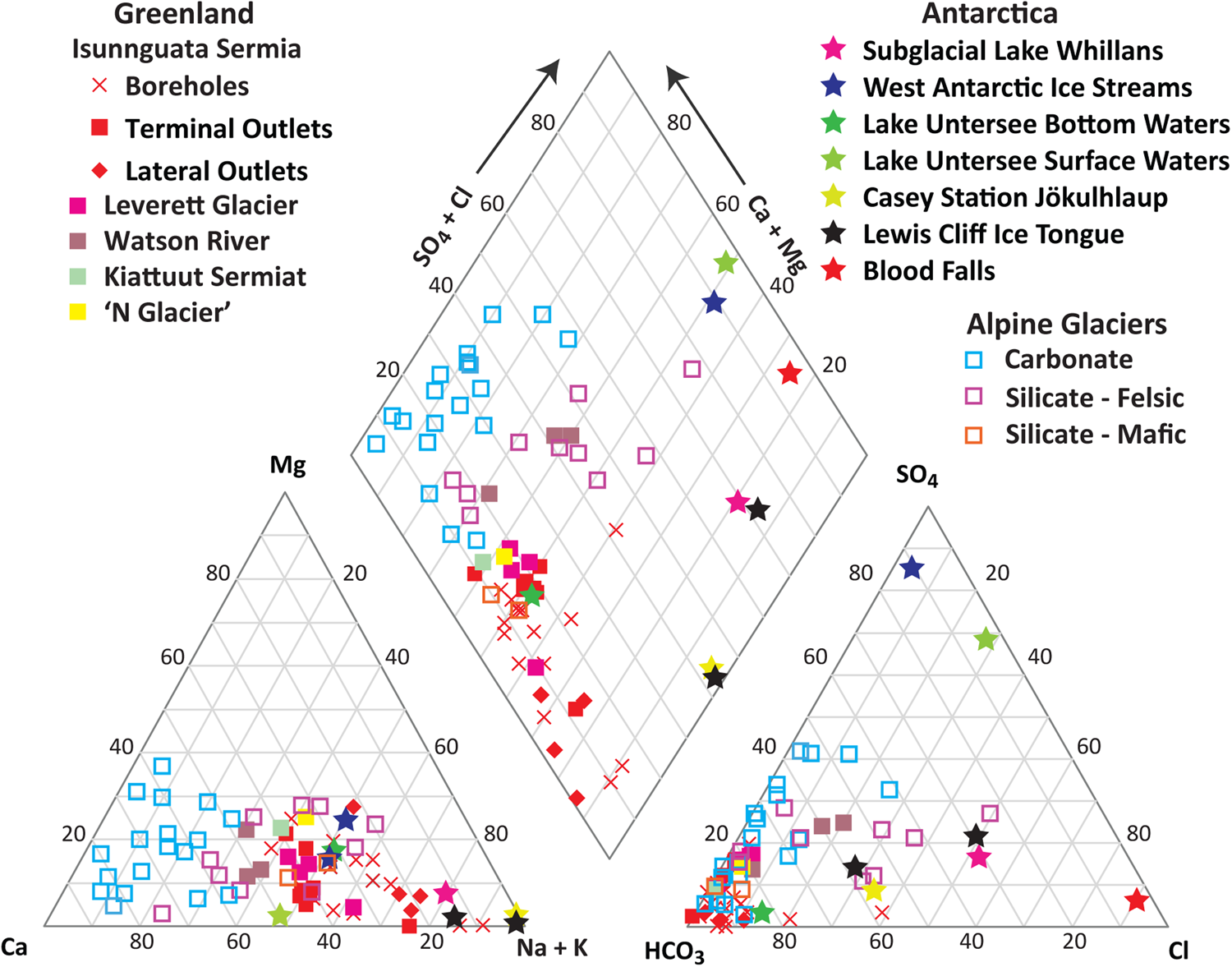
Figure 1. The major ion concentrations (in molar equivalents) of glacial waters from a wide range of studies (see sources in text; data in Supplementary appendix) presented on a Piper plot. Waters from alpine glaciers, the Greenland Ice Sheet and the Antarctic Ice Sheet generally plot on distinct regions of the diagram.
These differences can be broadly explained by the vastly different residence times of sediment and water within these respective settings. Both water and sediment are evacuated relatively quickly from the subglacial environments of alpine systems. This allows dissolution and oxidation of the most chemically reactive minerals to dominate the chemistry of the discharging water. These are typically sulfide and carbonate minerals, producing Ca2+ and Mg2+ as cations and HCO3− and SO42− as anions. Despite their unsurprising depletion in Ca2+ and HCO3−, the silicate bedded glaciers are still far more enriched in Ca2+ and Mg2+ than would be expected by their substrate composition. This implies dissolution of trace carbonate minerals, even in systems where an igneous or meta-igneous bedrock substrate implies <1% abundance of these reactive minerals.
The comparatively low abundance of Ca2+ and Mg2+ in Greenland Ice Sheet waters suggests a system depleted in reactive carbonate minerals, probably due to longer sediment residence times (Graly and others, Reference Graly, Humphrey, Landowski and Harper2014). Carbonate saturation could also play a role in limiting Ca2+ and Mg2+ abundance, however when sampled, the Greenland Ice Sheet waters were not at or close to calcite saturation (Graly and others, Reference Graly, Harrington and Humphrey2017a, Reference Graly, Drever and Humphrey2017b). Antarctic Ice Sheet waters can be highly saline. At Blood Falls, the most saline subglacial site sampled to date, Na+ and SO42− concentrations are at mirabilite saturation and total dissolved solids are around four times that of sea water (Mikucki and others, Reference Mikucki, Foreman, Sattler, Lyons and Priscu2004; Badgeley and others, Reference Badgeley2017). The porewaters of West Antarctic ice stream sediments are at gypsum and aragonite saturation, with 1/10th the total dissolved concentration of seawater (Skidmore and others, Reference Skidmore, Tranter, Tulaczyk and Lanoil2010). The subglacial or permanently ice-covered pro-glacial lakes are at calcite saturation. The precipitation reactions resulting from these saturation states remove Ca2+, Mg2+, HCO3− and SO42− from the waters, leaving them enriched in Na+, K+ and Cl− compared to other glacial systems.
The salinity question
The existence of highly saline waters in the Antarctic subglacial environment raises the question both of the origin and pervasiveness of subglacial brines. Some studies of subglacial brines have suggested that the brines originate either as seawater (Lyons and others, Reference Lyons2005) or from evaporite minerals hosted in underlying sedimentary rocks (Rutishauser and others, Reference Rutishauser2018). Freezing on the base of the glacier would enhance the salinity of seawater or dissolved evaporates through cryoconcentration, by which brine segregates from freezing water, and becomes increasingly concentrated in dissolved solids as water is removed. Alternatively, brine can be primarily lithogenic, with the sulfate forming from the oxidation of sulfides (Skidmore and others, Reference Skidmore, Tranter, Tulaczyk and Lanoil2010) and halides released from the weathering of minerals such as primary phosphates or halide-bearing silicates such as amphiboles and feldspathoids. Subglacial brines, such as Blood Falls, often have a demonstrable lithogenic component; i.e. Fe, Si and Sr are at far higher concentrations than could have originated from sea water (Mikucki and others, Reference Mikucki, Foreman, Sattler, Lyons and Priscu2004, Reference Mikucki2009). A similar process occurs within deep groundwaters, whereby fluids will progressively take on sulfate and halide anions with depth (Frape and others, Reference Frape, Blyth, Blomqvist, McNutt and Gascoyne2003). In most cases, the deep groundwater weathering of halide-bearing minerals only initiates at considerable depth (i.e. 100s to 1000s of m), suggesting a temperature requirement for the initiation of halide release (Frape and others, Reference Frape, Blyth, Blomqvist, McNutt and Gascoyne2003). Deep groundwater potentially plays a role in the formation of Blood Falls (Mikucki and others, Reference Mikucki2015), along with cryoconcentration as the fluids navigate englacial channels (Badgeley and others, Reference Badgeley2017). The pervasive presence of freshly ground mineral surfaces in the subglacial environment, along with the microbial communities they support, may allow for the weathering release of halides from silicates without high temperature conditions, allowing for lithogenic brine formation in the near surface.
Certain glacier hydrological phenomena may be attributable to the presence of brines at the ice–bed interface. Compilations of geophysical data from the Greenland Ice Sheet have shown a persistent mismatch between radar observations of liquid water at the ice-sheet bed and the basal hydrology predicted by geothermal heat flux and thermomechanical modelling (Rezvanbehbahani and others, Reference Rezvanbehbahani, Stearns, Van der Veen, Oswald and Greve2019). Basal water can be detected by strong bed-echo intensity corresponding to a rapid transition between a wet and dry bed (Jordan and others, Reference Jordan2018). The character of reflected radar signals changes under the presence of ponded water of >3 cm thickness (Oswald and others, Reference Oswald, Rezvanbehbahani and Stearns2018). Neither method is completely definitive, and basal water or ponded water may also occur where these radar phenomena are not observed. Nevertheless, basal water is consistently observed in regions of the Greenland Ice Sheet where the ice is too thin or slow moving to expect basal melt (Fig. 2). While different methods of estimating geothermal heat flux produce diverse melt maps for Greenland (MacGregor and others, Reference MacGregor2016; Rezvanbehbahani and others, Reference Rezvanbehbahani, Stearns, Van der Veen, Oswald and Greve2019), no model of geothermal heat flux is able to produce melt in these regions.

Figure 2. Approximate (20 km resolution) regions of unexplained basal meltwater in the Greenland Ice Sheet from Rezvanbehbahani and others (Reference Rezvanbehbahani, Stearns, Van der Veen, Oswald and Greve2019) compared to the underlying geology (a) and the subglacial topography (b). The geological map of Greenland's exposed and subglacial regions is simplified from Dawes (Reference Dawes2009). The subglacial topography map is from BedMachine V3 (Morlighem and others, Reference Morlighem2017).
We ran the SICOPOLIS model from the Eemian interglacial (−120 ka) to the present, following the methods of Rezvanbehbahani and others (Reference Rezvanbehbahani, Stearns, Van der Veen, Oswald and Greve2019). The model provides a map of basal temperatures of the Greenland Ice Sheet in 10 ka time intervals. In the areas where unexplained basal water is detected (i.e. Fig. 2), temperatures are modelled below −10°C. At many sites, temperatures are modelled below −15°C during earlier Pleistocene periods. If brines are able to remain liquid at these temperatures formed from cryoconcentration of seawater, they would need to be 2–3 times concentrated to remain liquid. Any body of water other than seawater would need a vast amount of cryoconcentration to achieve this level of freezing point depression. Saline proglacial lakes (e.g. those described in Henkemans and others, Reference Henkemans, Frape, Ruskeeniemi, Anderson and Hobbs2018) are 100–300 times less concentrated in halide minerals than the inferred brines. Proglacial waters (e.g. those in Graly and others, Reference Graly, Harrington and Humphrey2017a) would need to be concentrated in halide minerals >4000 times.
It is far more likely that brines under Greenland have a primarily lithogenic source. Most of the unexplained basal water occurs at high basal elevations, several hundred to 1500 m above sea level (Fig. 2b), where the thinner ice causes the model to predict cold-based conditions. Such topographically high locations are unlikely to be sites of sea water infiltration. The unexplained basal water occurs across every major type of bedrock geology found in Greenland (Fig. 2a). Phanerozoic sedimentary rocks contain evaporite beds that could facilitate brine formation (Scholle and others, Reference Scholle, Stemmerik, Ulmer-Scholle, Liegro and Henk1993). In the Precambrian, syenites and amphibolites would provide Cl-bearing minerals (Parsons, Reference Parsons1981). In the Paleogene volcanics, amphibole-bearing basalts could be a brine source. In theory, it is not difficult to make a substantially saline solution through the weathering of these rocks. Where they have been measured, volatiles (i.e. Cl, F, OH) make up ~1.5% by weight of syenites in West Greenland (Steenfelt, Reference Steenfelt1996). If syenite rock containing ~0.8 wt. % chloride were reduced to a saturated till with 2 g cm−3 density and porosity of 0.4, and all the halides from the rock were released into the water, the concentration of chloride in the water would be ~40 g l−1 (multiplying weight fraction by density and dividing by porosity), near that of seawater. Complete weathering of all mineral-bound halides in a till is highly unlikely, but this calculation illustrates that combined with other processes, such as reactive transport and cryoconcentration, these sorts of source rocks could well produce subglacial brines.
Conclusions and future directions
The synthesis of a wide range of major ion data from the world's glaciers and ice sheets suggests that the cycling of water and sediment under ice is the fundamental control on glacial geochemistry. Alpine glaciers, with their short water residence times and fast sediment cycling, are more chemically similar to each other than to any large glacial systems where water residence times are longer and sediments are depleted of reactive trace minerals. This controlling role of hydrology mostly overrides the role of bedrock geology in the chemical composition of subglacial waters. The Greenland and Antarctic ice sheets also differ dramatically from each other, as waters collected during the Greenland Ice Sheet's melt season are not significantly limited by chemical saturation.
The observed waters of Antarctica vary over a wide range of dissolved solids concentrations and major element compositions which are chiefly controlled by which minerals are at saturation in the subglacial environment. The set of conditions that cause the most saline solutions to develop is not fully understood, but there is some indication that such conditions may be widespread and may exist outside of Antarctica, i.e. under the Devon Ice Cap (Rutishauser and others, Reference Rutishauser2018) and Greenland Ice Sheet. If lithogenic subglacial brine formation is indeed widespread in Antarctica, Greenland and elsewhere, it has substantial implications for paleo ice-sheet behavior. Liquid at the ice–rock interface enables basal sliding and thereby faster ice-sheet response to climate signals. It also implies a wider distribution of subglacial microbial habitats and a larger impact of ice sheets on global geochemical cycling.
Future work on glacier geochemistry would benefit from a more complete understanding of chemical weathering mechanisms. The exact processes by which sulfates and halides are released into solution is relevant to the formation and prevalence of high salinity subglacial fluids. Furthermore, we understand that the easiest waters to sample, i.e. those naturally flowing out at land termini, are not fully representative of the diverse range of processes occurring under ice. Work accessing the glacial bed through drilling has characterized weathering regimes not present in marginal discharge; likewise, offseason water sampling can characterize a different weathering regime (Graly and others, Reference Graly, Humphrey and Licht2018). Glacial geochemistry has strong potential to enhance our understanding of subglacial processes, along with the ecology and global impacts of subglacial environments.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/aog.2023.56
Acknowledgement
We gratefully acknowledge Tavi Murry and the IGS for organising the online seminar series and inviting J Graly's talk, on which this paper is based. We also thank Martyn Tranter for editing and two anonymous reviewers for their constructive feedback.




